Polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalates and organotins in northern Atlantic Spain's coastal marine sediments
Blanca
Antizar-Ladislao
*
School of Engineering and Electronics, University of Edinburgh, William Rankine Building, Edinburgh, United Kingdom EH9 3JL. E-mail: B.Antizar-Ladislao@ed.ac.uk; Fax: +44131 6506781; Tel: +44131 6505712
First published on 16th October 2008
Abstract
Surface sediment samples (0–10 cm) from ten shallow marine sediments affected by industry and shipping traffic on the northern Atlantic Spanish coast were analysed to determine prevailing concentrations of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), phthalates and organotins. PAHs were detected at eight of the study areas (1.4 to 4.9 µg g−1), while PCBs were detected only at four of the study areas (2.9 to 37 ng g−1). PAHs concentration rations indicated that PAHs were mainly of pyrogenic origin. PCB congener patterns in all of the sediment samples were the same, and contained the less volatile congeners PCB-138, PCB-153 and PCB-180. Bis(2-ethyl hexyl)phthalate was the most abundant phthalate (190 to 2,600 ng g−1). Total organotin concentrations varied widely from 7.7 to 489 ng g−1. A significant correlation was found between PAH concentrations and sediment particle sizes (p<0.001). Peak concentrations of organotins have the potential to induce ecotoxicological impacts based on levels specified in international Sediment Quality Guidelines, although the majority of the stations analysed are included in the medium-low range of priority.
Introduction
Sediment is an essential, integral and dynamic natural resource, part of river basins, coastal zones and oceans, and of major strategic importance to Spanish economic and social development. It is generally accepted that sediments inadvertently constitute a sink for persistent organic pollutants (POPs), including polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), phthalates and organotins. POPs are of significant concern due to their high toxicity, long environmental half-lives and unacceptable risk to aquatic organisms, to wildlife and to humans through the ingestion of contaminated fish and shellfish.1–3 In fact, POPs are subject to bioaccumulation in the lipid fraction of biological tissues, thus leading to their biomagnification in the marine ecosystem.4,5 Hence, there is a need of identifying and quantifying levels of POPs such as PAHs, PCBs, phthalates and organotins in marine sediments around the world.PAHs are mainly incineration by-products.6,7 PCBs had a wide range of industrial applications in industry as dielectric and hydraulic fluids, prior to their ban in many countries. However, PCBs continue to be released particularly via the disposal of electrical waste in many countries.3 Phthalates have been used extensively as non-reactive plasticizers since the 1930s to increase the flexibility of many rigid polymers, including PVC.8 Phthalates have also been used in numerous industrial, medical, and consumer products. Recent studies have shown that phthalates might behave as carcinogens as well as endocrine disruptors.9 Ongoing release of phthalates from a range of products and their large-scale production has led phthalates to become ubiquitous environmental contaminants.8,10 Organotin compounds are mainly used as an antifouling agent in boat paints,1 but the International Maritime Organization (IMO) called for a global treaty that bans the application of TBT-based paints starting 1 January 2003, and total prohibition by 1 January 2008, in awareness of its undesirable effects.11,12 In Europe, the current Water Framework Directive is the major Community instrument for the control of point and diffuse discharges of dangerous substances. Decision no. 2455/2001/EC of 20 November 2001 of the European Commission Parliament, amending water policy directive 2000/60/EC defines 11 priority hazardous substances, including TBT compounds, subject to cessation of emissions, discharges and losses into water.
It is currently being investigated whether organotins might behave as endocrine disruptors.13–16 All of these compounds have the potential to enter the marine environment by leaching from waste deposits or other materials and via atmospheric deposition to the sea surface. A relatively large number of studies have involved surveys on the above mentioned priority substances distribution in the European sediments. Nevertheless, to date, a limited number of studies have focused on the occurrence of PAHs, PCBs and organotins in Spanish sediments,17–20 and data on the occurrence of phthalates in northern Spanish coastal marine sediments is nonexistent. It has been reported in several studies that marine sediments collected in other Mediterranean countries are extensively contaminated with POPs.21–23 Thus the objective of the present study was to collect northern Spanish shallow coastal marine sediments and determine the concentration of PAHs, PCBs, phthalates and organotins within the context of reported values from other Mediterranean countries and with reference to available sediment quality and ecotoxicological standards.
Materials and methods
Sampling
Shallow coastal marine sediment samples (about 10 cm depth) were collected in triplicates at low tide in July-September 2007 from 10 stations located in northern Spain, Cantabria, using a grab sampler. Samples from each station were thoroughly mixed with a stainless steel spatula in a glass beaker and sub-samples were collected for analysis. Sampling locations were selected considering the industrial and fishing activities of the area and historical shipping traffic (Fig. 1, Table 1). After collection, samples were placed in amber glass bottles, kept in a cooler box with ice, and transported to the laboratory where they were stored at 4 °C prior to analysis within one month.| Collecting station | Description | |
|---|---|---|
| Noa | Location | |
| a See Fig. 1 for sampling site location. | ||
| 1 | Lat 43°24′37″ Long 3°48′29″ | Location affected by the industrial effluents from a nearby shipyard and by the final effluent of municipal wastewater treatment plant (approx. 1,500 equivalent inhabitants), and receives water from rivers Solía and San Salvador. |
| 2 | Lat 43°25′7″ Long 3°48′22″ | Location affected by the industrial effluents from ferroalloys and foundry, by the traffic of boats and large ships, receives municipal untreated sewage from the Pedrosa island and also receives water from rivers Boo and Carmen. |
| 3 | Lat 43°25′21″ Long 3°46′43″ | Location affected by industrial effluents from a nearby chemical plant (rubber and black carbon). |
| 4 | Lat 43°27′25″ Long 3°45′54″ | Location affected by the traffic of boats and receives water from the river Miera. |
| 5 | Lat 43°26′6″ Long 3°27′8″ | Location affected by the traffic of boats and large fishing ships and receives waters from the river Asón. |
| 6 | Lat 43°24′42″ Long 3°48′22″ | Location affected by the industrial effluents from a nearby shipyard and a nearby yachting marina. Sediments were excavated in 2006 to build up coastal pathway. |
| 7 | Lat 43°25′5″ Long 3°48′24″ | Location affected by the industrial effluents from ferroalloys and foundry, by the traffic of boats and large ships, receives municipal untreated sewage from the Pedrosa island and also receives water from rivers Boo and Carmen. |
| 8 | Lat 43°25′21″ Long 3°46′43″ | Location affected by industrial effluents from a nearby chemical plant (rubber and black carbon). |
| 9 | Lat 43°24′54″ Long 3°49′9″ | Location affected by the industrial effluents from a nearby shipyard. Receives water from rivers El Astillero and El Carmen. |
| 10 | Lat 43°25′42″ Long 3°48′7″ | Location affected by the industrial effluents from a nearby shipyard and a nearby yachting marina. |
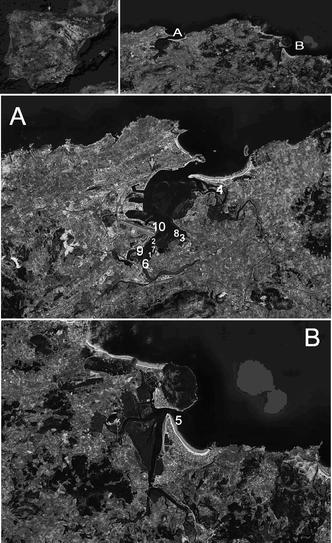 | ||
| Fig. 1 Map of the studied areas showing the locations of the 10 monitored stations in Cantabria, North of Spain. Location A, Santander Bay; location B, Santoña Bay. (See Table 1 for station description). | ||
Physical and chemical analysis of sediments
Physical and chemical analysis of the shallow coastal marine sediments collected from Cantabria were conducted following standard methods (including QC/QA procedures) at Alcontrol accredited laboratory for environmental analysis (Hoogvliet, The Netherlands). Briefly, the percentage dry weight of the sediments was determined after the sub-samples were oven-dried at 110 °C to a constant weight (NEN 6620). Total organic carbon (TOC) was determined by combustion at 600 °C. Sediments were also characterised by particle size distribution within the range 2µm–2cm (NEN 5753), pHH2O and conductivity (NEN 5749). Sn concentration was determined by decomposition with aqua regia followed by ICP-AES analysis (NEN 6966 and NEN-EN-ISO 11885).Sediment samples were also analysed for PAHs, PCBs, phthalates and organotins. The extraction of 16-EPA priority PAHs in sediments was done by soxhlet extraction with acetone/hexane. PAHs analysis was conducted by gas chromatography coupled with mass selective detector, GC/MS (PTV injection, SIM mode) and the limit of detection of each component was 0.02 µg g−1 d.w. (following standard methods NVN 5731 and NEN 5771). The extraction of PCBs was done by soxhlet extraction with acetone/pentane, following the extracts were concentrated (TurboVap) and cleaned-up on an alumina column. PCBs analysis was conducted by gas chromatography and tandem MS-MS detector (Programmed-temperature vaporization - PTV - injection) and the limit of detection of each component was 1 ng g−1 (following standard method NEN 5735).
The extraction of phthalates was done by soxhlet extraction with acetone/petroleum ether. Phthalates analysis was conducted by gas chromatography and MS detector (LVI injection) and the limit of detection of each component was 50 ng g−1. The extraction of organotins was done by soxhlet extraction with hexane, and the posterior conversion of ionic alkyl-tins into species that can be analysed by gas chromatography was based on in situ hybridisation (with sodium borohydride, NaBH4). Analyses of organotins were conducted by gas chromatography coupled with a MS detector (split/splitless injection) and the limit of detection of each component was 1 ng g−1 (following standard method RIKZ A65). For all the analysis, blanks (solvent) and spiked blanks (standards spiked into solvent) were routinely analysed. The calibration was frequently checked during the analysis of samples by the repeated analysis of quality control standards. Recoveries of surrogate standards and internal standards in samples were above 80% throughout all sample analyses.
Statistical analysis
Correlations between POPs concentration and physico-chemical properties of coastal marine sediments were investigated. All the statistical tests were executed with StatistiXL Version 1.8. A confidence limit of greater or equal to 95% was taken as being indicative of statistical significance between paired values.Results and discussion
Results of the physicochemical characterisation (medians) of sediments collected from the 10 different locations in northern Spain, Cantabria, are presented in Table 2. Furthermore, concentrations (medians) of POPs in shallow coastal marine sediments from the same 10 locations in Cantabria are given in Table 3. TOC values in these marine sediments ranged from 1.8% to 11.0% (d.w.); pH values ranged from 7.9 to 8.9; and conductivity values ranged from 3,500 to 9,800 µS cm−1.| Parameter | Station | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| % dry weight (d.w.) | 54.8 | 57.7 | 39.5 | 73.7 | 72.0 | 55.2 | 79.0 | 70.0 | 72.2 | 66.3 |
| Total organic carbon (% d.w.) | 5.7 | 4.6 | 11.0 | 2.0 | 2.0 | 7.2 | 1.8 | 5.2 | 3.4 | 5.4 |
| pH | 7.9 | 8.5 | 8.3 | 8.7 | 8.9 | 8.1 | 8.5 | 8.1 | 8.4 | 8.5 |
| Temperature/°C | 23.0 | 22.9 | 23.6 | 23.2 | 23.4 | 21.4 | 21.4 | 21.5 | 21.5 | 21.5 |
| Conductivity/mS cm−1 | 8.9 | 8.8 | 5.9 | 4.8 | 3.5 | 9.8 | 3.8 | 4.1 | 5.2 | 5.6 |
| Organic contaminant | Station | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| a n.a.: no data available; d.l.: detection limit. | ||||||||||
| PAHs/ng g−1 | ||||||||||
| Naphthalene | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 30 | <d.l. | <d.l. | 20 | <d.l. |
| Acenaphthylene | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 20 | <d.l. | <d.l. | 20 | <d.l. |
| Acenaphthene | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 20 | <d.l. | 30 | <d.l. | <d.l. |
| Fluorine | <d.l. | <d.l. | 30 | <d.l. | <d.l. | 20 | <d.l. | 20 | <d.l. | <d.l. |
| Phenanthrene | 90 | 70 | 150 | <d.l. | <d.l. | 140 | <d.l. | 70 | 80 | 80 |
| Anthracene | 30 | 30 | 60 | <d.l. | <d.l. | 60 | <d.l. | 50 | 50 | 30 |
| Fluoranthene | 160 | 140 | 420 | <d.l. | <d.l. | 320 | 40 | 470 | 160 | 210 |
| Pyrene | 140 | 140 | 490 | <d.l. | <d.l. | 400 | 40 | 440 | 150 | 160 |
| Benzo[a]anthracene | 90 | 80 | 190 | <d.l. | <d.l. | 190 | 30 | 220 | 120 | 140 |
| Chrysene | 80 | 70 | 160 | <d.l. | <d.l. | 200 | <d.l. | 210 | 100 | 110 |
| Benzo[b]fluoranthene | 240 | 310 | 340 | <d.l. | <d.l. | 1,100 | 120 | 230 | 760 | 270 |
| Benzo[k]fluoranthene | 110 | 130 | 150 | <d.l. | <d.l. | 500 | 50 | 100 | 330 | 120 |
| Benzo[a]pyrene | 120 | 150 | 230 | <d.l. | <d.l. | 510 | 50 | 180 | 370 | 150 |
| Dibenzo[a,h]anthracene | 40 | 50 | 50 | <d.l. | <d.l. | 170 | <d.l. | 40 | 130 | 30 |
| Benzo[g,h,i]perylene | 140 | 190 | 220 | <d.l. | <d.l. | 610 | 60 | 180 | 450 | 140 |
| Indeno[1,2,3-cd]pyrene | 140 | 190 | 160 | <d.l. | <d.l. | 590 | 80 | 100 | 430 | 130 |
| ΣPAHs (EPA, 16) | 1,400 | 1,600 | 2,600 | <d.l. | <d.l. | 4,900 | 520 | 2,400 | 3,200 | 1,600 |
| PCBs/ng g−1 | ||||||||||
| PCB 28 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 3.1 |
| PCB 52 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 1.8 | <d.l. | <d.l. | 1.3 | <d.l. |
| PCB 101 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 2.8 | <d.l. | <d.l. | 1.1 | <d.l. |
| PCB 118 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 2.7 | <d.l. | <d.l. | 2.2 | 1.5 |
| PCB 138 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 9.3 | <d.l. | <d.l. | 5.8 | 2.8 |
| PCB 153 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 9.2 | 1.7 | <d.l. | 6.8 | 3.7 |
| PCB 180 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 11 | 1.2 | <d.l. | 5.3 | 3.5 |
| ΣPCBs | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 37 | 2.9 | <d.l. | 23 | 15 |
| Phthalates/µg g−1 | ||||||||||
| Dimethyl phthalate | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Diethyl phthalate | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Di-n-butyl phthalate | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Butyl benzyl phthalate | n.a. | n.a. | n.a. | n.a. | n.a | 0.44 | <d.l. | <d.l. | <d.l. | <d.l. |
| Bis(2-ethyl hexyl) phthalate (DEHP) | n.a. | n.a. | n.a. | n.a. | n.a | 2.60 | 0.19 | 2.80 | 0.88 | 0.52 |
| Organotins/ng g−1 | ||||||||||
| Tributyltin | n.a. | n.a. | n.a. | n.a. | n.a | 330 | 10 | 3.4 | 23 | 34 |
| Dibutyltin | n.a. | n.a. | n.a. | n.a. | n.a | 19 | 2.8 | 1.5 | 4.1 | 4.5 |
| Monobutyltin | n.a. | n.a. | n.a. | n.a. | n.a | 140 | 5.5 | 2.8 | 11 | 14 |
| Triphenyltin | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Diphenyltin | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Monophenyltin | n.a. | n.a. | n.a. | n.a. | n.a | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
Station 6 located in Santander Bay (Fig. 1) corresponded with the highest POP concentrations, where PAHs (4.9 µg g−1), PCBs (37.0 ng g−1), phthalates (3.0 µg g−1) and organotins (480.0 ng g−1) where present. Most of the contamination at this station is likely to be the result of historic industrial activities, and also the nearby shipyard operating since the early 1900s. A decrease in POP concentrations with distance from the inner locations of Santander Bay was apparent, and higher concentrations were encountered in the west side as compared to the east side of the bay. This geographical distribution may be indicative of tidal action playing an important role in transporting particle-bound organic contaminants. Additionally, Station 6 presented elevated TOC (7.2%, d.w.) and conductivity (9.8 mS cm−1) values, which could have contributed to a higher sorption of hydrophobic contaminants to marine sediments.1 Stations 4 and 5 located in Santander Bay and Santoña Bay presented very low POP concentration levels, and low organic content (TOC < 2%, d.w.). A general correlation between POP concentration and organic content was not found in this study.
PAHs
PAHs in shallow coastal marine sediments from Cantabria were found at eight of the ten stations characterised, and concentrations ranged between 1,400 and 4,900 ng g−1 (Table 3). The concentration range of PAHs in this study was within the same range of the concentration of PAHs reported in sediments in the south east of France,25 and below those reported in the south of Italy, from 400 to 12,800 ng g−1 (d.w.).22 Nevertheless, higher concentrations of PAHs were found in northwestern Spanish coast within the range of 14.8 and 89.6 ng g−1 (d.w.) following the Prestige oil spill in 2002.31The geographical distribution clearly showed that all stations located at the inner parts of Santander Bay were more polluted than stations 4 and 5, where PAHs were below the detection limit. These trends suggest that PAHs were present in sediments collected from stations nearby the shipyard, influenced by heavy ship traffic, and exposed to smooth tides and sea waves. Furthermore, PAH contaminated stations are located very close to highly populated urban areas, thus atmospheric deposition can not be ruled out as a PAH source. Additionally, stormwater runoff from adjacent urban areas could also be a major pathway transporting PAHs into sediments.
PAH distribution patterns show the predominance of combustion derived high molecular weight (HMW) PAHs (four to six ring PAHs) over petrogenic low molecular weight (LMW) PAHs in all stations where PAHs were present. In general, HMW PAHs accounted for 70–83% of total PAHs in these stations. It has been reported that phenanthrene/anthracene (Phe/An) ratios of less than 10 indicates that combustion originated pyrogenic PAHs are predominant over petroleum related PAHs.7 Ph/An ratios in all samples ranged from 1.4 to 3.0, clearly indicating a higher input of pyrogenic PAHs. Benzo[b]fluoranthene to benzo[k]fluoranthene (B[b]F/B[k]F) and benzo[a]anthracene to chrysene (B[a]a/chrysene) ratios can indicate relative contribution of PAHs from coal burning and vehicle operation.32 B[b]F/B[k]F and B[a]A/chrysene ratios ranged between 2.2 to 2.4 and 1.0 to 1.3 respectively in all sediments, indicating that PAHs from coal-wood burning are dominant over PAHs originated from motor vehicles. This ubiquitous distribution of PAHs found in shallow marine sediments in Cantabria is thus due to continuous emission of large amounts of PAHs from fossil fuel combustion and wood burning. Therefore, unless we stop burning fossil fuels and wood as primary energy sources, PAH production is inevitable and a considerable amount of PAHs will be continuously introduced into the environment.
The highest PAH concentration (4,900 ng g−1) was observed at station 6, the closest station to the main shipyard located in Santander Bay. Intensive shipping traffic is likely to be the main source of these PAHs. The concentration of PAHs was lower at stations located at longer distances from the mentioned shipyard (i.e., PAH concentrations of 3,200 ng g−1 and 1,400 ng g−1 at stations 9 and 1, respectively and PAH concentration of 1,600 ng g−1 at stations 2 and 10). Stations 3 and 8 are influenced by industrial effluents from a nearby chemical plant, and at these stations PAHs concentration ranged between 2,400 and 2,600 ng g−1. Stations 2 and 7 are closely located, but PAH concentrations were 1,600 and 500 ng g−1 respectively, which may be explained by the influence of different hydrodynamics and thus particle size distribution of the sediments collected from both stations, where sediment collected at station 2 was characterised by higher percentage (d.w.) of small size particles than sediment collected at station 7 (Fig. 2). Sediments from station 2 presented a higher percentage of particles with sizes in the range 50 to 125 µm than sediments from station 7, and showed a significant correlation (p<0.001) between total PAH concentration and particle size within the mentioned range. These results suggested that higher concentrations of PAHs in marine sediments are influenced by adsorption and thus controlled by surface area of particles rather than by TOC concentration (p<0.05). Thus, future studies should consider the hydrodynamic transport of different particle fractions, since PAH concentrations have been found to be higher in smaller particles here and in other previous studies.33,34
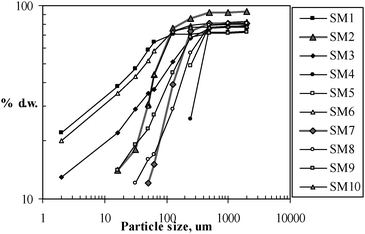 | ||
| Fig. 2 Particle size analyses of the marine sediments collected in Cantabria. | ||
PCBs
Concentrations of PCBs in shallow coastal marine sediments in Cantabria were monitored at stations 1–10 (Table 3). This study quantified 7 PCB congeners. PCB concentrations were lower than or close to method detection limits in stations 1–5 and 8, while high concentrations of PCBs were found in the sediments from stations 6, 7, 9 and 10 and ranged from 2.9 to 37.0 ng g−1. Thus, western sediments in Santander Bay contained higher levels of PCBs than eastern sediments, and their presence was likely from historic releases from adjacent industrial activities and railway operations. The concentration range of PCBs in this study was below the range of the concentration of PCBs reported in sediments in other Mediterranean areas (Table 4).| POP | Sampling | Concentration | Reference | |
|---|---|---|---|---|
| Location | Year | |||
| PAH | Spain, North coast | 2007 | <d.l.−4,900 | This study |
| Italy, Mar Piccolo | 2001 | 380–12,750 | 22 | |
| Italy, Trieste Gulf | 35–682 | 24 | ||
| France, Rhone Delta | 325–3,182 | 25 | ||
| PCB | Spain, North coast | 2007 | <d.l.−37 | This study |
| Spain, Catalan coast | 1.1–311 | 26 | ||
| Italy, Mar Piccolo | 2001 | 2–1,684 | 22 | |
| Italy, Venice Lagoon | 6–1,590 | 27 | ||
| Organotins | Spain, North coast | 2007 | 7.7–489 | This study |
| Spain, Barcelona harbour | 2002 | 423–17,243 | 28 | |
| France, South west | 2001 | 1.5–576 | 29 | |
| Italy, North west Sicilian coast | 1999–2000 | 7.2–66 | 30 | |
In this study, the sediment sample corresponding to lowest concentration of 2.9 ng g−1, station 7, was mainly of mineral composition and showed the lowest TOC value (<2.0%). It was observed that PCB values reported in this study were within the same range of PCB concentrations found in coastal sediments in Barcelona (Spain), which ranged from 2.3 to 44.0 ng g−1 (d.w.),17 but higher than PCB concentrations found in marine sediments in south Italy, which were lower than 1.7 ng g−1 (d.w.).22
The PCB source(s) is (are) likely to be located in the inner western part of Santander Bay which receives water from rivers Solía and San Salvador and also Boo and Carmen, both carrying effluents of nearby industry, and probably transported due to tidal action to the eastern side. As supporting evidence, PCB congener patterns in sediments from stations 6, 9 and 10 were the same. PCB congener patterns in sediments can be used as a fingerprint to identify sources of PCBs because each Aroclor has different PCB congener patterns and different characteristic PCB congeners.35 Shallow marine sediments collected in Cantabria contained the less volatile congeners PCB-138 (2.8 to 9.3 ng g−1), PCB-153 (1.7 to 9.2 ng g−1) and PCB-180 (1.2 to 11.0 ng g−1) and their concentration contributed to 68 to 100% of total PCBs monitored. This may be due to the greater tendency of more hydrophobic PCBs to be adsorbed onto the solids or because the greater leaching and biodegradation of less chlorinated congeners. Nevertheless, signature of highly chlorinated PCBs in this study could not be related to any Aroclor. This is the first available study which collected and analysed PCBs in Santander Bay.
Phthalates
Six phthalate compounds, namely, dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DBP), butylbenzyl phthalate (BBP), di-n-octyl phthalate (DOP) and di(2-ethylhexyl) phthalate (DEHP), have been classified as priority organic pollutants by the USEPA.36 Concentrations of phthalates in shallow sediments in Cantabria were monitored at stations 6 to 10 (Table 3). DEHP was present in all samples at a concentration range of 0.2 to 2.8 µg g−1, and contributed to 86% to 100% of total phthalates monitored. Sediments collected at station 6 presented the highest concentration of DEHP (2.6 µg g−1) and also BBP (0.4 µg g−1) was detected. Station 6 also presented the highest concentration of PAHs, PCBs and organotins.Concerning phthalate levels no data are available from other Mediterranean countries but for sediments collected in 1999, from the Netherlands levels for DEHP and DBP were 67.4 and 25.3 ng/g, respectively,37 indicating higher phthalate levels in northern Spanish's coastal marine sediments than in the Netherlands.
Organotins
Concentrations of three butyltins and three phenyltins in shallow coastal marine sediments in Cantabria were monitored at stations 6–10 (Table 3). Butyltins were found at five of the study areas (7.7 to 489 ng g−1) but no phenyltins were detected in any of the sediments under investigation. Organotin concentrations within the same range were reported in south west of France,29 but higher organotin concentrations have been reported in the north east of Spain, at Barcelona harbour in the range of 423–17,243 ng g−1 (d.w.).28The highest concentration of tributyltin (TBT) was located at station 6, where concentrations of TBT, dibutyltin (DBT), monobutyltin (MBT) and Sn were 330, 140, 19 and 7,000 ng g−1, respectively. The concentration of total inorganic tin at this station was a much higher value than organometallic tin. In fact higher concentrations of TBT, DBT and MBT have been reported in Barcelona harbour (north-eastern Spain) within the range of 239 to 11,473, 131 to 5,110 and 52 to 660 ng g−1 respectively (d.w.),38 and in Gipuzkoa (northern Spain) within the range of 122 to 13,370, 294 to 1,392 and 1,290 to 4,305 ng g−1 respectively (d.w.).19 Lower concentrations of TBT, DBT and MBT have also been reported in other Mediterranean countries. In the north west Sicilian coast of Italy TBT was found in concentrations within the range of 7.3 to 66 ng g−1 (d.w.) and no DBT or MBT concentrations were detected.30
These results make apparent that organotin contamination is widely distributed along coastal marine sediments in Cantabria affected by a heavy shipping traffic, but presenting lower concentrations than harbours with heavier shipping traffic in Spain, such as Barcelona harbour.38 Additionally, presence of DBT and MBT was indicative of TBT degradation. According to previous studies, TBT degradation can be evaluated following a degradation index, which also assists in predicting whether butyltin contamination is recent or not.28 Butyltin degradation index (BDI) was calculated as, BDI = [(DBT + MBT)/TBT], where MBT, DBT and TBT refer to their concentrations. Values of BDI lower than 1 indicate that butyltin contamination is recent, and values of BDI higher than 1 indicate that there were no recent inputs of butyltins to the sediments. Thus, the calculated BDI ranged from 0.48 to 1.26 in shallow sediments in Cantabria, which are in the same range as those reported in the Mediterranean Region in Spain within the range 0.15 to 2.9428 and Australia within the range 0.27 to 2.72.39 Only sediments collected from station 8 presented a BDI higher than 1 indicating that there were no recent inputs of butyltins to the sediments in this station, which is in agreement with the location of the station, far away from the water channel, as compared to the rest of the stations monitored.
Ecotoxicological concerns
In this study, the Sediment Quality Guidelines (SQG) were used to asses the potential ecotoxicological impacts for POPs measured in northern Atlantic Spanish coastal marine sediments. The weight of evidence approach to the derivation of empirically derived SQGs was originally developed in the US NOAA's National Status and Trends Program.40 Using this approach numerical SQGs two values are derived, the TEL and PEL values, which correspond with the threshold effects level and probable effects level. The TEL and PEL values define three concentration ranges for a chemical, including those that were rarely (below the TEL), occasionally (above the TEL, but below a PEL) and frequently (above the PEL) associated with adverse effects. Thus, when contaminant concentrations exceed PEL, sediment samples are predicted to be toxic.The US NOAA's SQGs for PAHs and PCBs were used in this study. The SQG proposed by the Dutch RIKZ (National Institute for Coastal and Marine Management) were used as reference values to assess the impact of organotin levels encountered in coastal marine sediments in Cantabria. Dutch guidelines are specified in terms of a maximum permissible concentration (PEL) and a negligible concentration (NEC or TEL).41 The comparison of POP concentrations found in this study with SQGs is presented in Table 5. For PAHs, contamination levels in 7 stations exceeded the TEL. For PCBs, contamination levels in two stations exceeded the TEL. For TBT, five stations tested for TBT concentration presented contamination levels exceeding the TEL, showing a high ecotoxicological risk. All stations (stations 6, 9, 10) with PCB contamination levels exceeding the TEL presented PAH and TBT contamination levels exceeding the corresponding TEL values. Thus, the comparison with the international SQGs shows the highest ecotoxicological risk for this area.
| POP | SQG TEL-PEL/µg kg−1 d.w. | Number of stations | ||
|---|---|---|---|---|
| <TEL | TEL-PEL | >PEL | ||
| a SQG by US NOAA. Mixture restricted to 13 PAHs (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene and benzo(a)pyrene. b SQG by US NOAA. c SQG by Dutch RIKZ. Standard sediment having 10% organic matter, or equivalently 5% organic carbon. | ||||
| PAHs | 655–6,676a | 3 | 7 | — |
| PCBs | 22–189b | 8 | 2 | — |
| TBT | 0.007–0.7c | — | — | 5 |
For assessing the potential effects of the POPs determined in coastal marine sediments in Cantabria, an approach based on mean PEL quotients calculation was applied. Briefly, PAHs, PCBs and TBT concentrations were divided by their respective PEL value. The concentrations of phthalates were not computed since no SGGs were for phthalates were available. Following, the mean of the quotients (mPELq) for all chemicals was calculated. The resulting index provides a method for the evaluation of synergic effects of contaminants in sediments.42 This approach defines four relative priority levels for contaminated sites: highest (mPELq > 2.3), medium-high (mPELq ranged from 1.51 to 2.3), medium-low (mPELq ranged from 0.1 to 1.5) and lowest (mPELq < 0.1). Concerning mPELq results, station 6 was included in the highest range, stations 7, 9 and 10 were included in medium-low range, and stations 4 and 5 were included in the low range. Additionally, considering only PAH quotients in all the stations, including the stations where other contaminants were not detected (or analysed), stations 1–3 and 6–10 were included in medium-low range. Thus, the majority of the stations analysed are included in the medium-low range, apart from station 6 included in the highest range and strongly influenced by industrial effluents from a nearby shipyard and a nearby yachting marina.
Conclusions
This is, to the knowledge of the author, the first time phthalate levels are reported in marine sediments in Spain. All samples were dominated by DEHP, with the highest value reported in station 6, located at the inner part of Santander Bay. PAHs were detected in eight out of 10 monitored stations, showing their ubiquity in coastal marine sediments. The highest contamination level was detected in station 6 with concentrations of PAH, PCBs, phthalates and organotin above the detection levels. The high concentrations found in samples from station 6 could be attributed to the high industrial impact of this area and the nearby shipyard. Station 6 corresponds to a regular fishing point, and thus the concentration levels found in this station may pose harmful effects to molluscs and other marine organisms, and ultimately these persistent organic contaminants may enter the trophic chain. There was an apparent decrease in organic contaminants concentration with distance from the inner locations of Santander Bay, and higher concentrations were encountered in the west side than in the east side of the bay. This was probably as the result of the tidal action playing an important role in transporting particle-bound organic contaminants.Acknowledgements
This research has been supported by the Spanish Ministry of Education and Science through the Ramón y Cajal programme. I am thankful to Mr D. Antizar and Mr J. L. Turrión for their assistance with surveying and sampling.References
- B. Antizar-Ladislao, Environ. Int., 2008, 34, 292–308 CrossRef CAS.
- K. C. Jones and P. De Voogt, Environ. Pollut., 1999, 100, 209–221 CrossRef CAS.
- O. Wurl and J. P. Obbard, Chemosphere, 2005, 58, 925–933 CrossRef CAS.
- S. Bayen, O. Wurl, S. Karuppiah, N. Sivasothi, K. L. Hian and J. P. Obbard, Chemosphere, 2005, 61, 303–313 CrossRef CAS.
- H. W. Vallack, D. J. Bakker, I. Brandt, E. Broström-Lundén, A. Brouwer, K. R. Bull, C. Gough, R. Guardans, I. Holoubek, B. Jansson, R. Koch, J. Kuylenstierna, A. Lecloux, D. Mackay, P. McCutcheon, P. Mocarelli, R. D. F. Taalman and N. G. Weavers, Environ. Toxicol. Pharmacol., 1998, 6, 143–175 CrossRef.
- B. Antizar-Ladislao, J. M. Lopez-Real and A. J. Beck, Crit. Rev. Environ. Sci. Technol., 2004, 34, 249–289 CrossRef CAS.
- H.-M. Hwang, P. G. Green and T. M. Young, Chemosphere, 2006, 64, 1383–1392 CrossRef CAS.
- C. A. Staples, D. R. Peterson, T. F. Parkerton and W. J. Adams, Chemosphere, 1997, 35, 667–749 CrossRef CAS.
- K. Marsee, T. J. Woodruff, D. A. Axelrad, A. M. Calafat and S. H. Swan, Environ. Health Persp., 2006, 114, 805–809 CAS.
- Q.-Y. Cai, C.-H. Mo, Q.-Y. Zeng, Q.-T. Wu, J.-F. Férard and B. Antizar-Ladislao, Env. Exp. Botany, 2008, 62, 205–211 Search PubMed.
- CD, O. J. European Communities, 2002, L183, 58–59 Search PubMed.
- IMO, International Marine Organisation, International Convention on the Control of Harmful Antifouling Systems on Ships, 2001 Search PubMed.
- L. Lagadic, M. A. Coutellec and T. Caquet, Ecotoxicol., 2007, 16, 45–59 CrossRef CAS.
- T. Nakanishi, J. Health Sci., 2007, 53, 1–9 CrossRef CAS.
- J. Oehlmann, P. Di Benedetto, M. Tilmann, M. Duft, M. Oetken and U. Schulte-Oehlmann, Ecotoxicol., 2007, 16, 29–43 CrossRef CAS.
- J. P. Sumpter, Acta Hydroch. Hydrob., 2005, 33, 9–16 Search PubMed.
- P. Castells, J. Parera, F. J. Santos and M. T. Galceran, Chemosphere, 2008, 70, 1552–1562 CrossRef CAS.
- M. Vila-Escalé, T. Vegas-Vilarrúbia and N. Prat, Water Res., 2007, 41, 2171–2179 CrossRef CAS.
- I. Arambarri, R. Garcia and E. Millan, Chemosphere, 2003, 51, 643–649 CrossRef CAS.
- C. Bancon-Montigny, J. L. Seidel, F. Brissaud and F. Elbaz-Poulichet, J. Environ. Monit., 2008, 10, 638–647 RSC.
- A. Gómez-Gutiérrez, E. Garnacho, J. M. Bayona and J. Albaigés, Environ. Int., 2007, 33, 867–876 CrossRef CAS.
- N. Cardellicchio, A. Buccolieri, S. Giandomenico, L. Lopez, F. Pizzulli and L. Spada, Mar. Pollut. Bull., 2007, 55, 451–458 CrossRef CAS.
- S. Galanopoulou, A. Vgenopoulos and N. Conispoliatis, Mar. Pollut. Bull., 2005, 50, 520–525 CAS.
- M. Notar, H. Leskovsek and J. Faganeli, Mar. Pollut. Bull., 2001, 42, 36–44 CrossRef CAS.
- E. Lipiatou and A. Saliot, Mar. Chem., 1991, 32, 51–71 CrossRef CAS.
- E. Eljarrat, J. Caixach and J. Rivera, Chemosphere, 2001, 44, 1383–1387 CrossRef CAS.
- M. Frignani, L. G. Bellucci, C. Carraro and S. Raccanelli, Chemosphere, 2001, 43, 567–575 CrossRef CAS.
- S. Diez, M. Abalos and J. M. Bayona, Water Res., 2002, 36, 905–918 CrossRef CAS.
- C. Bancon-Montigny, G. Lespes and M. Potin-Gautier, Water Res., 2004, 38, 933–946 CrossRef CAS.
- S. Chiavarini, P. Massanisso, P. Nicolai, C. Nobili and R. Morabito, Chemosphere, 2003, 50, 311–319 CrossRef CAS.
- M. A. Franco, L. Viñas, J. A. Soriano, D. de Armas, J. J. González, R. Beiras, N. Salas, J. M. Bayona and J. Albaigés, Mar. Pollut. Bull., 2006, 53, 260–271 CrossRef CAS.
- R. M. Dickhut, E. A. Canuel, K. E. Gustafson, K. Liu, K. M. Arzayus, S. E. Walker, G. Edgecombe, M. O. Gaylor and E. H. McDonald, Environ. Sci. Technol., 2000, 34, 4635–4640 CrossRef CAS.
- M. Ahrens and C. Depree, Mar. Pollut. Bull., 2004, 48, 341–350 CrossRef CAS.
- A. J. King, J. W. Readman and J. L. Zhou, Mar. Pollut. Bull., 2004, 48, 229–239 CrossRef CAS.
- G. M. Frame, Fresenius J. Anal. Chem., 1997, 357, 714–722 CrossRef CAS.
- USEPA, United States of America - Environmental Protection Agency, Integrated Risk Information System (IRIS), 2008 Search PubMed.
- W. J. G. M. Peijnenburg and J. Struijs, Ecotoxicol. Environ. Saf., 2006, 63, 204–215 CrossRef CAS.
- S. Diez, E. Jover, J. Albaiges and J. M. Bayona, Environ. Int., 2006, 32, 858–865 CrossRef CAS.
- E. D. Burton, I. R. Phillips and D. W. Hawker, Chemosphere, 2005, 59, 585–592 CrossRef CAS.
- E. R. Long and L. G. Morgan, The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program, NOAA Technical Memorandum, NOS OMA 52, 1990 Search PubMed.
- T. Crommentuijn, D. Sijm, J. de Bruijn, K. van Leeuwen and E. van de Plassche, J. Environ. Manag., 2001, 58, 297–312 Search PubMed.
- E. R. Long, C. G. Ingersoll and D. D. MacDonald, Environ. Sci. Technol., 2006, 40, 1726–1736 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
