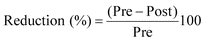Evaluation of HEPA vacuum cleaning and dry steam cleaning in reducing levels of polycyclic aromatic hydrocarbons and house dust mite allergens in carpets
Chang Ho
Yu
a,
Lih-Ming
Yiin
bc,
Zhi-Hua
(Tina) Fan
a and
George G.
Rhoads
*c
aExposure Science Division, Environmental and Occupational Health Sciences Institute, Piscataway, New Jersey 08854, USA
bDepartemnt of Public Health, Tzu-Chi University, Hualien, 97004, Taiwan ROC
cSchool of Public Health – University of Medicine and Dentistry of New Jersey, Room 128, 683 Hoes Lane West, Piscataway, New Jersey 08854, USA. E-mail: rhoads@umdnj.edu; Fax: +732-235-4814; Tel: +732-235-4353
First published on 6th November 2008
Abstract
Dry steam cleaning, which has gained recent attention as an effective method to reduce house dust mite (HDM) allergen concentration and loading in carpets, was evaluated in this study for its efficacy in lowering levels of polycyclic aromatic hydrocarbons (PAHs) as well as HDM allergens. Fifty urban homes with wall-to-wall carpets, mostly low-income and with known lead contamination, were studied in 2003 and 2004. Two carpet-cleaning interventions were compared: Repeated HEPA (High Efficiency Particulate Air filtered) vacuuming alone and repeated HEPA vacuuming supplemented with dry steam cleaning. Vacuum samples were collected to measure carpet loading of dust and contaminants immediately before and after cleaning. Paired comparisons were conducted to evaluate the effectiveness of the cleaning protocols in reducing the levels of PAHs and HDM allergens in carpets. The results indicated that both cleaning methods substantially reduced the loading of PAHs and HDM allergens as well as dust in carpets (p < 0.0001). The reductions in loading of dust (64.4%), PAHs (69.1%), and HDM allergens (85.5%), by dry steam cleaning plus repetitive HEPA vacuuming were larger than the reductions by regular HEPA vacuuming alone: dust (55.5%), PAHs (58.6%), and HDM allergens (80.8%), although the difference was statistically significant only for dust and PAHs. We conclude that intensive HEPA vacuum cleaning substantially reduced the loading of PAHs and HDM allergens in carpets in these urban homes and that dry steam cleaning added modestly to cleaning effectiveness.
Introduction
House dust is a repository for heavy metals, semi-volatile and non-volatile pesticides, polycyclic aromatic hydrocarbons (PAHs), persistent organic compounds, and viable biological particles.1 Once indoors, pollutants associated with dust persist for long periods, particularly if the dust is embedded in carpets. Previous studies have shown that carpets can harbor large amounts of lead, house dust mite (HDM) allergens, PAHs, and other chemical substances.2–5 Without proper cleaning, these contaminants are accessible and potentially harmful to young children.6–10Several previous studies have shown that dust lead contamination can be reduced with a vacuum cleaner, although significant reductions have generally required a thorough and repetitive vacuuming technique.11–13 It may also be difficult to remove HDM allergens and PAHs from carpets. One field study demonstrated that a HEPA-filtered vacuum cleaner with a dirt finder indicator was effective in reducing the carpet loading of dust mite allergens.13 The in-built dirt finder indicator provides a signal when the number of dust particles vacuumed per second falls below a preset level. The HEPA vacuum filtration is believed to prevent dust re-entrainment, which may re-contaminate carpet surfaces after using a non-HEPA vacuum cleaner. However, data demonstrating the importance of this are quite limited. Hot water extraction, another cleaning method for reducing lead dust in carpets, was not superior to dry vacuuming based on a limited number of field evaluation studies.5
A recent intervention study indicated that using a dry steam cleaner in addition to regular vacuuming could further reduce the levels of dust mite allergens for a period of up to eight weeks as compared to using regular vacuuming alone.4 Dry steam cleaning is believed to kill dust mites with high temperature vapor (above 100 °C), and simultaneously to soften and loosen sticky dust which is removed by a towel attached to the cleaning head or by subsequent regular vacuuming. In addition, the high temperature may help volatilize and remove PAHs and other chemicals in the carpets. The dry steam cleaner does not use detergents and only delivers dry vapor (in general, below 6% of water content) into the carpet pile, which allows carpets to dry within 15 minutes. Based on the foregoing, we proposed the use of dry steam cleaning with regular HEPA vacuum cleaning to enhance the removal of common toxicants from carpets.
We evaluated cleaning efficacy on carpets using repetitive HEPA vacuuming with or without dry steaming for PAHs, HDM allergens, and lead. These species were selected for study given their putative effects on health. PAHs are carcinogenic14 and their presence in house dust may be associated with cancer risk;9,15 HDM allergens contribute to allergic sensitization, development of asthma, and exacerbation of asthma symptoms;16 and lead is well known for its neurotoxicity, especially to young children.17 In addition, they represent three major types of pollutants that are associated with house dust.1 The dramatic differences among them in chemical and physical properties may lead to different cleaning efficiency using a given cleaning method. This paper focuses only on PAHs and HDM allergens; the results for reduction of lead in carpets have been published by Yiin et al.18
Methods
Recruitment and screening visit
We studied 50 homes, mostly low-income, urban residences, having at least one room with wall-to-wall carpeting. Families with children having elevated blood lead levels (10–25 µg dL−1) or higher were recruited via referrals from the New Jersey State Health Department or local health departments in northern New Jersey. The referred subjects were interviewed by phone or in person. Homes were screened to ascertain the presence of at least one wall-to-wall carpet. Baseline questionnaires were collected from the parent or caregiver to obtain demographic information, age of the house, and cleaning habits (e.g., how often, what method, and brand name of cleaning products). Informed consent was obtained from an adult member of each household. The study protocols and all supporting documentations were approved by the UMDNJ-Robert Wood Johnson Medical School Institutional Review Board.Interventions
Among carpeted rooms, the one in which a child would be presumed to spend the most time in a day was selected to test the effectiveness of two cleaning interventions: The HEPA–HEPA protocol consisted of HEPA vacuuming followed, after about two hours, by repeat HEPA vacuuming. The HEPA–steam–HEPA protocol consisted of an initial HEPA vacuuming followed by dry steam cleaning, followed by repeat HEPA vacuuming. Both protocols started with the same initial vacuuming that was applied to the whole carpet. The carpet was vacuumed twice in perpendicular directions at a rate of 10 ft2 min−1 using a HEPA vacuum cleaner (Self Propelled WindTunnel™ Ultra Upright, Hoover Company, Newton, IA) equipped with a dirt finder indicator. We used a high sensitivity setting for initial HEPA vacuuming, and used the indicator light as a signal to move the vacuum cleaner to the next area of carpet.After the first HEPA vacuuming, the carpet was divided into two halves to separate the interventions. Thirty to sixty minutes were allowed to permit disturbed dust inside the room to settle. A dry steam cleaner (VaporJet 2400, VaporTechnologies LLC, Sandy, UT) was then applied to one half of the carpet. The dry steam cleaning was performed at a rate of 4.3 ft2 min−1 according to the manufacturer's instructions for carpeting. After the dry steam cleaning a minimum of 15 minutes was allowed for drying, and the whole experimental area of carpet was then cleaned again with the same HEPA vacuum using a clean nozzle.
Dust sampling
Two pre-cleaning vacuum dust samples were collected from each of the 50 study carpets using a template, size 2.76 ft2, with a canister vacuum sampler (Metro Data-Vac/2, Metropolitan Vacuum Cleaner Co. Inc, Suffern, NY). The templates were located near the dividing line that separated the two cleaning strategies. One vacuum sample was for the analysis of PAHs, and one for HDM allergens, each providing a common baseline for the two cleaning strategies. The post-cleaning samples were collected separately on each half of the rug to assess the effects of the two cleaning strategies. These were collected using 2.76 ft2 templates located near the location of the pre-cleaning samples. Thus, in total, 50 pre-cleaning and 100 post-cleaning samples were collected for HDM analysis and an identical number for PAH analysis. Temperature and relative humidity were measured and recorded for each dust vacuum sample.In addition to assessing from the above samples whether there was an overall difference in cleaning efficacy when dry steam cleaning was added to repeated HEPA vacuuming, we were also interested to know whether a second HEPA vacuuming after dry steam cleaning removed a substantial further amount of dust and toxicants. To determine this we collected extra dust samples for HDM and PAHs after dry steam cleaning and compared these levels with the levels found after the subsequent (repeated) HEPA vacuuming. Because of budget limitations these extra samples were limited to 20 homes.
Laboratory analysis
The analysis of PAH content for dust vacuum samples was conducted in our laboratory. All pre/post-vacuum samples were blinded and delivered to the laboratory in identical, numbered containers. Each dust sample (0.1–1.0 g, depending on the amount available) was spiked with 1000 ng of four PAH surrogates, including naphthalene-D8, phenanthrene-D10, pyrene-D12 and benzo[a]pyrene-D12. After spiking with a known amount of acenaphene-D10 and anthracene-D10 as internal standards, the samples were extracted with 10 mL hexane for 30 min in a sonication bath. This procedure was repeated twice. After sonication, the extract was cleaned with a PTFE filter (pore size: 0.2 µm) to remove any particles in the solution. After cleaning, the extract was concentrated to ∼1 mL at 45 °C with a rotary evaporator. The extract was then transferred to a 1 mL clean vial and further concentrated to 500 µL under a gentle nitrogen gas stream. The sample extract were stored in the freezer at 4 °C for at least one day for any fine particles left in the extract to settle before injection on GC/MS.Prior to injection, 100 µL sample extract from the 500 µL available was filtered and transferred to a 200 µL glass insert. Internal standards, 200 ng of acenaphthene-D10 and anthracene-D10, were added to the 100 µL extract to monitor any instrumental variation during sample analysis. A 1 µL sample was injected on GC/MS for PAH analysis. The analytical conditions were as follows. The injection port temperature was 300 °C. The GC oven temperature program was: initially at 50 °C for 1.10 min, then a ramp increase of 25 °C min−1 to 125 °C, followed by a rate increase of 8 °C min−1 to 260 °C and 3 °C min−1 to final temperature of 300 °C with a holding time of 5:00 min. The transfer line temperature was 270 °C and the ion trap temperature was 220 °C.
Seven levels of PAH calibration standards were used for constructing the calibration curves. The calibration curves were constructed using the ratio of the response between the target PAHs and the internal standards, and the R2 was greater than 0.995. The precision of the instrument was determined by performing seven repeated analyses of a mid-level calibration standard. The standard deviation of these seven injections was < 25%. Additionally ten percent of lab blanks (N = 17) and solvent blank were analysed for QA/QC. In general, no PAHs were detected in the solvent blank or lab blanks. The analytical detection limits (ADLs) of PAHs using GC/MS ranged from 10–20 pg, and the method detection limits (MDLs), determined by ADLs and a final concentrated volume (500 µL) for each sample, were 5–10 ng g−1.
The PAH concentrations and the surrogate recoveries were quantified based on the calibration curves. The PAH concentrations were corrected with the recoveries of the surrogates. These considered the loss of PAHs during the sample processing. According to the volatility of each PAH species, the recovery of naphthalene-D8 was applied to correct the concentrations of naphthalene, acenaphthylene, acenaphthene and fluorene; the recovery of phenanthrene-D10 was used to correct the concentrations of phenanthrene and anthracene; the recovery of pyrene-D10 was used to correct the concentrations of pyrene, fluoranthene, benzo[a]anthracene and chrysene; and the recovery of benzo[a]pyrene-D12 was used for the correction of benzo[a]pyrene and the rest of compounds. The recovery of the surrogates was 179 ± 87% for naphthalene-D8, 136 ± 61% for phenanthrene-D10, 85 ± 53% for pyrene-D12 and 88 ± 83% for benzo[a]pyrene-D12. The large variability of the recovery may be due to the difference between each dust matrix so that the recovery of PAH from the dust samples differed.
The dust mite allergens analysis for dust vacuum samples was conducted by STL P&K Microbiology Services Inc. (Cherry Hill, NJ) which is accredited by the American Industrial Hygiene Association under the Environmental Microbiology Laboratory Accreditation Program (AIHA-EMLAP). ELISA (Enzyme-Linked Immunosorbent Assay) method was used to measure two common species of dust mite allergens: Dermatophagoides pteronyssinus allergen 1 (Der p 1) and Dermatophagoides farinae allergen 1 (Der f 1). The dust samples were prepared by extracting 100 mg of the fine dust in 2 mL PBS-T (phosphate buffered saline with 0.05% Tween 20; pH = 7.4). Extracts were clarified by centrifugation at 2500 rpm and the supernatants (1–1.5 mL) were decanted and stored at −20 °C until they were analysed. Individual HDM allergens were measured using monoclonal antibody-based enzyme linked immunosorbent assays (ELISAs) as described by Chapman et al.19 The MQL (method quantification limit) ranged between 0.26 and 0.80 µg g−1 throughout all test samples.
Statistical analysis
Many HDM allergen concentrations in pre-cleaning (70%) and post-cleaning (82%) samples were below the MQL. Carpets with pre-cleaning HDM allergen concentration below MQL were excluded from HDM analysis. To retain all of the remaining 15 sample pairs, we used a value of half of the MQL for carpets with a post-cleaning HDM level that was below MQL. HDM allergen (combination of Der p 1 and Der f 1) concentrations (µg g−1) and PAH concentrations (µg g−1) were converted to loadings (µg ft−2) by multiplying the mass of vacuumed house dust and dividing by the collection template area (2.78 ft2). The reduction percentages for loading of total dust, PAHs and HDM allergens by two cleaning interventions were calculated by the following equation:where, Pre is the pre-cleaning loading, and Post is the post-cleaning loading (either after HEPA vacuuming alone or after dry steaming plus HEPA vacuuming).
Descriptive statistical analyses were performed for loadings (micrograms per unit square foot) of PAHs and HDM allergens to examine their distributions. Most data for PAHs and HDM allergens were skewed to the right. Therefore, data for the levels of PAHs and HDM allergens were log-transformed (base 10) before conducting statistical analyses. Following the transformations, normality tests confirmed that the data were approximately normally distributed.
Paired t-tests were conducted for the pre-cleaning and post-cleaning loadings to estimate the efficacy of dry steam or HEPA vacuum cleaning on each carpet. The cleaning efficacy by dry steam cleaner was examined further by conducting paired t-test between post-HEPA cleaning and post-dry steam cleaning. Due to the asymmetric distribution of reduction percentages calculated, a non-parametric approach (Wilcoxon two-sample test) was used to determine the statistical difference in percent reductions. SAS v9.1 (SAS™, Cary, NC) was used to run all statistical analyses (α = 0.05).
Results
The descriptive statistics for loadings of PAHs and HDM allergens (sum of Der f 1 and Der p 1) obtained by vacuum sampler are provided in Table 1. Sixteen discreet PAHs were selected for the analysis in this study. The geometric means (GM) were calculated due to the skewness of PAH and HDM allergen loadings. Total PAHs calculated by the summation of all listed sixteen PAHs and dust loadings for three cleaning events are also provided in Table 1. Pre-cleaning samples ranged between 0.013 and 220 µg ft−2 for total PAH and between 0.22 and 10.7 µg ft−2 for HDM allergen loadings. Post-cleaning PAH levels were lower and ranged from 0.004 to 121 µg ft−2 following the HEPA–HEPA protocol and from 0.006 to 116 µg ft−2 following the HEPA–steam–HEPA protocol. HDM allergens were not detected in 35 of the 50 carpets tested. Among the 15 with detectable precleaning levels, loadings ranged from between 0.03 and 1.80 µg ft−2 and post-cleaning levels ranged (combining both protocols) between 0.03 and 1.76 µg ft−2. Dust loadings were also reduced from pre-cleaning samples (0.175–4.397 g ft−2) to (0.069–2.308 g ft−2) after HEPA–HEPA and to (0.072–2.459 g ft−2) after HEPA–steam–HEPA.| Carpet contaminantsa | Pre-cleaning | After HEPA–HEPA cleaning | After HEPA–steam–HEPA cleaning | |||
|---|---|---|---|---|---|---|
| N b | GMc | N b | GMc | N b | GMc | |
| a The unit of loading is µg ft−2 for PAHs and HDM allergens and g ft−2 for dust. b N = the number of samples. c GM = geometric mean. d The sum of all sixteen PAHs analysed in the study. See Methods for a description of the HEPA–HEPA and HEPA–steam–HEPA cleaning protocols. | ||||||
| Naphthalene | 44 | 0.024 | 42 | 0.012 | 41 | 0.012 |
| Acenaphthylene | 29 | 0.003 | 12 | 0.001 | 12 | 0.001 |
| Acenaphthene | 32 | 0.010 | 28 | 0.005 | 24 | 0.005 |
| Fluorene | 36 | 0.014 | 35 | 0.006 | 36 | 0.006 |
| Phenanthrene | 47 | 0.250 | 47 | 0.116 | 43 | 0.086 |
| Anthracene | 42 | 0.030 | 30 | 0.024 | 31 | 0.016 |
| Fluoranthene | 47 | 0.409 | 48 | 0.194 | 43 | 0.156 |
| Pyrene | 47 | 0.333 | 48 | 0.173 | 44 | 0.126 |
| Benzo[a]anthracene | 47 | 0.106 | 44 | 0.050 | 37 | 0.042 |
| Chrysene | 47 | 0.123 | 45 | 0.058 | 38 | 0.054 |
| Benzo[b]fluoranthene | 45 | 0.099 | 39 | 0.042 | 33 | 0.053 |
| Benzo[k]fluoranthene | 45 | 0.084 | 37 | 0.038 | 31 | 0.048 |
| Benzo[a]pyrene | 42 | 0.077 | 31 | 0.045 | 30 | 0.036 |
| Indeno[1,2,3-cd]pyrene | 34 | 0.054 | 27 | 0.027 | 22 | 0.034 |
| Benzo[g,h,i]perylene | 28 | 0.021 | 19 | 0.021 | 16 | 0.016 |
| Dibenz[a,h]anthracene | 40 | 0.050 | 27 | 0.029 | 25 | 0.032 |
| Total PAHsd | 49 | 1.57 | 50 | 0.66 | 48 | 0.45 |
| HDM allergens | 15 | 2.41 | 15 | 0.46 | 15 | 0.35 |
| Dust | 50 | 1.00 | 50 | 0.44 | 50 | 0.35 |
To evaluate the effectiveness of each carpet cleaning strategy, paired t-tests between pre-cleaning and post-cleaning samples are presented in Table 2. Further, PAH and HDM allergen reduction achieved by the HEPA–steam–HEPA protocol was compared to cleaning by the HEPA–HEPA protocol. Mean percent reduction for each set of comparisons and its 95% confidence intervals (CI) were calculated. HEPA–HEPA achieved mean reductions of 58.6% and 80.8% for total PAHs and HDM allergens, respectively, (both p < 0.0001). The HEPA–steam–HEPA protocol removed 69.1% and 85.5% of total PAH and HDM allergen loadings from the carpets (p < 0.0001). Compared to HEPA–HEPA, HEPA–steam–HEPA achieved further 31.7% and 24.7% reductions in total PAH and HDM allergen loadings, respectively. However, the addition of the dry steam cleaner did not make a statistically significant difference for many PAHs (except phenanthrene, pyrene, beozo[a]anthracene and benzo[a]pyrene) or for HDM allergens. The mean reductions for dust loading were 55.5% and 64.4% by HEPA–HEPA and HEPA–steam–HEPA protocols, respectively. The additional use of dry steam cleaning reduced total dust loading by 20.5% compared to the HEPA–HEPA protocol alone (p = 0.0012).
| Carpet contaminants | Reduction with HEPA–HEPA | Reduction with HEPA–steam–HEPA | Post-HEPA–HEPA − post-HEPA–steam–HEPA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N a | % reductionb | p valued | N a | % reductionb | p valued | N a | % differencec | p valued | |
| a N is the number of paired carpets analysed. b % reduction is the percentage decrease achieved on each carpet, as a mean value and 95% confidence intervals in parentheses. c % difference is the further reduction percentage achieved by HEPA–steam–HEPA protocol compared with the carpet cleaning by HEPA–HEPA protocol, as a mean value and 95% confidence intervals in parenthesis. d p value<0.05* and p value<0.01.** e The sum of all sixteen PAHs analysed in the study. f Limited to carpets with detectable pre-cleaning HDM allergens. See footnotes to Table 1. | |||||||||
| Naphthalene | 42 | 48.3 (31.9, 60.8) | <0.000** | 41 | 49.8 (31.8, 63.1) | <0.000** | 40 | 7.6 (−14.6, 25.4) | 0.463 |
| Acenaphthylene | 11 | 77.0 (45.9, 90.2) | 0.003** | 12 | 78.2 (58.1, 88.7) | 0.000** | 7 | 35.7 (−50.0, 68.9) | 0.249 |
| Acenaphthene | 23 | 45.0 (17.0, 63.6) | 0.006** | 19 | 48.8 (−4.2, 74.8) | 0.063 | 19 | 26.9 (−46.2, 63.4) | 0.355 |
| Fluorene | 29 | 62.8 (33.0, 79.3) | 0.002** | 29 | 62.0 (37.3, 77.0) | 0.001** | 30 | 16.7 (−19.7, 42.0) | 0.311 |
| Phenanthrene | 46 | 53.9 (34.4, 67.6) | <0.000** | 42 | 66.0 (46.4, 78.5) | <0.000** | 42 | 31.6 (1.1, 52.7) | 0.044* |
| Anthracene | 28 | 45.2 (19.9, 62.5) | 0.003** | 30 | 55.3 (30.8, 71.1) | 0.001** | 24 | 25.7 (−13.2, 51.3) | 0.159 |
| Fluoroanthene | 47 | 53.1 (34.8, 66.3) | <0.000** | 42 | 62.1 (42.5, 75.0) | <0.000** | 43 | 31.7 (−1.9, 54.2) | 0.061 |
| Pyrene | 47 | 48.2 (27.6, 63.0) | 0.000** | 43 | 62.3 (40.3, 76.2) | <0.000** | 44 | 32.7 (5.8, 51.9) | 0.022* |
| Benzo[a]anthracene | 43 | 55.4 (35.7, 69.1) | <0.000** | 36 | 65.0 (49.7, 75.6) | <0.000** | 36 | 27.3 (0.8, 46.7) | 0.045* |
| Chrysene | 44 | 57.4 (41.0, 69.2) | <0.000** | 37 | 64.0 (46.0, 76.0) | <0.000** | 37 | 9.7 (−28.5, 36.6) | 0.562 |
| Benzo[b]fluoranthene | 37 | 63.0 (39.2, 77.4) | 0.000** | 31 | 61.2 (34.6, 76.9) | 0.001** | 30 | 22.1 (−18.3, 48.8) | 0.232 |
| Benzo[k]fluoranthene | 35 | 60.9 (37.9, 75.5) | 0.000** | 29 | 60.9 (33.6, 77.0) | 0.001** | 27 | 16.7 (−35.5, 48.8) | 0.446 |
| Benzo[a]pyrene | 28 | 52.9 (19.5, 72.5) | 0.008** | 28 | 63.1 (33.8, 79.4) | 0.002** | 24 | 39.9 (1.6, 63.3) | 0.043* |
| Indeno[1,2,3-cd]pyrene | 24 | 61.8 (33.6, 78.1) | 0.002** | 19 | 54.1 (18.4, 74.2) | 0.011* | 17 | 31.7 (−26.8, 63.7) | 0.210 |
| Benzo[g,h,i]perylene | 13 | 20.8 (−68.3, 62.8) | 0.514 | 11 | 42.4 (−38.4, 76.0) | 0.191 | 12 | 28.0 (−72.6, 70.0) | 0.425 |
| Dibenzo[a,h]anthracene | 24 | 49.9 (18.7, 69.1) | 0.007** | 23 | 35.4 (−25.9, 66.8) | 0.188 | 20 | 18.6 (−49.6, 55.7) | 0.489 |
| Total PAHse | 49 | 58.6 (43.8, 69.5) | <0.000** | 47 | 69.1 (54.1, 79.1) | <0.000** | 48 | 31.7 (4.8, 51.0) | 0.025* |
| Dust | 50 | 55.5 (44.5, 64.4) | <0.000** | 50 | 64.4 (56.2, 71.5) | <0.000** | 50 | 20.5 (9.1, 30.5) | 0.001** |
| HDM allergens | 15 | 80.8 (64.1, 89.7) | <0.000** | 15 | 85.5 (72.3, 92.4) | <0.000** | 15 | 24.7 (−24.2, 54.4) | 0.244 |
| Dustf | 15 | 54.5. (34.8, 68.2) | 0.000** | 15 | 62.0 (46.1, 73.2) | <0.000** | 15 | 16.5 (−2.6, 32.0) | 0.081 |
In twenty homes, additional dust samples were collected after dry steam cleaning and before the final HEPA vacuum cleaning to assess the importance of the follow-up vacuuming after dry steam cleaning. Descriptive statistics and paired t-tests for total PAH and dust loadings are provided in Table 3. The HDM allergens were excluded from the analysis due to the very low number of available data sets (N = 3). The top row in three cleaning protocols shows data for the portion of the rug that was cleaned twice with the HEPA–HEPA protocol vacuum and had no dry steam cleaning. Calculating from the geometric means in Table 3, HEPA followed by dry steam cleaning (without repeat HEPA) yielded PAH loadings that were 16.6% lower than the levels produced by HEPA–HEPA. The best results (bottom row in three cleaning protocols) were achieved by the full sequence of HEPA–steam–HEPA, which reduced PAH loading by 31.9% compared to repeated HEPA vacuum cleaning alone. However, neither of these PAH differences was statistically significant.
| Carpet cleaning status | Total PAHs GMa | Dust GMa |
|---|---|---|
| a The unit of loading is µg ft−2 for total PAHs and g ft−2 for dust. b Loadings of total PAHs and dust before carpet cleaning. c HEPA–HEPA protocol. d HEPA–steam–HEPA protocol. e Differs significantly from HEPA vacuuming only (p = 0.0437) by paired t-test. | ||
| Baselineb | 1.01 | 0.77 |
| HEPA followed by repeat HEPAc | 0.48 | 0.37 |
| HEPA followed by dry steam without second HEPA vacuuming | 0.40 | 0.33 |
| HEPA followed by dry steam followed by second HEPA vacuumingd | 0.33 | 0.28e |
With respect to total dust loadings, HEPA followed by dry steam cleaning (without second HEPA vacuuming) yielded a geometric mean loading that was 9.2% lower than HEPA–HEPA, while HEPA–steam–HEPA yielded a loading that was 23.6% lower. Only the latter reduction was statistically significant (p = 0.04).
The cleaning results were analysed by carpet type to determine whether this affected cleaning efficacy. Among the 50 home carpets cleaned, 32 were identified as level-loop and 18 as cut-pile. The mean percent reductions by two carpet cleaning interventions for total PAH, HDM allergen and lead loadings were calculated and their reduction percentages were compared by carpet type (Wilcoxon two-sample test; two-sided). As shown in Table 4, the effect of carpet type did not approach significance (p > 0.10) for any of the carpet contaminants and cleaning methods tested in this study.
| Carpet contaminants | Cleaning interventions | Percent reduction | Level-loop vs. Cut-pile (p value)b | |||
|---|---|---|---|---|---|---|
| Level-loop | Cut-pile | |||||
| N | Mean (95% CI)a | N | Mean (95% CI)a | |||
| a The percentage decrease after carpet cleanings, as a mean value and 95% confidence intervals in parentheses. b Wilcoxon two-sample tests were conducted between reduction percentages by carpet type. See footnotes to Table 1. | ||||||
| Total PAHs | HEPA–HEPA | 31 | 58.8 (40.6, 71.4) | 18 | 58.3 (23.9, 77.2) | 0.267 |
| HEPA–steam–HEPA | 31 | 69.2 (50.4, 80.9) | 16 | 68.8 (31.9, 85.7) | 0.647 | |
| HDM allergens | HEPA–HEPA | 8 | 84.1 (63.8, 93.0) | 7 | 76.0 (19.7, 92.8) | 0.400 |
| HEPA–steam–HEPA | 8 | 84.9 (58.3, 94.6) | 7 | 86.1 (57.5, 95.5) | 1.000 | |
| Dust | HEPA–HEPA | 32 | 59.4 (45.3, 69.9) | 18 | 47.7 (26.3, 63.0) | 0.125 |
| HEPA–steam–HEPA | 32 | 65.0 (53.6, 73.6) | 18 | 64.0 (48.4, 74.8) | 0.284 | |
Discussion
The comparison of vacuum samples between the pre-cleaning and post-cleaning indicated that both cleaning sequences tested in this study reduced the levels of PAHs and HDM allergens significantly (p < 0.0001) in the carpets of these urban homes. This appeared to be mainly the result of the substantial (56% and 64%) reductions in geometric mean dust loadings, as shown in Table 2. Roberts et al.13 reported the reduction of dust loading for carpets in 11 homes ranged from 15 to 98% (GM = 72.7%), after intensive carpet cleaning with a vacuum cleaner equipped with HEPA filter bag, supporting the idea that substantial reduction in accessible carpet toxicants can be achieved by removing dust itself. Previous studies, evaluating the effectiveness of cleaning interventions to reduce lead burdens in children, confirmed the relationship of dust loading to lead loading and to blood lead levels.20In this study we detected HDM allergens before cleaning in only 15 (30%) of the homes. Despite this limited number, the decrease in loading of HDM allergens in home carpets was highly statistically significant for both cleaning protocols. HDM allergen loading is probably of more concern than HDM allergen concentration in carpet dusts, because it is more likely related to exposure. The percentage decrease in HDM allergens (81%–86%) was larger than the reduction in total dust (55%–62%), suggesting that the allergens are more superficial on the carpets or are associated for other reasons with particles that are relatively easily removed. While it must be noted that our analysis only included the 30% of homes with the highest levels of HDM allergens, the effectiveness of these vacuuming protocols in lowering these high levels was impressive. Our results are consistent with those of Vojta et al.4 who tested the effectiveness of a dry steam cleaner in reducing the HDM allergen levels in carpets for screened low-income, urban homes (N = 11; HDM allergen concentration > 10 µg g−1) and found that both intensive vacuuming and vacuuming plus dry steam cleaning could reduce high levels of HDM allergens. They reported greater and longer lasting reductions of allergens with dry steam cleaning than with vacuuming alone. We also achieved lower levels with the addition of dry steam cleaning although the difference from repeat vacuuming alone was not statistically significant.
Among the 16 PAHs analysed in this study, loadings for most were reduced significantly from pre-cleaning values by both cleaning protocols (Table 2). Some PAHs, such as acenaphthene, benzo[g,h,i]perylene and dibenzo[a,h]anthracene, were not significantly reduced after cleaning the carpets by either of the two methods. This may have been due to a smaller number of available pairs (pre–post cleaning samples; N ≤ 23). However, both cleaning methods reduced overall PAH carpet loadings significantly in these low-income, urban homes.
We found that adding dry steam cleaning to the HEPA–HEPA protocol led to further reductions of loading with PAHs (7.6% to 39.9%), HDM allergens (24.7%), and dust (20.5%) (Table 2). Significant differences between the protocols were obtained for total PAHs (p = 0.025) and four individual PAHs (phenanthrene, pyrene, beozo[a]anthracene and benzo[a]pyrene); for the remaining twelve PAHs, further reductions were obtained after dry steam cleaning, but the differences were not significant. Yiin et al.18 demonstrated further reduction of lead dust loadings (wipe samples; N = 50; p = 0.038) on carpet surfaces when dry steam cleaning was added to repetitive HEPA vacuuming (mean reduction = 40.4%) under this protocol.
An extra dust sample was collected in 20 homes to determine whether the second HEPA vacuuming after dry steam cleaning confers a substantial advantage (Table 3). The results showed that initial HEPA vacuuming followed by dry steam cleaning (without second HEPA vacuuming) reduced the loadings of dust and total PAHs compared to two consecutive HEPA vacuumings. However, with the smaller sample size, the differences were not significant. The addition of the second HEPA vacuuming after dry steam cleaning further reduced the loadings of total PAH (18.4%) and dust (15.9%), respectively, in carpets cleaned by initial HEPA vacuuming plus dry steaming only; however, these differences were not significant, either.
We found that carpet type (level-loop vs. cut-pile) didn't make a significant difference in cleaning efficacy, either by HEPA vacuum cleaning alone or HEPA plus dry steam cleaning. Causer et al.5 showed that carpet cleaning was more complete with low height, low density, and lightly worn carpets. However in the carpets studied here, level-loop carpets were usually low-height but densely piled, whereas cut-pile ones were high-height but loosely piled. These competing physical characteristics may limit any differences in the effects of the cleaning protocols on toxicant loadings.
Conclusion
A physical intervention study was conducted in 50 low-income, urban households to evaluate the efficacy of two cleaning protocols (HEPA followed by repeat HEPAvs.HEPA, dry steam, HEPA) proposed for reducing levels of PAHs and HDM allergens in wall-to-wall carpets.The results showed that both HEPA–HEPA and HEPA–steam–HEPA can result in significant reductions in loadings of PAHs and HDM allergens in carpets (p < 0.0001). We observed greater percentage reductions in PAHs and HDM allergens when dry steam cleaning was added to the repetitive HEPA vacuuming protocol, which was statistically significant for total PAHs and for dust (p < 0.05). There was no evidence that the efficacy of these cleaning protocols varied between level loop and cut pile carpets.
The cleaning methods tested in this study are effective and practical alternatives when compared to expensive physical abatement (e.g., removal or replacement of contaminated carpets) and are potentially feasible for families of modest means.
Acknowledgements
The authors wish to thank the entire ECSC field and study design team for their efforts in completing the many components of the study. We also want to thank Dr Kyung Hwa Jung for the analysis of PAHs in house dust vacuum samples. The research has been funded by U.S. Department of Housing and Urban Development (# NJLHH0111-02). Dr George G. Rhoads is in part supported by NIEHS center grant to UMDNJ (P30 ES05022).References
- P. J. Lioy, N. C. G. Freeman and J. R. Millette, Environ. Health Perspect., 2002, 110, 969–983 CAS.
- R. G. Lewis, C. R. Fortune, R. D. Willis, D. E. Camann and J. T. Antley, Environ. Health Perspect., 1999, 107, 721–726 CrossRef CAS.
- J. C. Chuang, P. J. Callahan, R. G. Menton and S. M. Gordon, Environ. Sci. Technol., 1995, 29, 494–500 CAS.
- P. J. Vojta, S. P. Randels, J. Stout, M. Muilenberg, H. A. Burge, H. Lynn, H. Mitchell, G. T. O'Connor and D. C. Zeldin, Environ. Health Perspect., 2001, 109, 815–819 CrossRef CAS.
- S. M. Causer, R. D. Lewis, J. M. Batek Sr and K.-H. Ong, J. Occup. Environ. Hyg., 2004, 1, 237–242 CrossRef.
- J. C. Chuang, P. J. Callahan, C. W. Lyu and N. K. Wilson, J. Exposure Anal. Environ. Epidemiol., 1999, 9, 85–98 Search PubMed.
- W. Butte and B. Heinzow, Rev. Environ. Contam. Toxicol., 2002, 175, 1–46 Search PubMed.
- C. Naspinski, R. Lingenfelter, L. Cizmas, Z. Naufal, L. Y. He, A. Islamzadeh, Z. Li, Z. Li, T. McDonald and K. C. Donnelly, Environ. Int., 2008, 34, 988–993 CrossRef.
- R. M. Maertens, X. Yang, J. Zhu, R. W. Gagne, G. R. Douglas and P. A. White, Environ. Sci. Technol., 2008, 42, 1747–1753 CrossRef CAS.
- R. M. Maertens, R. W. Gagne, G. R. Douglas, J. Zhu and P. A. White, Environ. Sci. Technol., 2008, 42, 1754–1760 CrossRef CAS.
- S. R. Hilts, C. Hertzman and S. A. Marion, Can. J. Public Health, 1995, 86, 345–350 Search PubMed.
- P. J. Lioy, L.-M. Yiin, J. L. Adgate, C. P. Weisel and G. G. Rhoads, J. Exposure Anal. Environ. Epidemiol., 1998, 8, 17–35 Search PubMed.
- J. W. Roberts, W. S. Clifford, G. Glass and P. G. Hummer, Arch. Environ. Contam. Toxicol., 1999, 36, 477–484 CAS.
- U.S. Agency for Toxic Substance and Disease Registry, Toxicological Profile for Polycyclic Aromatic Hydrocarbons, Atlanta, GA, 1995 Search PubMed.
- J. S. Colt, S. H. Zahm, D. E. Camann and P. Hartge, Environ. Health Perspect., 1998, 106, 721–724 CAS.
- R. Sporik, S. T. Holgate, T. A. E. Platts-Mills and J. J. Cogswell, N. Engl. J. Med., 1990, 323, 502–507 CAS.
- U.S. Agency for Toxic Substance and Disease Registry, Toxicological Profile for Lead, Atlanta, GA, 1999 Search PubMed.
- L.-M. Yiin, C. H. Yu, P. J. Ashley and G. G. Rhoads, J. Occup. Environ. Hyg., 2008, 5, 94–99 CrossRef CAS.
- M. D. Chapman, P. W. Heymann, S. R. Wilkins, M. J. Brown and T. A. E. Platts-Mills, J. Allergy Clin. Immunol., 1987, 80, 184–194 CrossRef CAS.
- G. G. Rhoads, A. S. Ettinger, C. P. Weisel, T. J. Buckley, K. D. Goldman, J. L. Adgate and P. J. Lioy, Pediatrics, 1999, 3, 721–726 Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |