Sodium borohydride versus ammonia borane, in hydrogen storage and direct fuel cell applications
Umit B.
Demirci
*abc and
Philippe
Miele
abc
aUniversité de Lyon, Lyon, F-69003, France
bUniversité Lyon 1, Lyon, F-69003, France
cCNRS, UMR 5615, Laboratoire des Multimatériaux et Interfaces, Villeurbanne, F-69622, France. E-mail: Umit.Demirci@univ-lyon1.fr; Fax: +33 4724 40618; Tel: +33 4724 48403
First published on 12th March 2009
Abstract
Since the late 1990s, sodium borohydride (NaBH4, denoted SB) is presented as a promising hydrogen storage material and an attractive fuel (aqueous solution) of the direct fuel cell (or direct liquid-feed fuel cell). In 2007, the U.S. Department of Energy recommended a no-go for SB for vehicular applications and suggested work on ammonia borane (AB), another promising hydrogen storage material, which is also considered as a fuel for the direct fuel cell. Both boron hydrides in hydrogen and fuel cell applications are the topics of the present paper. The basics, issues, solutions to the issues and state-of-the-art are tackled but the discussion aims to compare the hydrides for either application. It is shown that there are many similarities between SB and AB in their features and applications. Nevertheless SB and AB as hydrogen storage materials do not compete. Rather, SB is intended more to portable technologies while AB to vehicular applications. Otherwise, when these hydrides are utilised as fuels of direct fuel cell, one question arises: what can be the advantage of developing the AB-powered fuel cell when it seems to be less effective, practical, and more complex than the SB-powered fuel cell? These aspects are discussed. However that may be, it is concluded that both SB and AB are not mature enough for the applications considered.
 Umit Demirci | Educated at Strasbourg Louis Pasteur University (France), Umit Demirci received a BSc in Chemistry in 1997, a MSc in Physics and Chemistry of Materials in 1999, and a PhD in Physical Chemistry with a specialization in heterogeneous catalysis in 2002. Then, through various experiences from 2002 to 2007, he worked on automotive exhaust gas post-treatment and fuel cells. Now, an Assistant Professor of Chemistry at Claude Bernard University of Lyon (France), he works on “Chemistry and Materials for Energy”, focusing on hydrogen (storage/generation), fuel cells, inorganic (transition metals and their oxides) materials and especially boron-based materials. |
 Philippe Miele | Philippe Miele studied inorganic chemistry at the University Montpellier 2 and was awarded his PhD in 1993 in the Laboratory of Materials and Membranes Process. In 1994, following a postdoctoral fellowship with Prof. W. S. Rees at Georgia Institute of Technology (Atlanta, USA), he became Assistant Professor at the University Claude Bernard in Lyon and was promoted Professor in 2001. He became the leader of the research group “Molecular Precursors and Inorganic Materials” in the Laboratory of Multimaterials and Interfaces. In January 2003, he was appointed to his present position as head of this laboratory. He is also professor of inorganic materials at the University Claude Bernard in Lyon. Its main research interest lies in the synthesis and characterisation of non-oxide advanced ceramics in specific forms using the pyrolysis of preceramic polymers (Polymer Derived Ceramics route). He is a member of the “Institut Universitaire de France” (IUF) and of various national and international scientific committees. |
Broader contextSodium borohydride (NaBH4) and ammonia borane (NH3BH3) are two boron hydrides which capacities, as hydrogen storage materials and fuel of the direct liquid-feed fuel cell, have attracted much attention since the early 2000s. This attention falls within the current energy context, in which climate change, depletion of fossil fuels resources, and sustainability are among our main concerns. Hydrogen and fuel cells are today considered as promising technologies that could tomorrow play a major role in energy applications. However their development is hindered by several issues like hydrogen storage, which both technologies share. One of the solutions that has been suggested is the utilisation of complex hydrides like NaBH4 and NH3BH3. Both are hydrogen carriers capable of indirectly powering a fuel cell. And both are energy carriers capable of directly powering a fuel cell (if in aqueous solution). In fact, NaBH4 and NH3BH3 have many similarities in their properties and their applications. Hence one question arises. What are the advantage(s) of developing one hydride when the other is less effective, practical, safe, and more complex? An answer can be given by virtue of the state-of-the-art. |
1. Introduction
As an energy carrier, hydrogen has advantages (e.g. energetic and clean) but also drawbacks because it has to be produced, purified, stored, distributed and finally stored on-board. Each of these steps is problematic. Hydrogen storage is especially problematic, representing a scientific, technical, and technological challenge. Various storage solutions are studied1 in order to meet the targets set by the U.S. Department of Energy (US DOE).2 However it appears that complex hydrides like e.g. some boron hydrides are among the most promising storage systems by virtue of their high gravimetric/volumetric hydrogen storage capacities.3Sodium borohydride (NaBH4; denoted SB) was discovered in the 1940s.4 Without delay its hydrogen releasing capacity attracted U.S. Army attention.5 However in the energy context of the 1960s SB was forgotten about until the late 1990s, when the growing interest towards hydrogen as a renewable energy carrier brought it again to attention. The potential utilisation of SB as a hydrogen storage material6 and as a fuel of direct fuel cell7 were shown. Since then many papers showing the increasing interest in SB have been published.5,8–10
In November 2007, the US DOE published an independent report that recommended a no-go for SB for on-board vehicle hydrogen storage.11 This realistic decision is not surprising because the aqueous solution of SB does not meet US DOE criteria in terms of storage capacity, spent fuel recycling and cost.11 The report's conclusion is not completely negative. It consistently remarks that the improvements obtained for SB can benefit ammonia borane (NH3BH3; denoted AB). Actually, AB is today the most promising boron hydride for on-board hydrogen storage while SB has still potential for portable applications.12
Now SB and AB are both considered as hydrogen storage materials and also as fuels for direct fuel cells. Due to the fact that the US DOE has in a way compared SB and AB (especially in the framework of the former application), it may be beneficial and interesting to compare these hydrides in terms of their basic and their state-of-the-art for either application. Accordingly the present paper is a short review which discusses these aspects.
2. Basics
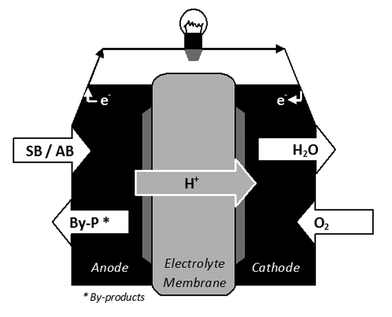
SB is a versatile boron hydride (Fig. 1). It is widely utilised in industrial processes (e.g. pharmaceutical, paper bleaching, and wastewater treatment). Accordingly it is produced in large amounts. Ever since the late 1990s it has also been suggested as a promising source of hydrogen because it contains 10.8 wt% of hydrogen.7 Stored hydrogen can be released by thermolysis or hydrolysis. Otherwise, SB can be oxidised to liberate eight electrons and thus it can power the direct borohydride fuel cell (denoted DBFC). Unlike SB, the utilisation of AB is not widespread. For example, it finds a use in organic chemistry (Fig. 1) as an air-stable derivative of diborane.13 This is especially detrimental for its production cost. Actually AB is a promising material by virtue of its gravimetric hydrogen storage capacity of 19.5 wt%. Besides this, it is the fuel of the direct ammonia borane fuel cell (denoted DABFC).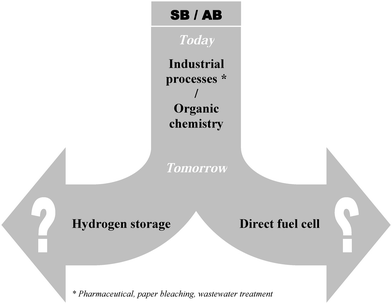 | ||
| Fig. 1 SB and AB: existing and potential applications. | ||
For both boron hydrides, finding cost-effective production routes with the prospect of application is one of the main objectives. Investigations are in progress but none of the proposed routes has reached sufficient efficiency.14,15 Various synthesis routes have been reported in some review papers: for SB in references [14,16] and for AB in references [15,17]. One way to reduce the cost is to recycle the spent fuel (i.e. the reaction by-products) back to the hydrides. Both hydrides suffer from their high cost and AB is even more expensive than SB. Hence a detailed comparison of the respective costs is not really relevant. One has no choice but to admit that there is no outstanding improvement to be made in this way. With respect to the cost issue, there is another interesting aspect that Crabtree18 discussed, this aspect has never been tackled elsewhere (at least, in the references cited throughout the present paper). Today boron is a cheap element because demand is low. And, it is a rare element in terms of world production. Accordingly, if one of the boron hydrides reaches a commercialisation phase, the price of boron will most likely rise sharply, making the cost issue worse. This point actually stresses the crucial need to recycle the by-products in a cheap, efficient, and effective way.
Safety is important for chemicals intended for large-scale utilisation. Information on safety is generally available in the chemicals' material safety data sheet. The few about AB are incomplete (see ref. [19]). The ones on SB are much more complete.20 Lane15 discussed the safety aspects relating to AB. Table 1 reports safety information and properties of SB and AB (for reviews of the properties, see references [21–23] and [17,24,25], respectively). SB and AB are white solids. They are recognised as being safe and stable at room temperature if stored in a closed vessel and anhydrous medium. Both are moisture sensitive, hydrolysing and generating hydrogen; and oxidising agents and acids must be avoided. To be stable and safe, AB must be of high purity. The main difference between SB and AB is the thermal stability. AB decomposes at very low temperatures in relation to SB.
| SB | AB | |
|---|---|---|
| Properties | ||
| Molecular formula | NaBH4 | H3NBH3 |
| State at room temperature | Solid | Solid |
| Molecular weight (g mol−1) | 37.8 | 30.8 |
| Hydrogen storage capacity (wt%) | 10.8 | 19.5 |
| Hydrogen storage capacity (gH2 L−1) | 133 | 152 |
| Density (g cm−3) | 1.07 | 0.78 |
| Solubility (g per 100 g(H2O)) | 55.0 | 33.6 |
| Melting point (°C) | 505 | 112 |
| Decomposition temperature | 565 | 78 |
| Safety | ||
| Stability | Moisture sensitive | Moisture sensitive |
| Stability of aqueous solution | Self hydrolysis (stabilized with addition of NaOH) | High stability |
| Chemicals to avoid | Acids, oxidising agents | Acids, oxidising agents |
Safety is also important from a green standpoint. Green chemistry is an emerging concept that addresses hazards (such as flammability, carcinogenicity and climate change) as inherent properties of a molecule.26 Accordingly green chemistry is engaged in addressing energy needs through the development of sustainable energy technologies such as hydrogen and fuel cells. Green chemistry can be defined through twelve principles, which are prevention, atom economy, less hazardous chemical synthesis, safer chemicals, safer solvents and auxiliaries, energy efficiency, use of renewable feedstocks, reduction of derivatives, catalysis, design for degradation, real-time analysis for pollution prevention, and safer chemistry for accident prevention.27 Simply, green chemistry advocates safe chemicals and safe chemistry. Hence the relative safety of both SB and AB conforms to the green chemistry concept.
Green chemistry is also engaged in sustainability. It intensely supports the utilisation of renewable feedstocks. Boron sources and, accordingly, both SB and AB are not renewable (Fig. 2). Nevertheless they are recyclable (even if the processes are still too expensive; discussed hereafter). From this point of view, SB and AB may be regarded as being relatively green.
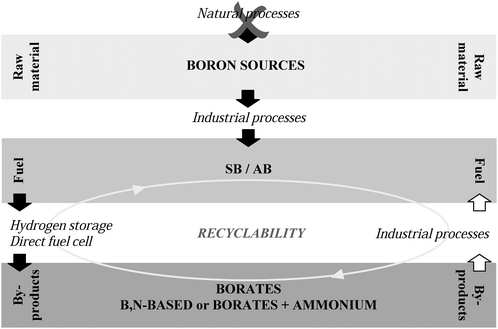 | ||
| Fig. 2 Non-renewability but recyclability of SB and AB. | ||
To summarise, the safety aspects, the high theoretical hydrogen storage capacities, and the hydrogen generation capacities are in favour of both hydrides. It is thus evident that they have a potential as hydrogen/energy carriers. The questions that then arise are: what are the effective capacities of SB and AB for such applications? And is SB competing with AB?
3. Hydrogen storage
In order that an application is expected, a potential hydrogen storage material has to show some basic technical requirements: i.e. high gravimetric/volumetric hydrogen storage capacities, hydrogen generation capacities, and reversibility of the storage. State-of-the-art relating to these are reported hereafter.3.1. Storage capacities
The US DOE has set targets to be reached by the years 2007, 2010 and 2015 (Table 2). SB and AB meet the targets by virtue of theoretical gravimetric hydrogen storage capacities of 10.8 and 19.5 wt%, respectively, and theoretical volumetric hydrogen storage capacities of 113 and 152 gH2 L−1, respectively. Nonetheless the effective capacities are well below requirements. Hydrolysis of SB to release hydrogen has been studied more than thermolysis because the latter reaction needs too high temperatures to be considered suitable for applications (i.e. >400 °C). In consequence, only the former reaction is discussed below.| GHSC (wt%) | VHSC (gH2 L−1) | References | |
|---|---|---|---|
| a Reported so far. b Liquid water provided. c Vapour water provided. d Not given. e At 85 °C (target set by the US DOE). f Theoretical values. g Assuming that the aqueous solution has a density of 1 kg m−3. | |||
| US DOE targets | |||
| 2007 target | 4.5 | 36 | 2 |
| 2010 target | 6.0 | 45 | 2 |
| 2015 target | 9.0 | 81 | 2 |
| SB highest effective capacitiesa | |||
| SB (aq) | 6.7 | 80 | 29 |
| SB (s)b | 9.0 | 188 | 30 |
| SB (s)c | n.g.d | n.g.d | 31 |
| AB highest effective capacitiesa | |||
| AB thermolysise | 5.9 | 46 | 35 |
| AB hydrolysisf | 4.9 | 66g | — |
To improve the effective capacity, SB can be stored as solid and then either liquid water (implying a capacity of 10.8 wt%)30 or vapour water (implying a capacity of 21.3 wt%, steam being recovered from a fuel cell)31 is provided when hydrogen is needed. The highest effective capacity ever measured is 9.0 wt% with liquid water30 while no data has yet been reported for steam. Nevertheless the value 21.3 wt% cannot be reached because of the hydration of the hydrolysis by-product.31,32 Indeed, the volume expansion of the by-product has to be taken into account and thus the expected highest capacity can only be 10.7 wt%.
To summarise, it seems that SB may meet the targets set by the US DOE (Table 2). However the present analysis (and all of the analyses reported in the open literature) do not take into account the storage system as a whole. If this was done, then the given capacities must be divided by a factor 22 and none of these SB-based storage solutions would meet the 2010–2015 targets. One can then easily admit the no-go recommendation.11 All the same, SB has still potential for portable applications, for which the specifications are less severe.33
The thermal decomposition of AB is a complex process involving several reactions34 (discussed in Section 3.2.2). The net AB decomposition initiates at 70 °C and reaches a maximum at about 110 °C.34 About 1 mol(H2) per mol(AB) is released, this means capacities of 6.5 wt% and 51 gH2 L−1. However this temperature is too high by comparison with the target 85 °C. Bluhm et al.35 reported that 0.9 mol(H2) per mol(AB) is generated after 17 h at 85 °C. Although the generation rate is slow, this result shows that an equivalent of 5.9 wt% of hydrogen can be recovered at 85 °C. The second mol(H2) per mol(AB) is released over a broad temperature range 110–200 °C25 (capacities of 13.0 wt% and 102 gH2 L−1). The third hydrogen can be recovered at temperatures up to 500 °C.17 Solutions have been proposed for improving the effective storage capacity as well as the hydrogen releasing rate:24,25 thermolysis of AB in ionic liquid35 or in organic solvents,36 insertion of AB into a mesoporous silica (SBA-15),37 transition metal complex catalysis,38 and solid state catalysis using a Ni–Pt alloy.39 These solutions decrease the hydrogen releasing rate35,36,37,39 and the decomposition temperature by 10–15 °C.35,37,39 However undesirable by-products35,39 may be generated, so decreasing the effective storage capacity.35 The primary objective of these studies was to accelerate hydrogen generation while decreasing the decomposition temperature.
AB has great potential because it stores 19.5 wt% of hydrogen, a third of which can be released at 85 °C. The hydrogen generation rate appears to be more critical. Anyway improvements can be legitimately expected since the investigations are in their early stages.
Until now there has been little investigation into the hydrolysis of AB. Few research groups40–42 are involved. They mainly focus on the catalytic materials, but where experimental conditions favour the catalytic reactivity to the detriment of the storage capacity. Hence the highest effective gravimetric hydrogen storage capacities reported so far are only of few tenths wt%. Accordingly it is not relevant to discuss the published studies on these terms.
To conclude, the highest capacity of AB in aqueous solution is <5.0 wt%. This value is lower than the highest effective capacity of SB in aqueous solution. Therefore, the aqueous solution of AB can only be attractive for portable applications but, frankly speaking, SB should be more favourable from this point of view.
3.2. Hydrogen releasing
To the best of our knowledge, there is no specific target for the hydrogen generation rate. In fact, this rate is closely linked to the fuel cell capacity. The hydrogen storage system must be capable of releasing hydrogen in sufficient amounts and at a sufficient rate for to power a fuel cell.| NaBH4 + 4 H2O → NaB(OH)4 + 4 H2 | (1) |
Overall, SB conversion is >90%.10,43 The most effective catalyst reported so far is 10 wt% PtRu–LiCoO2. A hydrogen generation rate of 560 LH2 min−1 gPtRu−1 was measured at 25 °C for an aqueous solution with an effective capacity of 2.5 wt%.44 However Pt is a noble metal. Hence cheaper metals have been investigated. The most attractive non-noble metal is cobalt.10 For example, a 10 wt% Co–C catalyst showed a hydrogen generation rate of about 130 LH2 min−1 gCo−1 at 20 °C.45
Efforts have been devoted to searching for an outstanding metal catalyst suitable for SB hydrolysis. Today, the reactivity and the cost of the non-noble catalytic systems are no longer issues. However, all of the catalysts suffer from a short lifetime and therefore the main challenge today is to improve catalyst durability.10
| nNH3BH3 → [NH2BH2]n + nH2 | (2) |
| [NH2BH2]n → [NHBH]n + nH2 | (3) |
| [NHBH]n → BN + nH2 | (4) |
One notices that if AB was to release all of its hydrogen it would form boron nitride (BN), an unpromising material for the recycling step.18 As reported in section 3.1.2, Bluhm et al.35 observed that 0.9 mol(H2) per mol(AB) was generated after 17 h at 85 °C. This releasing rate is obviously unacceptable for any application. Solutions have been proposed in order to increase this rate.24,25
For example, the thermolysis of AB within mesoporous silica scaffolds took 85 min at 50 °C while the thermolysis of neat AB lasted 290 min at 80 °C.37 The thermolysis onset temperature of AB was decreased by about 15 °C in relation to that of neat AB. Chen et al.39 catalysed the AB thermolysis with Ni0.88Pt0.12 alloy. This resulted in enhanced decomposition of AB, with a lowered heating temperature and an increased rate of mass loss. Bluhm et al.35 assessed the AB thermolysis in an ionic liquid, i.e. 1-butyl-3-methylimidazolium chloride (equal weights of dried ionic liquid and AB). The dehydrogenation showed no induction period and 0.95 mol(H2) per mol(AB) was released at 85 °C in 3 h (evolution of 3.1 wt% of hydrogen). The most outstanding result was reported by Denney et al.38 With an iridium pincer (POCOP)Ir(H)2 with POCOP = [η3-1,3-(OPtBu2)2C6H3], 1 mol(H2) per mol(AB) was completely released within 14 min at room temperature. However it is important to note here that AB was dissolved in THF at a concentration of 0.5 M and that the catalyst loading was 0.5 mol%. This implies that the gravimetric hydrogen storage capacity of the fuel was <1 wt%. According to Stephens et al.,24 a rapid rate and an acceptable extent of dehydrogenation have not yet been achieved, despite the utilisation of a catalyst.
Further optimisations and innovations should bring improvements. But, today, compared with SB, the thermolysis of AB is achieved under milder conditions and is more efficient.
| NH3BH3 + H+ + 3H2O → B(OH)3 + NH4+ + 3H2 | (5) |
| NH3BH3 + 2H2O → BO2− + NH4+ + 3H2 | (6) |
The few studies on AB hydrolysis40–42,46 have started a screening process in order to find the most efficient catalyst. Xu and co-workers,40,47 highly involved in AB hydrolysis, have listed most of the metal catalysts they have tested. The best one was 2 wt% Pt–γAl2O3, in which the reaction completed in 45 s. For a ratio Pt/AB of 0.009, the hydrogen generation rate was 26 LH2 min−1 gPt−1. However, very recently, Simagina et al.41 reported a hydrogen generation rate of 122 LH2 min−1 gRh−1 for 1 wt% Rh–TiO2.
As discussed above for the SB hydrolysis, improvements are always possible in terms of catalyst reactivity. However catalyst durability is an aspect that should be more deeply studied. Now it must be stated in the defence of AB that the current studies are only first attempts. The highly efficient catalysts are noble metal-based but, from a cost point of view, cheaper metals need to be utilised.42,46 Interestingly, Xu and co-workers42 have reported that iron nanoparticles with no protective shell were capable of generating hydrogen at a rate of 1.5 LH2 min−1 gFe−1. This is a promising catalytic result.
3.3. Storage reversibility
Automotive applications are often cited when discussing the potential of energy technologies because the huge car fleet all over the world implies a considerable need for energy. Fuels must therefore be cheap, stable, relatively safe, easily stored, easily distributed, and easily refuelled. In other words, hydrogen storage must be reversible. This is not the case for the boron hydrides. SB and AB do not reversibly store hydrogen and their decomposition leads to by-products (B(OH)4−, BO2−, B(OH)3, [NH2BH2]n, [NHBH]n, BN, and other intermediates such as borazine, diborane, and so on; this is not discussed in the present paper; for more details, see references [17,24,25]) that have to be recovered. Alternatively, by-products can be recycled back to the hydrides. The storage is then recyclable, want of being reversible (Fig. 2). A target for the regeneration efficiency has been set. It is 60%.2Regarding SB hydrolysis, spent fuel recycling has not shown enough efficiency, which made the US DOE recommend a definitive no-go for automotive applications.11 The high energy penalty and the high cost of regenerating sodium borate (NaBO2) back to SB are worrying. The reaction conditions are far from being mild and effective and the yields are lower than the target 60%.16,48 Still today it is believed that the SB synthesis has merit through its by-product recycling. Liu and Li10 especially stressed the fact that recycled by-products is a key point to lower SB production costs. Several papers reviewed the various recycling processes (i.e. direct thermal reductions, multi-step thermal reductions or electrochemical methods) and for more information reader can refer to references [14,16,48–50]. The report by the US DOE did not analyse waste generation implied in some of the recycling approaches.11 This is understandable because the US DOE focused as a priority on the cost of the processes. Nevertheless a recycling process that would reach the 60% criterion and that could be applied commercially on a vast scale should be analysed in terms of waste generation, that is, in terms of green chemistry.
While specifying the reasons for their no-go decision, the US DOE acknowledged that the SB recycling challenge is rather difficult and that all of efforts could be of benefit for hydrogen storage via AB.11 And actually, the regeneration of AB is as crucial as the regeneration of SB for the prospect of automotive applications.24,25,51 Up to now, several regeneration processes (acidic digestion and reduction, electrochemical, and so on), which depend on the by-products, have been tested. They were largely reviewed by Stephens et al.24 and Peng and Chen.25 None of the suggested regeneration processes has shown enough efficiency. According to Crabtree,18 the AB regeneration is not at all straightforward. An alternative solution was recently suggested. Instead of recycling the by-products of AB, it was proposed that new uses of spent fuel could be a solution but this would defeat the sustainability objectives (depletion of boron deposits).51
As a conclusion, let us cite Peng and Chen.25 According to these authors, the reversibility/recyclability of AB is still one of the main challenges to its practical use. This is also true for SB.
3.4. Summary and conclusions
Neither boron hydride is mature enough to envisage applications, especially automotive applications.11 Both suffer from low (not-optimised) hydrogen storage capacities, inefficiency in spent fuel recycling, and catalyst inefficiency in terms of durability. Moreover, with respect to the AB thermolysis, the hydrogen generation rates are too low. Note that this problem does not concern SB because its thermal decomposition is even chemically unlikely under mild conditions. The picture that is drawn here may seem quite pessimistic but it is one of today. Research on boron hydrides as hydrogen carriers is in its early stages, especially for AB. Improvements have been shown. For example, highly efficient catalysts, with hydrogen generation rates well above 100 L(H2) min−1 g−1(metal), have been prepared. One may expect further improvements.The issues discussed above are the main ones.11 There are many other issues that are more or less significant10,24,25,43 and that can hinder more or less severely the hydrides' development: e.g. purity of generated hydrogen, by-products recovery, by-product (NaBO2) crystallisation (in the case of hydrolysis), thermal management (especially for SB hydrolysis), and refuelling of fresh hydride. To this list, another issue must be added. When the hydrogen storage capacities, especially the gravimetric ones, are calculated, the net storage system as a whole is always neglected. The capacities are assessed on the basis of the hydride (solid or in solution) and not on the basis of the whole system. This is a mistake since the US DOE have set their specifications in relation to the net storage system. Maybe should we more cautious about that, especially when potential applications are considered?
Table 3 tentatively compares SB and AB in terms of net storage capacity (the net storage system is taken into account and it is assumed that the hydride accounts for 50% of the total weight) and hydrogen generation rate. This table confirms the non-maturity of the hydrides for automotive applications. However portable applications could be considered. Table 4 suggests potential applications for SB and AB while considering thermolysis and hydrolysis. Although, to the best of our knowledge, this has never been done, we have considered stationary applications for the thermolysis of the hydrides. From Tables 3 and 4, and taking into account the state-of-the-art, four observations stand out (the main reasons are given in parenthesis). First, the SB hydrolysis has a higher potential than the AB hydrolysis for portable applications (commercially available, and high storage capacity). Second, SB is not viable for automotive applications (low storage capacity, and far from targets). Third, AB thermolysis has the highest potential for automotive applications (high storage capacity, and potential for improvements). According to Marder,51 AB is potentially consistent with the 2015 target if progress can be made on a number of fronts. Fourth, both hydrides could be used for stationary applications that utilise high-temperature fuel cells. In conclusion, SB is not really competing with AB. Each may be intended to specific applications: e.g. SB for portable and AB for automotive applications.
| SB | AB | |||
|---|---|---|---|---|
| Thermolysis | Hydrolysis | Thermolysis | Hydrolysis | |
| a Gravimetric hydrogen storage capacity (wt%) from Table 2; taking into account the net storage system (hydride weight accounting for 50% of the total weight). b Volumetric hydrogen storage capacity (gH2 L−1) from Table 2 (without considering the net storage system). c Hydrogen generation rate (LH2 min−1 gcatalyst/metal−1) reported in section 3.2. | ||||
| Cost | ||||
| Availability | Large amounts | Large amounts | Not commercial | Not commercial |
| Storage capacities | ||||
| GHSCa | — | 4.5 | 3.0 | 2.5 |
| VHSCb | — | 188 | 46 | 66 |
| H2 releasing | ||||
| HGRc | — | 560 | 342 | 122 |
| Over cycles | — | Degradation | — | Degradation |
| Spent fuel | ||||
| Reversibility | — | No | No | No |
| Recyclability | — | Inefficient | Inefficient | Inefficient |
| SB | AB | |||
|---|---|---|---|---|
| Thermolysis | Hydrolysis | Thermolysis | Hydrolysis | |
| Portable applications | ✗ | ✓ | ✓ | ✓ |
| Automotive applications | ✗ | ✗ | ✓ | ✗ |
| Stationary applications | ✓ | ✗ | ✓ | ✗ |
4. Direct fuel cell
SB and AB as hydrogen storage materials are indirect fuels of fuel cell. Indirect because they have to be dehydrogenated and then generated hydrogen powers the fuel cell. Otherwise, SB and AB have a potential as direct fuels of fuel cell (Fig. 3). In this case, an aqueous solution of the hydride powers the fuel cell, which is basically based on the hydride anodic electrooxidation. This is the principle on which the direct liquid-fed fuel cell (denoted DLFC) works. Note that many other chemicals have been considered as direct fuels so far (e.g. methanol, ethanol, propanol, formic acid, ammonia, hydrazine, dimethyl ether, trioxane, and so on).22,23 In fact, the DLFC is none other than the polymer electrolyte membrane fuel cell (denoted PEMFC), which has been initially constructed to be powered with hydrogen. DLFC is mainly intended to portable applications (e.g. mobile phone, laptop, and portable power generator).While the direct borohydride fuel cell (DBFC) has been much investigated (since the late 1990s), the direct ammonia borane fuel cell (DABFC) is a recent technology. The present section discusses the basics, the issues and the state-of-the-art relating to both fuel cells.
4.1. Direct borohydride fuel cell
| Anode BH4− + 8OH− → BO2− + 6H2O + 8 e− | (7) |
| Cathode 2O2 + 4H2O + 8 e− → 8OH− | (8) |
| Overall BH4− + 2O2 → BO2− + 2H2O | (9) |
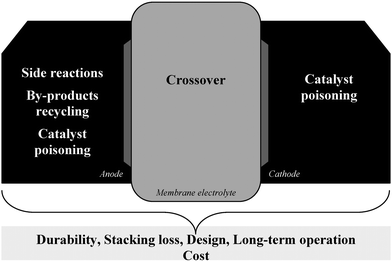 | ||
| Fig. 4 Issues of the DBFC and likely of the DABFC. | ||
The cost issue is significant.8,9,21,53,54 For example, Liu et al.52 remarked that the fuel contribution to the electricity cost for the DBFC is 100 times in comparison with hydrogen. Actually, a discussion about the SB cost is not necessary because the arguments are identical to those given for SB in sections 2 and 3.
The DBFC is highly promising because the oxidation–reduction reactions are ideally based on the transfer of 8 electrons (Eqn 7). However the number of electrons utilised per borohydride oxidised is less than 8 because of the anode catalyst.52 With metals like e.g. Pt, Ni or Pd, the number of electrons is 4 to 6 because SB hydrolysis is competing with SB oxidation.8,9,52 Hydrogen evolution is detrimental to DBFC performance. Noble metals with high hydrogen over-potential, such as Au and Ag, permit an 8-electron oxidation but these are not effective enough in terms of electrode kinetics and power density.9 Alternatively, composite catalysts such as ZrCo0.8Ni1.255 and AB5-type alloys such as LaNi4.5Al0.556 are capable of near 8-electron oxidation with relatively high efficiency of fuel utilisation. Generally speaking, these materials, i.e. Au, Ag, composites and ABX-type alloys, are commonly accepted as being potential solutions which have 8-electron reactions.8,9 Furthermore, actions (e.g. optimisation or addition of molecules inhibiting the hydrogen generation) on the anolyte (i.e. the aqueous alkaline solution of SB) may decrease hydrogen evolution.8,9 However Jamard et al.57 considered that it would be preferable to run the hydrogen evolution down in order to keep a balance between the amount of hydrogen generated and the amount of oxidised hydrogen by the DBFC. This is maybe the best solution today.
The decrease of SB utilisation efficiency is also due to borohydride crossover through the electrolyte membrane. This crossover is detrimental to the performance of the cathode electrocatalyst. It is generally suggested that more selective membranes should solve this issue,8,21,53 another solution being to use borohydride-resistant cathode materials like e.g. MnO2.8,53 Yet Liu and Li9 are pessimistic. According to these authors, it is very difficult to synthesise a membrane with high selectivity. Indeed the membrane must allow efficient hydroxide ions transport, while it must simultaneously block the borohydride ions transport.8 Finally, a final solution could be to not use a membrane.53
The issues discussed above are the main ones. To these, it may be added the following ones: durability of each DBFC component,9 by-product crystallisation at anode after use,8,9,21,53 stacking loss,9 DBFC system design,8,9,21 by-product recycling,8,9,21,53 and long-term operation.53
To conclude the present section, let us cite some authors. In 2006, Ponce de Leon et al.53 noticed that “considering the relatively small number of person years of R&D invested in direct borohydride fuel cells, the performance of borohydride fuel cells must be considered promising. There are real opportunities for improvements.” In 2007, Demirci8 added that “the DBFC technology interests more and more but it is still in the development phase and there are several problems to solve.” And, in 2008, Liu and Li9 confirmed: “the DBFC technology has attracted more and more attention but it is still at its initial development phase.” In other words, one has no choice but to admit that there is no outstanding development in the R&D devoted to the DBFC.
Constructing a DBFC or even any fuel cell (single cell or stack) is a very complex operation. Demirci8 reported that all the construction steps are crucial and that fuel cell performance is closely dependent on the materials utilised. It is even truer that a fuel cell is made up of several components that have to be prepared, conditioned and/or optimised: e.g. catalysts, electrodes, diffusion layers, membrane, membrane–electrode-assembly, bipolar plates, collectors, fuel, and fuel supplying. This does not make the cross-comparison of DBFCs easy. The performances of lab-prepared DBFCs have been widely reviewed.8,21,22,52–54 The best performance ever reported is 290 mW cm−2 at 60 °C.52 However this performance has been surpassed by a DBFC powered by SB and hydrogen peroxide (instead of oxygen):
| Cathode 4H2O2 + 8H+ + 8 e− → 8H2O | (11) |
| Overall BH4− + 4H2O2 → BO2− + 6H2O | (12) |
The theoretical potential of this overall reaction is 3.01 V versus SHE, which implies a theoretical energy density of 17060 Wh kg−1. In relation to the classical DBFC, the theoretical energy density is higher by about 85%. Very promising performances were reached.8,9 For example, Miley et al.59 reported a power density above 600 mW cm−2 for a SB–H2O2 DBFC employing a palladium anodic catalyst and a gold cathodic catalyst. Moreover it was shown that a 14-cell stack was capable of producing power of 500 W. Hence such a DBFC could be envisaged for e.g. two-wheeled vehicle applications (if H2O2 storage and safety are not taken into account).
Real improvements have been achieved with respect to DBFC. However it still suffers from high cost due to all its components (specifically SB, catalysts, and membrane). Furthermore, durability has not been optimised. To conclude, the DBFC is attractive and has a real potential for various applications.9,22 Nonetheless, the above-mentioned issues critically hinder its development towards commercialisation.
4.2. Direct ammonia borane fuel cell
| Anode NH3BH3 + 6OH− → BO2− + NH4+ + 4H2O + 6 e− | (13) |
| Cathode 3/2O2 + 3H2O + 6 e− → 6OH− | (14) |
| Overall NH3BH3 + 3/2O2 → BO2− + NH4+ + H2O | (15) |
From the standard enthalpies, the standard potential of the AB oxidation has been calculated as being −1.22 V versus SHE.60 The standard potential of the DABFC is thus −1.62 V versus SHE and its theoretical specific energy is 8400 Wh kg−1.60 The pure compound capacity is about 5200 Ah kg−1. Despite similar potentials (−1.62 versus −1.64 V), a lower number of electrons (6 versus 8) makes the DABFC less energetic than the DBFC.
The DABFC is similar to the DBFC. It consists of a hydride-fed anode, an O2-fed cathode and a membrane electrolyte. According to Xu and co-workers,60 AB for the DABFC combines the following advantages: stability, cheapness, availability, solubility in water, environmental safety of the discharge products, and recyclability of the by-products. The picture drawn entirely benefits the DABFC. We would be more cautious with respect to the cost, the availability, the recyclability (as discussed previously) and the safety of the by-products (see ref. [22,23]).
Presently there are very few papers dealing with DABFC, an insufficient number to discuss relevantly state-of-the-art of DABFC. This fuel cell appears to be fairly similar to the DBFC. They are similar with regard to their theoretical capacities, their advantages (e.g. aqueous solution as fuel, fuel stability, membrane utilisation, and so on) and their issues (at least for the ones experimentally in evidence). The side reaction (hydrolysis), the crossover and the cost are common issues. One may add the following, which are also given for DBFC: durability of the fuel cell components, by-products crystallisation at anode after use, stacking loss, DABFC system design, by-products recycling, and long-term operation (Fig. 4).
To our knowledge, there is only a single group of researchers working on the DABFC.60–62 The first study published dates to 2007. There are most likely opportunities for improvements but many more research groups should be involved. The case of DBFC is a good example. Even after nearly ten years of R&D efforts it is commonly admitted that DBFC is still at an initial phase of development.8,9,53
The only conclusions we can make are as follows. First, there are opportunities for performance improvements. Second, the amount of utilised Pt is high and this is detrimental to the fuel cell cost. Searching for efficient non-noble metals should be a priority. Third, the hydrogen evolution and the crossover are significant issues and the experience with the DBFC has shown that without addressing these problems the fuel cell cannot be technically, commercially viable.
4.3. Summary and conclusions
The DABFC is a novel technology. Only one group works on its development. Hence the present section compares the DBFC with the DABFC but the huge difference in R&D efforts makes the comparison quite difficult. The comparison is thus based on theoretical information. Table 5 compares few properties. Three observations stand out:| DBFC | DABFC | |
|---|---|---|
| a NaOH-stabilized b borohydride c species not clearly specified yet | ||
| Properties | ||
| Standard electrode potential (V vs SHE) | 1.64 | 1.62 |
| Number of electrons involved | 8 | 6 |
| Theoretical specific energy (Wh kg−1) | 9300 | 8400 |
| Pure compound capacity (Ah kg−1) | 5700 | 5200 |
| Others | ||
| Fuel (aqueous solution) stability | Yes a | Yes |
| Cost | High | High |
| By-products | BO2− | BO2−, NH4+ |
| By-products recyclability | Inefficient | Inefficient |
| Side reaction | Hydrolysis | Hydrolysis |
| Crossover | Yes b | Yes c |
• The DBFC is superior to the DABFC in terms of theoretical performances;
• With respect to the fuel stability and the by-product (except NH4+), both fuel cells are equivalent;
• The presence of NH4+ in the anolyte of the DABFC has to be clarified in terms of reactivity, recyclability, and crossover.
In fact, one question arises from Table 5. What can the advantage be of developing the DABFC when it is less effective, less practical (because of NH4+), and more complex than the DBFC?
The experience of the DBFC may benefit the DABFC because, in our opinion, all of the issues the DBFC encountered face the DABFC. For the DABFC, the presence of NH4+ is a further issue. Very few studies have dealt with the DABFC and thus there are improvement opportunities. For example, a AB–H2O2 DABFC could be a solution to increase the fuel cell performances:
| Overall NH3BH3 + 3H2O2 → BO2− + NH4+ + 4H2O | (16) |
The standard electrode potential is 2.99 V versus SHE, which implies a theoretical specific energy of 15500 Wh kg−1 and a pure compound capacity of 9600 Ah kg−1.
To summarise, the DABFC is an interesting fuel cell but the DBFC appears more attractive and less complicated.
5. Conclusion
In the energy field, the history of SB is longer than that of AB but both have potential. The present review has discussed the potential of each, focusing on their capacities as hydrogen storage materials and as fuels for direct fuel cell. The basics, issues, solutions (to the issues) and state-of-the-art have been reviewed and discussed.SB and AB are hydrogen carriers, which store 10.8 and 19.5 wt% of hydrogen. However their effective gravimetric storage capacities are below the theoretical values because the hydrogen release is inefficient. Both suffer from low effective hydrogen storage capacities, inefficiency in spent fuel recycling and inefficiency of catalyst in terms of durability. With respect to SB, the most efficient process is hydrolysis. With respect to AB, the most efficient hydrogen release reaction is thermolysis. This has consequences with regard to their potential applications. It appears that SB is more suitable for portable applications if it is hydrolysed while AB is suitable for automotive applications if it is thermally decomposed. In other words, SB is not really competing with AB because they are intended for different, specific applications. However, neither is mature and both are far from commercialisation.
SB and AB are energy carriers, i.e. potential fuels of the direct liquid-fed fuel cell. The DBFC and the DABFC are both more suited to portable applications. The DBFC has been studied since the late 1990s but it is still in its initial development phase. The DABFC is a novel technology and thus discussing its state-of-the-art is irrelevant. However, from theoretical considerations, it appears that the DBFC is superior to the DABFC. In fact, one question has arisen. What can the advantage be of developing the DABFC when it appears to be less effective, less practical (because of NH4+), and more complex than the DBFC? The present question does not aim to cast doubt over the DABFC but it rather aims to show that interest in developing this technology has to be more clearly specified. Further it aims to highlight the fact that only one group is investing in R&D devoted to this fuel cell.
However that may be, SB and AB are not mature enough yet either as hydrogen carriers or as fuels for fuel cells. Real improvement opportunities still exist. Yet both have potential in specific applications. They are not in competition, especially as hydrogen storage materials.
References
- A. van den Berg and C. O. Areán, Chem. Commun., 2008, 668 RSC.
- U.S. Department of Energy, Hydrogen Program, http://www.hydrogen.energy.gov/ Search PubMed.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353 CrossRef.
- H. I. Schlesinger, H. C. Brown, A. E. Finholt, J. R. Gilbreath, H. R. Hoekstra and E. K. Hyde, J. Am. Chem. Soc., 1953, 75, 215 CrossRef CAS.
- U. B. Demirci and P. Miele, C. R. Chim., 2008 DOI:10.1016/j.crci.2008.08.002.
- S. C. Amendola, P. Onnerud, M. T. Kelly, P. J. Petillo, S. L. Sharp-Goldman and M. Binder, J. Power Sources, 1999, 84, 130 CrossRef CAS.
- S. C. Amendola, S. L. Sharp-Goldman, M. S. Janjua, N. C. Spencer, M. T. Kelly, P. J. Petillo and M. Binder, Int. J. Hydrogen Energy, 2000, 25, 969 CrossRef CAS.
- U. B. Demirci, J. Power Sources, 2007, 172, 676 CrossRef CAS.
- B. H. Liu and Z. P. Li, J. Power Sources, 2008, 187, 291.
- B. H. Liu and Z. P. Li, J. Power Sources, 2008, 187, 527.
- U.S. Department of Energy. Independent review. November 2007. Go/no-go recommendation for sodium borohydride for on-board vehicular hydrogen storage. NREL/MP-150-42220 Search PubMed.
- G. Principi, F. Agresti, A. Maddalena and S. L. Russo, Energy, 2008 DOI:10.1016/j.energy.2008.08.027.
- G. C. Andrews, in Borane-ammonia, Encyclopedia of reagents for organic synthesis, ed. Paquette L., John Wiley & Sons, New York, 2004 Search PubMed.
- Y. Wu, M. T. Kelly and J. V. Ortega, Review of chemical processes for the synthesis of sodium borohydride ( 2004), http://www.hydrogen.energy.gov/ Search PubMed.
- C. F. Lane, Ammonia-borane and related N–B–H compounds and materials: safety aspects, properties and applications, U.S. Department of Energy Chemical Hydrogen Storage Centerhttp://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/nbh_h2_storage_survey.pdf Search PubMed.
- Ç. Çakanyildirim and M. Gürü, Int. J. Hydrogen Energy, 2008, 33, 4634 CrossRef CAS.
- H. W. Langmi and G. S. McGrady, Coord. Chem. Rev., 2007, 251, 925 CrossRef CAS.
- R. H. Crabtree, Energy Environ. Sci., 2008, 1, 134 RSC.
- Sigma Aldrich, Material safety data sheet of AB, Product number 287717.
- Sigma Aldrich, Material safety data sheet of SB, Product number 452173.
- J. H. Wee, J. Power Sources, 2006, 155, 329 CAS.
- U. B. Demirci, J. Power Sources, 2007, 169, 239 CrossRef CAS.
- U. B. Demirci, Environ. Int., 2009, 35, 626 CrossRef CAS.
- F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2007, 2613 RSC.
- B. Peng and J. Chen, Energy Environ. Sci., 2008, 1, 479 RSC.
- P. T. Anastas, Green Chem., 2003, G29 Search PubMed.
- P. C. Anastas and J. C. Warner, in Green chemistry: Theory and practice, ed. Oxford University Press, New York, 1998 Search PubMed.
- Y. Kojima, K. I. Suzuki, K. Fukumoto, M. Sasaki, T. Yamamoto, Y. Kawai and H. Hayashi, Int. J. Hydrogen Energy, 2002, 27, 1029 CrossRef CAS.
- D. Hua, Y. Hanxi, A. Xinping and C. Chuansin, Int. J. Hydrogen Energy, 2003, 28, 1095 CrossRef CAS.
- Y. Kojima, Y. Kawai, H. Nakanishi and S. Matsumoto, J. Power Sources, 2004, 135, 36 CrossRef CAS.
- E. Y. Marrero-Alfonso, J. R. Gray, T. A. Davis and M. A. Matthews, Int. J. Hydrogen Energy, 2007, 32, 4117.
- L. Zhu, D. Kim, H. Kim, R. I. Masel and M. A. Shannon, J. Power Sources, 2008, 185, 1334 CrossRef CAS.
- D. K. Slattery M. D. Hampton, Complex hydrides for hydrogen storagehttp://www1.eere.energy.gov/hydrogenandfuelcells/pdfs.32405b34.pdf Search PubMed.
- V. Sit, R. A. Geanangel and W. W. Wendlandt, Thermochim. Acta, 1987, 113, 379 CrossRef CAS.
- M. E. Bluhm, M. G. Bradley, R. Butterick III, U. Kusari and L. G. Sneddon, J. Am. Chem. Soc., 2006, 128, 7748 CrossRef CAS.
- T. Wideman and L. G. Sneddon, Inorg. Chem., 1995, 34, 1002 CrossRef CAS.
- A. Gutowska, L. Li, Y. Shin, C. M. Wang, X. S. Li, J. C. Linehan, R. S. Smith, B. D. Kay, B. Schmid, W. Shaw, M. Gutowski and T. Autrey, Angew. Chem., Int. Ed., 2005, 44, 3578 CrossRef CAS.
- M. C. Denney, V. Pons, T. J. Hebden, D. M. Heinekey and K. I. Goldberg, J. Am. Chem. Soc., 2006, 128, 12048 CrossRef CAS.
- F. Y. Cheng, H. Ma, Y. M. Li and J. Chen, Inorg. Chem., 2007, 46, 788 CrossRef CAS.
- Q. Xu and M. Chandra, J. Alloys Compd., 2007, 446–447, 729 CrossRef CAS.
- V. I. Simagina, P. A. Storozhenko, O. V. Netskina, O. V. Komova, G. V. Odegova, Y. V. Larichev, A. V. Ishchenko and A. M. Ozerova, Catal. Today, 2008, 138, 253 CrossRef CAS.
- J. M. Yan, X. B. Zhang, S. Han, H. Shioyama and Q. Xu, Angew. Chem., Int. Ed., 2008, 47, 2287 CrossRef CAS.
- J. H. Wee, K. Y. Lee and S. H. Kim, Fuel Process. Technol., 2006, 87, 811 CrossRef CAS.
- P. Krishnan, T. H. Yang, W. Y. Lee and C. S. Kim, J. Power Sources, 2005, 143, 17 CrossRef.
- D. Xu, P. Dai, X. Liu, C. Cao and Q. Guo, J. Power Sources, 2008, 182, 616 CrossRef CAS.
- Q. Xu and M. Chandra, J. Power Sources, 2006, 163, 364 CrossRef CAS.
- M. Chandra and Q. Xu, J. Power Sources, 2007, 168, 135 CrossRef CAS.
- Y. Kojima and T. Haga, Int. J. Hydrogen Energy, 2008, 28, 989.
- B. H. Liu, Z. P. Li and J. K. Zhu, J. Alloys Compd., 2008 DOI:10.1016/j.jallcom.2008.09.057.
- B. H. Liu, Z. P. Li, N. Morigasaki and S. Suda, Int. J. Hydrogen Energy, 2008, 33, 1323 CrossRef CAS.
- T. B. Marder, Angew. Chem., Int. Ed., 2007, 46, 8116 CrossRef CAS.
- Z. P. Li, B. H. Liu, K. Arai and S. Suda, J. Alloys Compd., 2005, 404–406, 648 CrossRef CAS.
- C. Ponce de Leon, F. C. Walsh, D. Pletcher, D. J. Browning and J. B. Lakeman, J. Power Sources, 2006, 155, 172 CAS.
- J. H. Wee, J. Power Sources, 2006, 161, 1 CrossRef CAS.
- S. M. Lee, J. H. Kim, H. H. Lee, P. S. Lee and J. Y. Lee, J. Electrochem. Soc., 2002, 149, A603 CrossRef CAS.
- L. Wang, C. Ma, X. Mao, J. Sheng, F. Bai and F. Tang, Electrochem. Commun., 2007, 7, 1477.
- R. Jamard, A. Latour, J. Salomon, P. Capron and A. Martinent-Beaumont, J. Power Sources, 2008, 176, 287 CrossRef CAS.
- F. de Bruijn, Green Chem., 2005, 7, 132 RSC.
- G. H. Miley, N. Luo, J. Mather, R. Burton, G. Hawkins, L. Gu, E. Byrd, R. Gimlin, P. J. Shrestha, G. Benavides, J. Laystrom and D. Carroll, J. Power Sources, 2007, 165, 509 CrossRef CAS.
- X. B. Zhang, S. Han, J. M. Yan, M. Chandra, H. Shioyama, K. Yazuaki, N. Kuriyama, T. Kobayashi and Q. Xu, J. Power Sources, 2007, 168, 167 CrossRef CAS.
- X. B. Zhang, J. M. Yan, S. Han, H. Shioyama, K. Yasuda, N. Kuriyama and Q. Xu, J. Power Sources, 2008, 182, 515 CrossRef CAS.
- X. B. Zhang, S. Han, J. M. Yan, H. Shioyama, N. Kuriyama, T. Kobayashi and Q. Xu, Int. J. Hydrogen Energy, 2009, 34, 174 CrossRef CAS.
- N. Maffei, L. Pelletier and A. McFarlan, J. Power Sources, 2008, 175, 221 CrossRef CAS.
- U. B. Demirci, Electrochim. Acta, 2007, 52, 5119 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |