Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica
Christopher W.
Marshall
and
Harold D.
May
*
Medical University of South Carolina, 171 Ashley Avenue, Charleston, SC 29425, USA. E-mail: mayh@musc.edu
First published on 31st March 2009
Abstract
Microbial fuel cells (MFCs) are bioelectrochemical devices capable of converting chemical energy to electrical energy using bacteria as the catalysts. Mechanisms of microbial electron transfer to solid electrode surfaces are not well defined in most electrochemically-active microorganisms, particularly for Gram-positive bacteria. In this study, we investigated the electrochemical characteristics of the Gram-positive, thermophilic bacterium Thermincola ferriacetica strain Z-0001. This organism was capable of transferring electrons from acetate to the anode of an MFC to generate an electric current. T. ferriacetica exhibited rapid recovery of current following medium exchanges, recovering to near-maximum current output in less than three hours. The recovery of electrons from acetate was 97% in air-cathode MFCs inoculated with T. ferriacetica. Further insights into the anode reduction by these biofilms were gained through cyclic voltammetry (CV). A continuous steady-state current was reached above −0.1 V vs.Ag/AgCl reference electrode in CV scans of an established T. ferriacetica biofilm. A catalytic wave with a midpoint potential consistently near −0.28 V indicated a continuous electron-transporting interface between the attached microbial biofilm and the electrode surface. Additionally, no significant peaks were observed when scanning cell-free spent medium from active MFCs. These data suggest that T. ferriacetica directly transfers electrons to an electrode through a mechanism that is tightly associated with the biofilm that forms on the electrode. This is the first mechanistic insight into how Gram-positive extracellular electron transfer might occur without the addition of soluble electron shuttling mediators. These mechanistic evaluations will be essential for the improvement and application of such biocatalysts in microbial fuel cells and other bioelectrochemical systems.
Broader contextMicrobial fuel cells (MFCs) generate electricity using microorganisms as the catalysts and have the potential to be an inexpensive renewable energy resource. Understanding the varied microbial physiologies that support electrode reduction is of utmost importance in improving electricity yields from MFCs. Most of the research on the bacteria in MFCs has been conducted on Gram-negative mesophilic bacteria, but recent evidence suggests that other groups of bacteria may play important roles in electricity generation. Recent investigations have shown that operating MFCs at elevated temperatures with robust thermophilic microorganisms may increase electricity production and decrease contamination; a common problem that lowers the efficiency of MFCs. Although a relatively small amount of data is available on thermophilic electrode reduction, Gram-positive bacteria frequently dominate thermophilic anodic communities. Most Gram-positive bacteria require soluble electron-shuttling compounds in order to generate electricity, but this study reports evidence to the contrary using the thermophilic Gram-positive bacterium Thermincola ferriacetica as the catalyst. This study provides the first mechanistic insight into electrode reduction by a thermophilic Gram-positive bacterium. |
Introduction
Improving renewable energy technologies is imperative to reduce the world's dependence on fossil fuels. One technology that has great potential is bioelectrochemical systems (BESs) used to generate electricity (microbial fuel cells, MFCs), produce valuable chemicals and fuels, or serve as remote sensors.1,2 The hallmark of BESs is the biocatalytic transfer of electrons to or from an electrode.3 Although this is the key feature of a BES, the extracellular electron transport between a bacterial cell and an electrode is a poorly understood process.4 MFCs are an increasingly studied variation of a BES that uses the extracellular electron transport capabilities of bacteria to generate electricity. Investigations into direct electrode reduction in MFCs have narrowly been focused on mesophilic, Gram-negative microorganisms, but surprisingly few studies have been conducted on a wider range of microbial phenotypes and physiologies. Two underrepresented bacterial groups with immense promise for electrocatalysis are the Gram-positive and thermophilic bacteria.Understanding the bacterial catalysts in MFCs has become essential to maximize power output and make these bioelectrochemical reactors a practical renewable energy resource. While engineering design of the reactors still need to be improved,5 the electrical conduit from the bacterial cell wall or membrane to the anode surface arguably remains the most nebulous component of MFC-based electricity generation. Little is definitively known about the mechanisms of electron transfer from the bacteria to the electrode, which is a major limiting step in increasing currents and thus improving the practical viability for MFCs.6
The current knowledge about extracellular electron transfer by microorganisms in MFCs has been primarily elucidated in mesophilic, Gram-negative bacteria.7 By far the two most extensively studied mesophilic Gram-negative bacteria are Geobacter spp. and Shewanella spp. Consequently, the prevalent theories of extracellular electron transport have been developed based on the experiments completed with these two genera of microorganisms. Studies on Geobacter sulfurreducens have led to the hypothesis that multiple mechanisms may be employed by the bacterium to transfer electrons onto the anode. Two of these mechanisms include outer membrane c-type cytochromes6,8,9 and conductive pili called nanowires.10 Similarly, S. oneidensis has been shown to possess nanowires11 and cytochromes,12 but it has also been shown that electrons are indirectly transferred to the anode through the secretion of the electron-mediating shuttle riboflavin.13,28 The considerable attention that Gram-negative bacteria receive on the various mechanisms of electron transport highlights the fact that there is a conspicuous absence of data on these mechanisms in Gram-positive bacteria.14 Despite this absence of data, Gram-positive bacteria have been shown to be prevalent in electricity-generating communities.15–17 Thus, it is essential to explore mechanisms in a wide variety of microorganisms with diverse physiologies and phenotypes to discover the bacteria or cellular features that could be employed as catalysts in an MFC.
The deficiency of data on extracellular electron transport in Gram-positive bacteria may be because they do not have an outer membrane. The current theory of direct electrode reduction proposes a localization of electron transport chain components to the outer membrane of Gram-negative bacteria.7 These electron transport proteins accumulate on the outside of the cell envelope and can directly interface with an electrode to donate or receive electrons. Due to their lack of outer membrane electron transport proteins, Gram-positive bacteria are thought to only play a supporting role in electricity generating communities.14 The necessity of incubating Gram-positive organisms such as Brevibacillus sp.18 and Desulfitobacterium hafniense19 with soluble electron-carrying mediators is consistent with this idea. The data presented in this paper support a contrary hypothesis that Gram-positive bacteria are capable of direct electrode reduction. No mediator was added or detected in MFCs producing electricity with a thermophilic, Gram-positive bacterium and the electrochemical properties of the bacterium suggest a novel mechanism of direct electrode reduction.
In a previous study completed by this laboratory, Gram-positive bacteria selected from marine sediment from Charleston Harbor, South Carolina were shown to predominate an electricity-generating, thermophilic microbial community in MFCs.16 The results of that study concluded that the most abundant microorganisms in the selected community were closely related to the Gram-positive bacterium Thermincola ferriacetica strain Z-0001. These results were corroborated by a recent study that found Gram-positive bacteria to dominate a thermophilic community enriched in an MFC with sediment from San Francisco Bay, and an isolate of a Thermincola sp. from this community would generate electricity.17 Finding the same genus of thermophilic, Gram-positive microorganisms catalyzing electrode reduction on opposite coasts of North America is remarkable, given that the genus is only recently described after an isolation in Southern Siberia in Russia.20 This suggests a broader role for these organisms in the environment than previously believed, and provides greater impetus for them to be studied in MFCs. Additionally, both MFC studies show that thermophilic bacteria produce higher levels of current than mesophilic bacteria in the same reactor. MFCs operated at elevated temperatures may also enrich for robust electrode reducers and help prevent loss of efficiency due to fouling by contaminating bacteria. These competitive advantages of utilizing thermophilic bacteria in MFCs advocate further inquiry into anode reduction mechanisms. Due to the evidence of electricity generation by Thermincola sp. in anode respiring bacterial communities, the purpose of the present study was to characterize electrochemical properties of the Gram-positive, thermophilic bacterium T. ferriacetica in bioelectrochemical cells and gain insight into the method of electrode reduction employed by this bacterium.
Experimental
Culture conditions
Thermincola ferriacetica strain Z-0001 (DSM 14005) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) and maintained in the DSMZ medium 962 at pH 7.0 with the following exclusions from the medium: meat extract, resazurin, and sodium fumarate. The medium contained per liter: 0.33 g NH4Cl, 0.33 g KH2PO4, 0.33 g MgCl2, 0.33 g CaCl2, 0.33 g KCl, 0.05 g yeast extract, 0.70 g NaHCO3, 10 ml of trace mineral solution, 10 ml of red vitamin solution, and 1 ml of selenite–tungstate solution (per liter: 0.5 g NaOH, 3 mg Na2SeO3 × 5 H2O, 4 mg Na2WO4 × 2 H2O). Medium 962 was prepared and dispensed into culture bottles under anoxic conditions in an atmosphere of N2 : CO2 (80 : 20). Culture bottles containing autoclaved 962 medium were supplemented with 10 mM sodium acetate and 15 mM amorphous iron (III)-hydroxides39 as the electron donor and acceptor. Batch cultures were incubated at 60 °C and subcultured by a 1% transfer every 4–6 days.Single-chamber fuel cells
Single-chamber microbial fuel cells were constructed essentially as described previously by Mathis et al.16 and Milliken and May19 with an 1000-Ω external resistor. The 25 ml capacity anode compartment contained a solid graphite block electrode (6.7 cm2 surface area). The graphite anode was connected to a marine-grade wire using conductive silver epoxy (Chemtronics). The fuel cells were assembled with a cation exchange membrane (Nafion117) clamped to a carbon–platinum (10%) cloth cathode (1.7 cm2 surface area) and autoclaved for 50 min and placed in an anaerobic Coy chamber for at least 24 h. Upon inoculation of culture and medium, the medium would soak through the membrane to the cathode as the two were tightly clamped together. The single-chamber fuel cells were inoculated with either a 5% (v/v) culture transfer from T. ferriacetica grown on sodium acetate and insoluble iron-hydroxides, 100% culture transfer, or by transferring 5–10 ml of iron-hydroxide-free spent medium from one T. ferriacetica MFC into the anode compartment of an uninoculated single-chamber MFC containing 10–15 ml of fresh medium. All microbial fuel cells were incubated at 60 °C with replacement of spent medium with 20 ml fresh medium and 10 mM sodium acetate every 3–5 days unless otherwise noted. Voltage measurements were taken every 60 min as previously described by Mathis et al.,16 unless otherwise noted.Electron recovery and analysis
The Coulombic efficiency (Ec) was calculated as Ec = (Cex/Cth) × 100, where Cex is the total amount of Coulombs derived experimentally by integrating the area under the curve of a voltage vs. time plot, calculated as C = (Σ (V*t))/R, where V is the measured voltage, t is the time in seconds, and R is the external resistance measured in ohms (Ω). Cth is the theoretical amount of Coulombs that can be derived from acetate and was calculated as Cth = F*b*m, where F is Faraday's constant (96,485 C mol−1 electrons), b is the number of moles of electrons produced per mol of acetate (b = 8), and m is the moles of acetate consumed by the microorganism. These calculations were made as described by Oh and Logan.40 The microorganisms in MFC were starved for acetate through at least two exchanges before each Coulombic efficiency experiment in an attempt to eliminate acetate stored in the bacterial cell that could interfere with the electron recovery data. Acetate concentrations were below detection limits (less than 50 µM) and the voltage was less than 4 mV before substrate addition. Measurements below this voltage and acetate concentration indicated completion of the electron recovery analysis. Fuel cells for these experiments were also maintained without the 0.005% (w/v) of yeast extract ordinarily present in the medium to ensure no other carbon and energy source was supplied. Acetate concentrations were determined with a gas chromatograph (GC) equipped with a flame ionization detector and a 30 m × 0.53 mm × 1 µm HP-FFAP capillary column. Samples were filtered through a 0.2 µm syringe and acidified using 10% formic acid before analysis.41 The GC method had a starting temperature of 60 °C for 1 min, increased to 120 °C at 20 °C min−1, and then increased to 220 °C for 2 min at 6.13 °C min−1. The injector and detector temperatures were set at 230 °C. Helium was used as the carrier gas.Scanning electron microscopy
Anodes from MFCs were sacrificed and fixed in 2% gluteraldehyde in 0.1 M sodium cacodylate buffer with a 2% osmium tetraoxide postfix wash and then chemically dehydrated with a series of graded ethanol washes (25, 50, 75, 95, 100%). Samples were then sputter coated with a SC7640 (Polaron) sputter coater and viewed using a JEM-5410LV scanning electron microscope (JEOL).Electrochemical analyses
The polarization and power density curves were created to analyze voltage and power density as a function of current densities. This analysis is valuable for comparison with other MFC studies as well as to evaluate potential losses associated with operating MFCs.24 The curves were calculated using a variable resistor decade box to change the external resistance and a multimeter to measure the voltage over increasing resistances (100–100,000 Ω). The current and power calculations were then normalized to the surface area of the anode (6.7 cm2). The MFC was allowed to equilibrate to each change in resistance for 3 min before recording the voltage.Cyclic voltammetry (CV) was performed using an electrochemical analyzer (CH Instruments 660a). CV experiments were run in a dual chamber MFC setup similar to the above description with a graphite block anode as the working electrode and a carbon–Pt cloth cathode as the counter electrode. A second chamber was clamped to the MFC configuration in order to place an Ag/AgCl reference electrode (3 M KCl, CH Instruments) submerged in 3 M KCl. T. ferriacetica was grown in these modified MFC reactors (without the 3 M KCl catholyte until the start of CV experiments) and were exchanged every 3–5 days with media excluding insoluble iron exactly as described above. Electrochemical potential sweeps measuring the current produced by T. ferriacetica cells were completed at 60 °C on the biofilms from at least 50 day old MFCs. CV was also performed on MFCs immediately after medium exchanges. In this case, spent medium was extracted from the MFC and either stored for subsequent CV analysis or discarded. Sterile 962 medium heated to 60 °C was then gently rinsed over the anode to remove any loosely associated compounds from the electrode. This medium was extracted and discarded. Finally, 20 ml of heated sterile 962 media were injected along with 10 mM sodium acetate into the MFC, which was then analyzed by cyclic voltammetry. Additionally, cell-free spent medium was examined in the same electrochemical cell design, except with sterile electrodes. In these experiments, the spent medium was extracted from an electricity-generating MFC and then centrifuged for 10 min at 5040 × g to remove planktonic bacteria. The supernatant was withdrawn and used as inoculum for CV analyses to determine if soluble mediating compounds were present in the spent medium. Sterile medium without vitamins was also analyzed in the same MFC configuration to elucidate any background peaks that may be present from components of the MFC (graphite, conductive epoxy, wiring). The potential range of all scans (all potentials will be reported versus Ag/AgCl reference electrode) was −800 mV to 200 mV. All CV experiments were conducted at 60 °C. Scan rate was 1 mV s−1. All reported scans are representative of at least 3 full sweeps done in at least triplicate.
Results
Electricity generation
Thermincola ferriacetica strain Z-0001 was capable of sustained electricity generation in semi-batch MFCs (Fig. 1). The current density observed in an MFC inoculated with T. ferriacetica was steady around 400 mA m−2, depending upon the age of the fuel cell. This current could be maintained by repeated exchanges of the medium with the addition of acetate for over 3 months. T. ferriacetica MFCs were minimally affected by these medium exchanges, reestablishing current to near maximum in less than three hours following an exchange of the medium (Fig. 1 inset). Reinoculating 20 ml of spent medium from current generating MFCs into freshly exchanged MFCs did not recover the current, nor did the addition of 10 µM riboflavin to MFCs at any stage of the batch experiment have any effect on the measured current (data not shown). Moreover, once an electrode reducing biofilm was established, eliminating yeast extract and vitamins from the medium had no effect on the current. The characteristically rapid current recovery shown in Fig. 1 and the apparent absence of soluble mediators in the medium is typical of microorganisms known to directly transfer electrons to an electrode.21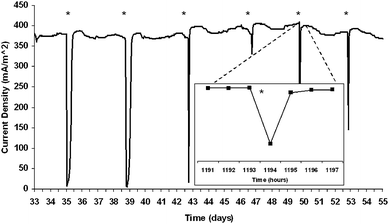 | ||
| Fig. 1 Sustainable current generation by T. ferriacetica in a thermophilic MFC 33 days after initial inoculation. Each star represents an exchange of the medium. This level of current could be maintained for over 3 months. Inset shows time it took in hours to reestablish maximum current after a medium exchange. | ||
The maximum power density and open circuit potential calculated from a power density and polarization curve were 146 mW m−2 (4.9 W m−3) and 460 mV, respectively (Fig. 2). The point of maximum power was measured at an external resistance of 400 Ω, and the slope of the linear portion of the polarization curve was 424 Ω. These two measurements designate an approximation of the internal resistance of the MFC reactor used in these experiments. This internal resistance is quite high compared to other improved reactor designs,22,23 and could be a major factor limiting the maximum power density with this organism. In addition, the external resistance should be set equal to the internal resistance,24 but the MFCs operated in this study were initiated with 1000 Ω external resistors. Further investigations into increasing power densities with T. ferriacetica in MFCs will need to take reactor design and internal resistance into careful consideration.
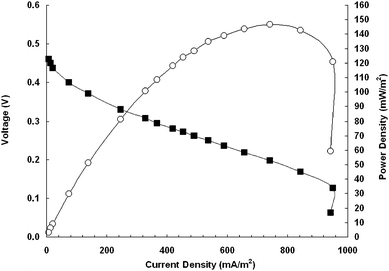 | ||
| Fig. 2 Polarization (■) and power density (○) curve. The voltage was measured at each external resistance (100–100,000 Ω) after three minutes of equilibration time. | ||
The microbial recovery of electrons from a particular substrate, known in electrochemical systems as the Coulombic efficiency, is an indicator of complete or incomplete oxidation of substrate as well as the efficiency of the biocatalyst to transfer electrons to the electrode.21,25,26 In each electron recovery experiment with T. ferriacetica, the electric current was allowed to dissipate to baseline levels before the addition of acetate (2 mM), which was measured before and after substrate addition, and at the end of the experiment. The Coulombic efficiency was 97%.
Spatial orientation on the anode
In order to assess biofilm coverage on an electrode, anodes from MFCs were sacrificed after three and six months of incubation. The biofilm of the three-month old fuel cell had developed primarily in and around the crevices of the graphite block, leaving portions of the electrode surface uncolonized (Fig. 3A). Higher magnification (Fig. 3B, uncolonized anode Fig. 3C) revealed appendages or extracellular structures that the microorganisms formed on the anode. Although the extracellular material appears to be some kind of matrix and could include biopolymers important to electron transfer, the nature and role of this material cannot be concluded from these images alone. The 3-month-old anodic biofilm coverage was divergent from what was observed in the 6-month-old fuel cell, which had an anode heavily coated with multiple layers of biomass (Fig. 3D). This increase in bacteria growing on the anode is correlated with bacteria that couple their growth to electrode reduction. It is clear from these images and the low number of planktonic cells observed by light microscopy (not shown) that T. ferriacetica developed an affinity for establishing a biofilm on the anode's surface.![Scanning electron microscopy images of T. ferriacetica on the anode of an MFC sacrificed at three months [(A), 1500×; (B), 5000×], an uninoculated graphite block [(C), 1500×], and a MFC sacrificed at 6 months [(D), 1000×]. Size bars: (A), 10 µm; (B), 5 µm; (C), 10 µm; (D), 10 µm.](/image/article/2009/EE/b823237g/b823237g-f3.gif) | ||
| Fig. 3 Scanning electron microscopy images of T. ferriacetica on the anode of an MFC sacrificed at three months [(A), 1500×; (B), 5000×], an uninoculated graphite block [(C), 1500×], and a MFC sacrificed at 6 months [(D), 1000×]. Size bars: (A), 10 µm; (B), 5 µm; (C), 10 µm; (D), 10 µm. | ||
Voltammetric evaluation of T. ferriacetica
Cyclic voltammetry (CV) was performed on T. ferriacetica-inoculated MFCs operating in semi-batch mode. A MFC with an established biofilm (generating current for at least 50 days) produced a catalytic current with an onset potential near −0.42 V and displayed a maximum steady-state current above −0.1 V (Fig. 4, green line). The midpoint potential of the catalytic current was −0.28 V, which can easily be visualized by a plot of the first derivative (Fig. 4 inset). In addition to the major peak seen from the catalytic wave, two more possible peaks around −0.35 V and −0.42 V were evident from analyzing the first derivative. These peaks appeared to be outside of the formal potential range of the major species responsible for electron transport, but may still play a role in electrode reduction. The catalytic current was significantly higher and more distinct than any signal seen from sterile medium that did not contain vitamins (Fig. 4, red line) or from cell-free spent medium harvested at maximum current (Fig. 4, blue line). This absence of a significant redox peak in spent medium coupled with the catalytic wave seen with biofilms is indicative of biofilm-associated direct electrode reduction.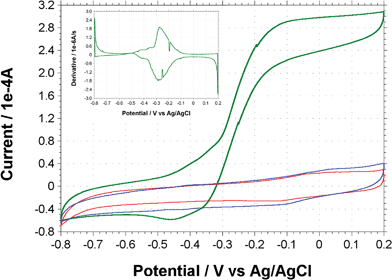 | ||
| Fig. 4 Cyclic voltammograms of T. ferriacetica biofilms (green line), sterile medium (red line), and spent medium from MFC (blue line). Inset picture is of the first derivative of the biofilm-associated catalytic wave represented by the green line. Scan rates were 1 mV s−1. | ||
In order to further ascertain biofilm-associated electron transfer capabilities, CV was performed on MFCs immediately after exchanging the spent medium with sterile fresh medium (Fig. 5). The MFCs were first rinsed gently with sterile medium, which was subsequently removed before supplying 20 mL of fresh sterile medium to conduct the experiment. Close to 30 min after the electrode was washed with the fresh medium, a midpoint catalytic current was observed at −0.28 V; which was similar to the midpoint potential of the catalytic wave in Fig. 4. In addition to the major catalytic wave, two peaks similar to those seen at peak current (Fig. 4) were again evident from the first derivative (Fig. 5 inset). Thus, a persistence of the catalytic current was observed after an exchange of the medium. This is in accordance with the data presented in Fig. 4, suggesting that the dominant redox active compound is associated with the biofilm and not available in the spent medium.
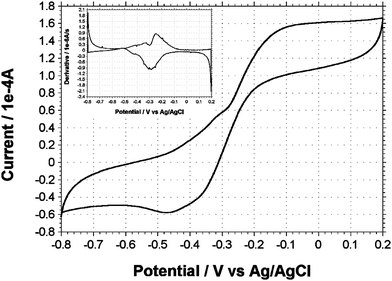 | ||
| Fig. 5 Cyclic voltammogram of T. ferriacetica MFCs less than 30 min after an exchange of the medium. Inset picture is the first derivative clearly delineating midpoint potential of redox components. Scan rates were 1 mV s−1. | ||
Discussion
Thermincola ferriacetica was capable of generating an electric current at thermophilic temperatures in an air-cathode microbial fuel cell. This microorganism produced a current without the addition of electron-shuttling mediators and the data presented here suggest that current was produced without a soluble mediator secreted by the bacteria. This is the first characterization of the electrochemical properties and electron transporting capabilities of a thermophilic, Gram-positive bacterium.Mathis et al.16 with their mixed thermophilic cultures and Wrighton et al.17 with mixed thermophilic cultures and isolates of Thermincola sp. demonstrated electrode reduction without adding mediators. However, neither group examined the electrochemical properties of these thermophiles or determined if the mediation was consistent with direct electrode reduction or through the production of soluble mediators. Here, when MFCs were washed with fresh medium and substrate, the recovery of current was rapid (Fig. 1 inset). This is divergent from what is seen in MFCs operating with bacteria that produce and excrete their own soluble electron-mediating compounds. An example of this lag in current-recovery time is the Gram-negative bacterium Shewanella oneidensis that produces flavins to aid in current production.13 The time it takes for S. oneidensis pure cultures to regenerate the current to maximum levels after a medium exchange is approximately 72 h. This is presumably to accumulate increasing concentration of mediators (flavins). Furthermore, replacing the spent medium from S. oneidensis back into the freshly exchanged MFC or the addition of riboflavin can restore the current in this mediated reactor. T. ferriacetica-inoculated MFCs, on the other hand, can reestablish the maximum current within a few hours, and the replacement of spent medium or addition of riboflavin does not hasten current regeneration by T. ferriacetica. Thus, the current recovery data from T. ferriacetica MFCs resemble that of previously reported pure culture MFCs exhibiting direct electron transfer with Gram-negative organisms such as Geobacter sulfurreducens21,27 instead of resembling the slow current regeneration seen with microorganisms that excrete soluble mediators like flavins13,28 or phenazines29 for electron transfer. Additional correlations related to direct electron transfer is apparent when comparing the Coulombic efficiencies of different microorganisms. G. sulfurreducens is the most commonly studied electrically active bacterium known to directly reduce an anode, and it has a Coulombic efficiency of near 100%.30 Conversely, the flavin-mediated anode reducing bacterium S. oneidensis commonly has a Coulombic efficiency below 60%.31 The Coulombic efficiency of T. ferriacetica determined in this study was very high (97%), indicating nearly all electrons were diverted to electrode reduction and not to the production of reduced organic compounds. This distinction of high Coulombic efficiency is important when considering electron transfer mechanisms and the capabilities of these microorganisms to be effective biocatalysts.32 Overall, the current recovery data and the Coulombic efficiency for T. ferriacetica are consistent with data presented on direct electron transfer as seen in G. sulfurreducens and not characteristic of mediated electron transfer displayed by S. oneidensis.
Another interesting observation was the presence of thick biofilms on the electrode containing an intricate connective structure. Previous studies have shown that pili production in electricity generating communities may play a conductive role in electron transport.10,33 These conductive nanowire pili are a potential mechanism of direct electron transfer in G. sulfurreducens10 and S. oneidensis,11 and could be a factor contributing to current production by T. ferriacetica. However, it is undetermined whether this is the case or if the extracellular matrix is simply a feature of adherence in this Gram-positive biofilm. Despite these speculations, it is clear that T. ferriacetica establishes contact with the electrode while increasing biofilm coverage on the electrode over time.
The most compelling evidence that T. ferriacetica employs a direct electron transfer mechanism instead of soluble electron-shuttling mediators is the cyclic voltammetry data. These data revealed a redox-active component around −0.28 V only when a biofilm was present. A rate limiting current was reached at a potential above −0.1 V, indicating that a continuous connection of electrical current was established between the biofilm and the electrode above this potential. The establishment of a continuous, steady state current observed in CV scans is remarkable since this has not been reported for a Gram-positive bacterium.
Reinforcing the hypothesis of direct electron transport in the absence of a soluble mediator, the cell-free spent medium had no distinct voltammetric profile. No peaks were detected that could be responsible for the level of current seen from the biofilm, and no peaks were seen in the range of the biofilm-associated catalytic wave. Only minor inflections, most likely due to a background signal, were seen outside of the range of the catalytic current. Due to the absence of oxidation or reduction peaks in the CV scans of cell-free spent medium (Fig. 4), it is possible to conclude that no electron shuttle was added exogenously (i.e.iron or vitamins in the growth medium) or produced in significant quantities by the bacterium itself. The lack of redox active compounds in the spent medium of MFCs is further evidence of direct electrode reduction capabilities by T. ferriacetica.
As well as not detecting a redox active compound in the cell-free spent medium, the biofilms of T. ferriacetica could not be gently washed of their electron transporting capacities. CV of an operating MFC immediately after a medium exchange showed that T. ferriacetica maintained its catalytic capabilities despite washing away the medium surrounding the cells (Fig. 5). The redox peak at the same potential as the catalytic wave seen at maximum current production indicates that the conduit of electron transport was not washed away during medium exchanges, but remained intact with the biofilm. Thus, the data for a direct electron transfer mechanism is strengthened because biofilms of T. ferriacetica retain their electrode-reducing capability after the washing away of potential mediating compounds.
Including the primary signal at −0.28 V, the first derivative of all CVs revealed one or two more features (−0.35 V and −0.42 V) associated with current generation. Similarly, multiple redox peaks have been reported for G. sulfurreducens, which directly transfers electrons to an electrode.34 This characteristic has been attributed to the possibility of two components on the electron transport chain (for example two different cytochromes) transferring electrons to the electrode.35 Another explanation may be one protein or contact point capable of transferring electrons at two different formal potentials (via different catalytic protein or cofactor centers). The data presented for T. ferriacetica are similarly consistent with the idea of more than one redox active biological component of this Gram-positive thermophile being capable of electron exchange with an electrode, and are highly suggestive of some form of direct electron transport to an electrode in association with the CV feature with a midpoint around −0.28 V. However, different approaches will be needed to further elucidate the role of the redox active component(s) involved in electron transfer by T. ferriacetica.
Conclusions
The CVs presented in this study are the first to be applied to a thermophilic Gram-positive microorganism. The slow scan rates used represent steady-state conditions and characterize the enzymatic activity of the microorganisms that cannot be adequately analyzed at increased scan rates.34,35 Park et al. reported a CV for Clostridiumbutyricum EG3, a mesophilic Gram-positive fermentative bacterium, but we are not able to compare it with our electrochemical data due to the high scan rate used in that study.36 Thus, it is unknown how Gram-positive bacteria transfer electrons extracellularly without the presence of an electron-shuttling mediator, yet it is hypothesized that such a mechanism exists.37 The experiments completed using CV in this study provide the initial framework describing external electron transport by T. ferriacetica, components of which may be targeted for future improvement and specialization of MFCs and other BESs. Thermincola ferriacetica, is a logical choice for future investigations into thermophilic, Gram-positive electrode reduction because it has been physiologically characterized after an isolation on Kunashir Island in the Sea of Okhotsk,38 and is closely related to electrochemically active microorganisms found in marine sediments on the east and west coasts of North America.16,17 The ubiquity of the genus indicates that it could be used as a microbial catalyst in BESs around the globe. It is our hope that this electrochemical characterization of T. ferriacetica will generate interest in Gram-positive extracellular electron transfer so that different mechanisms can be discovered and manipulated to enhance the performance of MFCs. We predict that these discoveries will lead to increased power production from MFCs or more product yield from BESs and thus push these technologies closer to viable applications.Acknowledgements
The authors thank Microbial Fuel Cell Technologies, LLC for its support through DOE STTR Grant DE-FG02-07ER86319.References
- C. Melhuish, I. Ieropoulos, J. Greenman and I. Horsfield, Autonom. Robots, 2006, 21, 187–198 Search PubMed.
- L. M. Tender, S. A. Gray, E. Groveman, D. A. Lowy, P. Kauffman, J. Melhado, R. C. Tyce, D. Flynn, R. Petrecca and J. Dobarro, J. Power Sources, 2008, 179, 571–575 CrossRef CAS.
- U. Schroder, Phys. Chem. Chem. Phys, .2007, 9, 2619–2629 Search PubMed.
- J. A. Gralnick and D. K. Newman, Mol. Microbiol., 2007, 65, 1–11 CrossRef.
- Z. Du, H. Li and T. Gu, Biotechnol. Adv., 2007 Search PubMed.
- D. E. Holmes, S. K. Chaudhuri, K. P. Nevin, T. Mehta, B. A. Methe, A. Liu, J. E. Ward, T. L. Woodard, J. Webster and D. R. Lovley, Environ. Microbiol., 2006, 8, 1805–1815 CrossRef CAS.
- D. R. Lovley, Curr. Opin. Biotechnol., 2008, 19, 564–571 CrossRef CAS.
- D. E. Holmes, T. Mester, R. A. O'Neil, L. A. Perpetua, M. J. Larrahondo, R. Glaven, M. L. Sharma, J. E. Ward, K. P. Nevin and D. R. Lovley, Microbiology, 2008, 154, 1422–1435 CrossRef CAS.
- B. C. Kim, B. L. Postier, R. J. Didonato, S. K. Chaudhuri, K. P. Nevin and D. R. Lovley, Bioelectrochemistry, 2008, 73, 70–75 CrossRef CAS.
- G. Reguera, K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen and D. R. Lovley, Nature, 2005, 435, 1098–1101 CrossRef CAS.
- Y. A. Gorby, S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson and J. K. Fredrickson, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11358–11363 CrossRef CAS.
- O. Bretschger, A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson and K. H. Nealson, Appl. Environ. Microbiol., 2007, 73, 7003–7012 CrossRef CAS.
- E. Marsili, D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick and D. R. Bond, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3968–3973 CrossRef CAS.
- K. Rabaey, J. Rodriguez, L. L. Blackall, J. Keller, P. Gross, D. Batstone, W. Verstraete and K. H. Nealson, ISME J., 2007, 1, 9–18 CrossRef CAS.
- P. Aelterman, K. Rabaey, H. T. Pham, N. Boon and W. Verstraete, Environ. Sci. Technol., 2006, 40, 3388–3394 CrossRef CAS.
- B. J. Mathis, C. W. Marshall, C. E. Milliken, R. S. Makkar, S. E. Creager and H. D. May, Appl. Microbiol. Biotechnol., 2008, 78, 147–155 CrossRef CAS.
- K. C. Wrighton, P. Agbo, F. Warnecke, K. A. Weber, E. L. Brodie, T. Z. DeSantis, P. Hugenholtz, G. L. Andersen and J. D. Coates, ISME J., 2008, 2, 1146–1156 CrossRef CAS.
- T. H. Pham, N. Boon, P. Aelterman, P. Clauwaert, L. De Schamphelaire, L. Vanhaecke, K. De Maeyer, M. Hofte, W. Verstraete and K. Rabaey, Appl. Microbiol. Biotechnol., 2008, 77, 1119–1129 CrossRef CAS.
- C. E. Milliken and H. D. May, Appl. Microbiol. Biotechnol., 2007, 73, 1180–1189 CAS.
- T. G. Sokolova, N. A. Kostrikina, N. A. Chernyh, T. V. Kolganova, T. P. Tourova and E. A. Bonch-Osmolovskaya, Int. J. Syst. Evol. Microbiol., 2005, 55, 2069–2073 Search PubMed.
- D. R. Bond and D. R. Lovley, Appl. Environ. Microbiol., 2003, 69, 1548–1555 CrossRef CAS.
- Y. Fan, H. Hu and H. Liu, J. Power Sources, 2007, 171, 348–354 CrossRef CAS.
- B. Logan, S. Cheng, V. Watson and G. Estadt, Environ. Sci. Technol., 2007, 41, 3341–3346 CrossRef CAS.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS.
- H. P. Bennetto, G. M. Delaney, J. R. Mason, S. D. Roller, J. L. Stirling and C. F. Thurston, Biotechnol. Lett., 1985, 7, 699–704 CAS.
- B. E. Logan, Microbial Fuel Cells, John Wiley & Sons, Inc, Hoboken, 2008 Search PubMed.
- S. Srikanth, E. Marsili, M. C. Flickinger and D. R. Bond, Biotechnol. Bioeng., 2008, 99, 1065–1073 CrossRef CAS.
- H. von Canstein, J. Ogawa, S. Shimizu and J. R. Lloyd, Appl. Environ. Microbiol., 2008, 74, 615–623.
- K. Rabaey, N. Boon, M. Höfte and W. Verstraete, Environ. Sci. Technol., 2005, 39, 3401–3408 CrossRef CAS.
- K. P. Nevin, H. Richter, S. F. Covalla, J. P. Johnson, T. L. Woodard, A. L. Orloff, H. Jia, M. Zhang and D. R. Lovley, Environ. Microbiol., 2008 Search PubMed.
- M. Lanthier, K. B. Gregory and D. R. Lovley, FEMS Microbiol. Lett., 2008, 278, 29–35 CrossRef CAS.
- D. R. Lovley, Nat. Rev. Microbiol., 2006, 4, 497–508 Search PubMed.
- G. Reguera, K. P. Nevin, J. S. Nicoll, S. F. Covalla, T. L. Woodard and D. R. Lovley, Appl. Environ. Microbiol., 2006, 72, 7345–7348 CrossRef CAS.
- E. Marsili, J. B. Rollefson, D. B. Baron, R. M. Hozalski and D. R. Bond, Appl. Environ. Microbiol., 2008, 74, 7329–7337 CrossRef CAS.
- K. Fricke, F. Harnisch and U. Schroder, Energy Environ. Sci., 2008, 1, 144–147 RSC.
- H. S. Park, B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park and H. I. Chang, Anaerobe, 2001, 7, 297–306 CrossRef CAS.
- H. L. Ehrlich, Geobiology, 2008, 6, 220–224 CrossRef CAS.
- D. G. Zavarzina, T. G. Sokolova, T. P. Tourova, N. A. Chernyh, N. A. Kostrikina and E. A. Bonch-Osmolovskaya, Extremophiles, 2007, 11, 1–7 CrossRef CAS.
- J. W. Moon, Y. Roh, R. J. Lauf, H. Vali, L. W. Yeary and T. J. Phelps, J. Microbiol. Met., 2007, 70, 150–158 Search PubMed.
- S. Oh, B. Min and B. E. Logan, Environ. Sci. Technol., 2004, 38, 4900–4904 CrossRef CAS.
- S. Freguia, K. Rabaey, Z. Yuan and J. Keller, Environ. Sci. Technol., 2007, 41, 2915–2921 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
