CO2 capture and storage: are we ready?
Franklin M.
Orr, Jr.
*
Global Climate and Energy Project, Stanford University, Yang and Yama saki Building, Suite 324, 473 Via Ortega, Stanford, California 94305-4230, USA. E-mail: fmorr@stanford.edu.; Fax: +1 650 725 9190; Tel: +1 650 725 6270
First published on 13th March 2009
Abstract
Options for capture and storage of CO2 that would otherwise be released into the atmosphere by combustion of fossil fuels are considered. This paper assesses whether CO2 can be captured, whether sufficient potential capacity exists for storage in geologic formations, describes physical mechanisms that can prevent escape of the CO2 from the subsurface, delineates methods for monitoring the movement of CO2 in the subsurface and for detecting leaks, and describes field experience with CO2 injection. While much remains to be learned about the design of specific storage projects, the current state of knowledge of carbon capture and storage is sufficient to permit testing at the scale of large power plants.
Broader contextOne option for reducing emissions of CO2 to the atmosphere is the capture and storage of CO2 from carbon combustion reactions or from industrial sources. Processes to capture CO2 have been demonstrated at commercial scale, though the energy required to separate the CO2, and therefore the cost, is significant. To have an impact on overall CO2 emissions, large quantities of CO2 will have to be stored in porous rocks in the deep subsurface: oil and gas reservoirs, deep formations that contain salt water, coal beds, and possibly basalt formations. Geologic storage of CO2 in oil reservoirs and deep saline formations has also been demonstrated on a commercial scale. The next important step is to demonstrate the integration of carbon capture and storage at the scale of large power plants in a variety of geologic settings. |
Introduction
Around 86% of world primary energy use is currently based on fossil fuels, coal, petroleum, and natural gas, and energy use is the dominant contributor to greenhouse gas emissions associated with human activities. Concentrations of two greenhouse gases associated with energy use have increased significantly: CO2 concentration in the atmosphere has increased by a third over preindustrial levels, from about 280 ppm to 385 ppm, and the concentration of methane has more than doubled.1 The impact of these emissions can already be discerned in global average temperatures, high latitude ice cover, changes in snow cover, and a decrease in upper ocean pH, among many others, but what matters more is the potential impact of future emissions, which are currently projected to increase substantially in this century in the absence of significant changes in energy systems. Recent estimates by the Intergovernmental Panel on Climate Change1 indicate that deep reductions from current CO2 emissions will be required by midcentury if we are to stabilize atmospheric concentrations of CO2, even at levels substantially higher than at present but low enough to limit predicted global average temperature rises to about 2 °C.The obvious first option for emissions reduction is to make more efficient use of energy, in homes, businesses, vehicles, and industrial processes. Improved energy efficiency has the potential to reduce CO2 emissions relatively rapidly and quite significantly, and often at costs that are more than offset by reduced energy costs. Use of renewable energy resources other than hydroelectric generation (wind, solar, biomass, and geothermal, for example) is growing rapidly, but from a relatively small base. Given the size of the world's energy systems, projected growth in energy demand, and the magnitude of investments in energy infrastructure that will be required, it is likely that worldwide transitions to large-scale use of those resources will take decades. The existing infrastructure for fossil fuels, particularly coal, is so large that it is likely that methods to reduce CO2 emissions from fossil fuel use, particularly for electric power generation, will be needed in addition to the worldwide effort to substitute primary energy resources that are not based on fossil carbon.
US CO2 emissions account for about a quarter of world emissions, and coal-fired power plants provide about half of electric power generation. Hence, examination of the potential role of CCS in the US illustrates many of the issues that will have to be faced. How is CO2 emitted in the US? Fig. 1 shows the CO2 emissions associated with various sectors of the US economy. Electric power generation is the largest source of CO2 emissions for the US (2.4 billion metric tons, Gt CO2, in 2007, mainly from coal and natural gas2,3), but transportation, at 2 Gt CO2, is a close second.3 The quantities of CO2 reported for industrial, residential, and commercial sectors include the CO2 that comes from electric power generation.
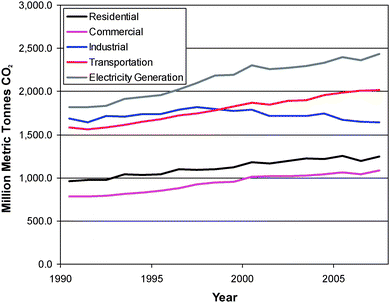 | ||
| Fig. 1 US CO2 emissions by sector.3 Note that the total electric power generation for each sector is also included in the values for that sector. Data for 2007 are preliminary. | ||
Power plants are large, stationary sources of CO2, which are obvious targets for CO2 capture. though other industrial processes (petroleum refining, ammonia, cement, and steel, production, natural gas cleanup, for example) that emit about 1 Gt CO2 in addition to the CO2 from electricity offer opportunities as well. It is also possible that transportation will make greater use of electricity (particularly if battery energy density and durability improve) through plug-in hybrids and electric vehicles. Residential and commercial uses (largely for space heating with natural gas) each account for about 0.6 Gt CO2 in addition to electricity generation. As a first step towards reducing CO2 emissions, it makes sense to focus on relatively large stationary sources, where quantities large enough to make a difference and economies of scale might be available.
One option for reducing emissions is to capture a portion of the CO2 that would otherwise be emitted and store it in a location that isolates it from the atmosphere on time scales of centuries to millennia. Whether carbon capture and storage (CCS) can be done at large scale in the decade ahead depends on the answers to questions that include:
• Can we capture the CO2 efficiently and at an acceptable cost?
• Do we have a sufficient variety of geologic settings and sufficient volume to store enough CO2 to have an impact on emissions distributed around the globe?
• Are there sufficient physical mechanisms that will trap the CO2 in the subsurface to allow design of safe storage projects that will not leak?
• Can the movement of fluids in the subsurface be monitored and leaks be detected and if necessary, remediated?
• Do we have enough experience with actual operations to undertake storage at a scale large enough to have an impact on emissions?
This paper offers an assessment of where we stand on these questions.
CO2 capture
Fig. 2 shows that a range of options is currently available for CO2 separation from effluent gases from electric power production or industrial processes. Any one of several separations can be used to obtain relatively pure CO2 for storage: CO2 from N2 or from CH4, O2 from N2, or H2 from CO2.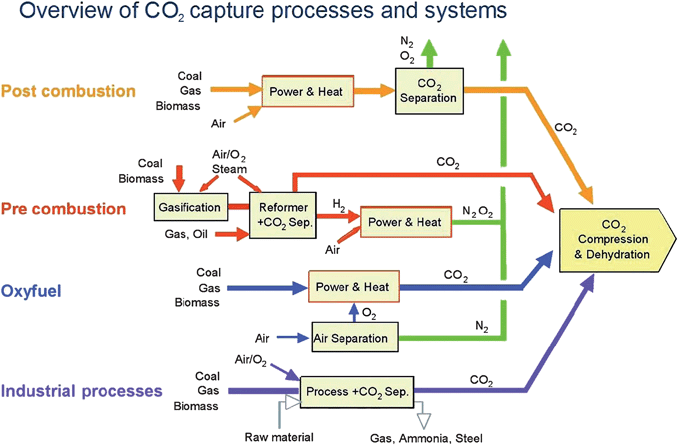 | ||
| Fig. 2 CO2 capture options (IPCC 2005: IPCC Special Report on Carbon Capture and Storage, prepared by Working Group III of the Intergovernmental Panel on Climate Change, Figure TS.3, Cambridge University Press).4 In post-combustion capture, CO2 is separated from nitrogen and oxygen in combustion product gases. In pre-combustion capture, oxygen is separated from air for gasification of a fuel, and then CO2 is separate from hydrogen produced by shift reactions. An alternative is to use the oxygen directly for combustion, with separation of the CO2 by condensing water present in the combustion products. | ||
If CO2 is to be separated from some gas stream, the starting point will often be combustion product gases, which typically contain from 3–15% CO2 along with nitrogen and traces of other gases. Natural gas combustion turbines produce lower CO2 concentrations (because excess air is used to control temperatures in the hot section of the turbines). Coal combustion generates concentrations at the high end of the range. Other gas streams may also provide opportunities. Still higher CO2 concentrations (15–30%) can occur in cement production, where a fossil fuel is typically burned to convert calcium carbonate to calcium oxide, which liberates CO2 from the calcium carbonate in addition to the CO2 from combustion. Natural gas sometimes contains CO2 as a contaminant with widely varying concentrations. High concentration gas streams are likely to be the early favorites for CO2 capture because they provide a larger concentration driving force for CO2 separation.
Post combustion capture and separation of CO2 from natural gas typically make use of chemisorption, often in solvents such as an ethanolamine. The gas stream is mixed with solvent in a contacting tower, the CO2 transfers to the liquid phase, and the liquid solution is then separated physically from the remaining gas. Next, the separated solution is heated to recover the CO2, and the regenerated solvent is then recycled to absorb more CO2 in the contacting tower. The energy required to move the fluids, recover the CO2, and compress it for transportation by pipeline to a storage site reduces the energy available for electric power production. For a subcritical pulverized coal electric power plant, according to estimates of a recent study, the addition of 90% carbon capture reduces the thermal efficiency of the power plant from about 34% to about 25%, a significant penalty2 that implies a corresponding increase in the estimated levelized cost of electricity, from about 4.8 to about 8.2 cents kWh−1 (2006 dollars), an increase of almost 70%. For a more efficient, ultrasupercritical plant, which operates with higher boiler temperatures and pressures, the estimated reduction is from about 43% to 34%, and the estimated cost of electricity increases from 4.7 to 7.3 cents kWh−1. Thus, the addition of post-combustion capture with amines would increase the low cost of electric power from coal significantly, though the increased cost would still be significantly lower than current estimates of the cost of electricity for photovoltaics and solar thermal electricity and roughly comparable to current cost estimates for electric power from wind turbines.
Precombustion and oxyfuel schemes for CO2 capture typically start with a cryogenic separation of O2 from air (see Fig. 2). That separation also requires energy, of course. Oxyfuel combustion produces CO2 and H2O (and small amounts of contaminants), and the separation of the H2O is accomplished by condensation of water as a liquid. Some recycling of CO2 to dilute the O2 is typically required to control temperature of combustion. Gasification methods (typically in a form known as integrated gasification and combined cycle, or IGCC, power plants) use partial oxidation of a feedstock such as coal, biomass, or petroleum coke to make a synthesis gas (CO and H2), which can than be combined with steam to produce H2 and CO2 with a shift reaction. The CO2 is separated, and the H2 can then be used as a fuel for a gas turbine or for other chemical syntheses. Significant commercial experience in gasification has been gained in the manufacture of hydrogen for use in ammonia synthesis, for example.
Because the gasification and shift reactions are often performed at elevated pressure, the separation can be done with physical solvents (selexol or rectisol, for example), and the separated CO2 is also captured at an elevated pressure, which reduces the total amount and the cost of compression to the pressure required for transportation and storage compared to that for lower pressure separations. Relatively recent cost and efficiency estimates2 indicate that oxyfuel and IGCC plants could produce electricity with 90% capture of CO2 for about 7.0 and 6.5 cents kWh−1, estimated costs that are slightly lower than similar estimates for pulverized coal plants. It should be noted that construction costs for new plants rose sharply in 2007 and early 2008 but may have declined since then due to the worldwide economic downturn. Operation of IGCC plants at full commercial scale with carbon capture and storage has not yet been demonstrated.
The CO2 and O2 separation processes described above are all currently used at commercial scale, though not at the scale that would be required for a large power plant (say 500 MW or more). Other approaches are being investigated, however. Improved solvents, membrane separations, oxygen separation with ion transport membranes and ionic liquids, solid sorbents, and chemical looping are among the methods being developed. The solvent-based separations that are currently used are thermodynamically relatively inefficient, requiring six or seven times as much energy as the minimum based on the chemical potentials of the mixed and separated gases. Thus, there is room for improvements that would reduce the cost and energy penalties of separations. Most estimates indicate that the cost of separation is the largest component of the overall cost of CCS, so improvements in the energy efficiency and cost of capture could have significant impact on the extent to which CCS can be implemented at acceptable cost.
Geologic settings and potential storage volumes
Porous rocks that might be used for storage are widely distributed around the world, as Fig. 3 shows, but they are not collocated everywhere with large CO2 sources. Fig. 4 indicates, however, that the United States is well endowed with regions that include the three types of geologic formations most commonly considered for storage: oil and gas reservoirs, deep formations that contain salt water, and coal beds that are too deep to be mined. Storage in basalts, deep ocean sediments, and olivine, settings that are less well defined, has also been proposed.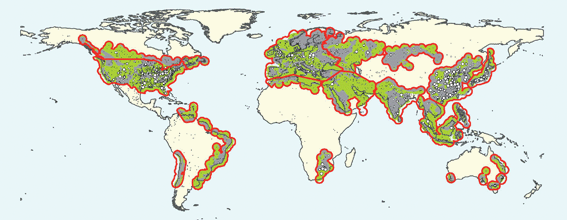 | ||
| Fig. 3 Sedimentary basins that have formations potentially suitable for CO2storage. White dots are significant sources of CO2 emissions. Green regions include sedimentary rocks with storage potential, grey zones do not, and white areas have not been assessed. Areas with large CO2 sources (white dots) within 300 km are outlined in red.31 | ||
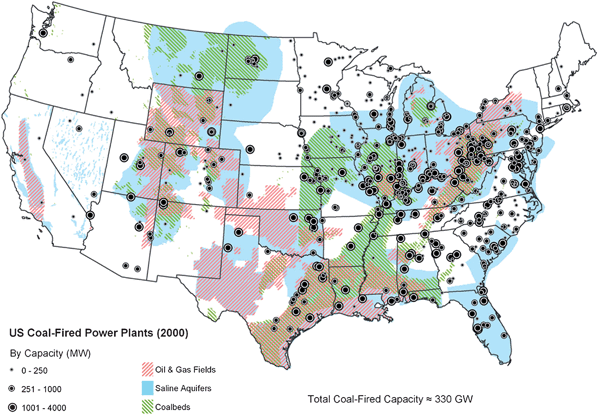 | ||
| Fig. 4 US locations of large CO2 sources and geologic formations (oil and gas fields, deep saline aquifers, and deep coal beds) potentially suitable for storage.2 (a) Spatial distributions of permeability. (b) Spatial distributions and fraction of pore space occupied by injected CO2. | ||
The existence of porous rocks alone does not guarantee that they will be suitable for storage, of course, and specific sites will have to be selected and evaluated carefully.4 Sites that are appropriate will have rock layers above the storage formation that prevent vertical flow to the surface. Oil and gas fields always have such seal rocks (also called caprocks). If they did not, the buoyant oil and gas would have long since escaped. Specific saline aquifers or coal beds may or may not have suitable seal rocks. Porous formations will need to be deep enough that the pressure is sufficient to make the CO2 relatively dense: depths below about 800–1000 m are generally sufficient for oil, gas, and aquifer settings. Coal beds must be deep enough that there is no possibility they will be mined, but depths shallower than 1000 m could be considered because the storage mechanism for coals involves adsorption rather than storage of CO2 as a free phase. Formations will need to be large enough that a significant quantity of CO2 can be stored without exceeding the pressure that would fracture the rocks or activate faults that might leak. Areas with potential leak paths (nonsealing faults or unsealed abandoned wells) will clearly be unsuitable. The permeability (a measure of how rapidly a fluid flows in a rock under a given pressure gradient) will also need to be large enough that the CO2 can be injected at a reasonable rate in a reasonable number of wells.
DOE-supported regional teams have completed a preliminary assessment of the potential storage volume resource.5Table I reports the results of those assessments along with related estimates compiled by Vidas et al.6 that include offshore formations as well as shales and basalts. The total potential storage capacity (in billion metric tons of CO2, Gt CO2) is large compared to the current annual CO2 emissions from electric power generation and industrial sources in the US (about 3 Gt CO2). It is important to remember that these are resource estimates, and specific site evaluation will be required to determine which subset of formations will meet cost, impact of surface facilities, safety, and other constraints.
Oil and gas reservoirs, if they are available within a reasonable distance, will be preferred sites, because an effective seal exists, because some of the required infrastructure (wells, for example) may be available, because there is an existing regulatory structure for CO2 injection, and because it may be possible to offset some of the cost of storage by the value of additional oil or gas recovered. But oil and gas reservoirs are not as widely distributed as deep saline aquifers (see Fig. 4), and their capacity is limited.
Deep saline aquifers offer much larger volumes, however, and the combination of the potential storage volumes available in all three storage settings means that many CO2 sources will be located within reasonable distance of a potential storage formation. Dooley, et al.7 estimated that 95% of the 500 largest CO2 sources in the US are within 80 km of a potential storage formation. A variation on the idea of storage in saline aquifers is injection of CO2 into deep sea sediments.8 This approach takes advantage of the low temperature and high pressure at depths below 2700 m in the ocean. At those conditions, CO2 is more dense than sea water, and it forms hydrates rapidly.9 Below the ocean-bottom sediment surface, temperature rises with depth due to the geothermal gradient. Hence CO2 injected at greater depths, where CO2 is less dense than brine, would rise due to buoyancy, but would then cool, become dense enough to stop further rise, and form solid hydrates. Storage in sediments at shallower depths where CO2 would be buoyant but less dense and less mobile than in deep onshore saline aquifers has also been proposed.10
Some investigators have also proposed that basalt formations could also be used to store CO2.11 That possibility has been investigated much less extensively, however, and it has not yet been tested in the field. Further study will be needed to refine estimates of potential storage volumes in that setting.
The combination of potential storage volumes and locations that are widely distributed suggests that there is sufficient potential capacity available to allow storage at a scale large enough to have an impact on emissions if other constraints can be met.
Physical mechanisms of storage
In a well-designed geologic storage project, there will be multiple physical mechanisms that limit the movement of CO2, and the security of storage will increase with time.4 Injected CO2 will be a relatively dense supercritical phase at typical subsurface conditions, but it will buoyant compared to oil or water, so it is essential that seal rocks prevent vertical migration while other mechanisms act to limit the potential for subsequent escape of the CO2. Thus the first line of defence against leakage of CO2 to the near surface zone will be rock layers with low permeability, which occurs when they have very small pores. If the permeability is low, the flow through the porous rock that is induced by a pressure gradient occurs slowly. In addition, a bubble of supercritical CO2 can penetrate into such a layer only if its pressure is significantly higher than the pressure in surrounding brine (this pressure difference is called the capillary pressure) in order to overcome the effects of interfacial tension at the curved interface of the bubble as it enters the pore. Good sites will have multiple low-permeability barriers to flow (shales, evaporites, or sealing faults) with high capillary entry pressures that prevent significant vertical movement of injected CO2.High pressure CO2, at depths typical of those considered for storage, has a relatively low viscosity (a few hundredths of a centipoise). The low-viscosity CO2 will flow preferentially through layers with higher permeability than surrounding layers, and the brine in those layers will be displaced relatively inefficiently. And the difference in density between CO2 and brine will cause formation of a gravity “tongue”, as the CO2 makes its way upwards to the seal rocks and flows horizontally away from the injection well.
Fig. 5a illustrates some of these effects in simulations of CO2 injection into vertical cross sections of saline formations with two spatial arrangements of permeability.12 The first is a set of random variations, and the second is a three layer system in which two high permeability layers are separated by a lower permeability layer. CO2 is injected over the bottom quarter of the left side until about 7% of the pore volume of the formation has been injected. In these displacements, gravity is quite important, and despite the fact that the CO2 is injected at the bottom of the formation, it quickly flows upward and forms a gravity tongue under the impermeable layer that bounds the top of the porous formation in both cross sections (see Fig. 5b, part (i)). In the layered example, a second tongue forms beneath the low-permeability layer. Thus, the combination of gravity segregation and heterogeneity means that the CO2 does not displace the brine uniformly. Instead, it sweeps only a relatively small fraction of the formation, and it has high saturation (the fraction of the pore space occupied by CO2) at the top of the formation just beneath the caprock.
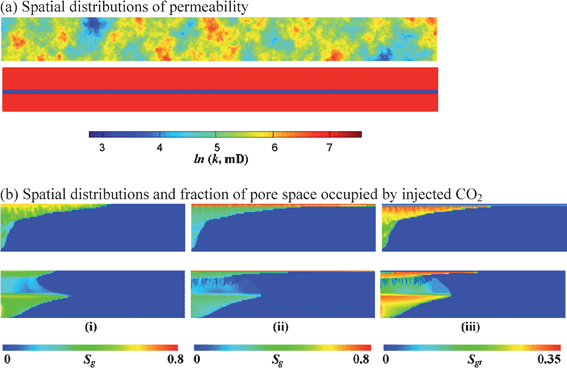 | ||
| Fig. 5 Permeability and CO2 saturations in two vertical cross sections of deep saline formations. (a) The two cross sections have differing permeability distributions. One is heterogeneous with random variations in permeability, and the other consists of two high permeability layers with a low permeability layer between them. (b) The saturation maps for the two permeability arrangements in (a). Part (i) shows the location of injected CO2 at the end of the injection period, typically multiple decades in a full-scale project. Parts (ii) and (iii) show CO2 saturations at the end of the gravity relaxation period. Part (ii) shows the total saturation of CO2, and (iii) shows the saturation of CO2 that is unable to flow because it has been trapped by capillary snap-off. (note the different scale for trapped saturation). In these simulations, the CO2 remains in a separate phase, and the effects of dissolution are ignored. | ||
When injection ceases, a slow process of gravity-driven redistribution begins. The location of the injected CO2 at the end of the injection period is shown in part (i) of Fig. 5b, and the corresponding locations of CO2 at the end of the gravity-relaxation period are shown in part (ii). As the CO2 migrates upward, its saturation at the base of the gravity tongue declines as water invades from below and replaces the CO2 moving upwards. As that happens, an important mechanism known as capillary trapping begins to immobilize CO2 in isolated bubbles that occupy a few pores. This trapping is the result of an instability that occurs as a bubble of CO2 is displaced through a pore. The curvature of the CO2–brine interface at a pore throat causes brine to flow through thin films on rock surfaces to form a neck in the pore throat that snaps off to isolate the bubble. Once trapped, these bubbles are very difficult to move, so any trapped CO2 is not available to leak. This mechanism is well studied because it is a primary way that oil can be trapped and left behind during the secondary recovery process of waterflooding to displace oil from reservoir rocks.13 Part (iii) of Fig. 5b shows the distribution of trapped CO2 at the end of the relaxation period (note the different saturation scale for these panels). The trapped saturation varies spatially because the amount that is trapped depends on the maximum CO2 saturation reached before the brine began to invade behind the CO2. Capillary trapping can immobilize a significant fraction of the injected CO2 on time scales of a few injection periods in horizontal formations.12 Those periods are likely to be decades in full scale projects. Water injection after CO2 injection can speed trapping further,12 a process that is similar to walter-alternating-gas or WAG injection in enhanced oil recovery by gas injection. In formations that are not horizontal, even a few degrees of inclination can also aid trapping.12,14
CO2 is relatively soluble in brine, and hence, the injected CO2 will dissolve over time. At typical subsurface conditions, about 20 volumes of brine will be required to dissolve a given volume of CO2. In most storage projects, the CO2 will displace a small enough fraction of the brine that sufficient brine will remain to dissolve a large fraction of the CO2. How long that will take depends on the setting. Consider, for example the distributions of mobile and trapped CO2 in Fig. 5. Brine that is in contact with CO2 will be saturated quickly. Surprisingly, brine saturated with CO2 is slightly more dense than brine alone. As a result, CO2 dissolving in the neighborhood of the gravity tongue will cause more-dense saturated brine to lie above less-dense brine, an unstable situation. Fingers of dense brine will then descend through uncontacted brine, speeding dissolution over the rate that would be observed if diffusion alone were responsible for transport of CO2 away from zones of high saturation.15,16Fig. 6 illustrates what will happen, according to high resolution, high order simulations, at least. Many fingers develop initially, but nonlinear interactions reduce the number and produce larger-scale plumes at later times. The length of time it takes for plumes to initiate, develop, and descend through the formation can be weeks to thousands of years, depending on permeability (with shorter times in high permeability formations), and the size (width) can be less than 1 m to greater than 100 m.16 Thus, dissolution removes the driving force for CO2 to migrate upwards toward the surface, though the time required is generally long enough that an effective seal is still required.
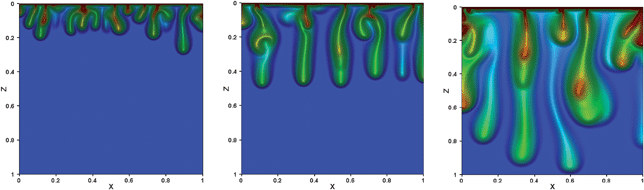 | ||
| Fig. 6 Simulation of the mixing of low-density CO2 with brine in a porous sedimentary formation.12 The CO2 saturates the brine at the top boundary (high concentrations of dissolved CO2 are indicated by the red color), forming a mixture that is slightly more dense than brine alone. The resulting gravitational instability leads to downward convection as fingers or plumes. | ||
The effect of CO2 injection on pressure in the subsurface formation and displacement of brine must also be considered. Some deep formations that contain brine are connected over very long distances. In those settings, the overall compressibility of the rock/brine system, even though it is small, is sufficient to accommodate injection of large quantities of CO2 with small overall pressure increases, and the amount of lateral displacement of brine is small compared to the scale of the formation. In smaller, confined formations, however, total CO2 injection will be limited to insure that pressure in the formation does not rise to the point of fracturing the formation and caprock or activating pathways for flow along faults.17,18 Assessment of the impact of injection on formation pressure and geomechanics is part of the evaluation of a potential storage site.
Geochemical reactions can also act to immobilize dissolved CO2 in the form of solid minerals. When CO2 dissolves it lowers pH and forms both carbonate (CO32−) and bicarbonate (HCO3−) ions, which can react to dissolve CaCO3 or silicate minerals. Dissolution of silicate minerals can raise pH enough to precipitate Ca, Fe, or Mg carbonates, among other minerals, depending on the details of brine composition and the chemical composition of the sedimentary rocks and cements.11 These reactions can occur as dissolved CO2 encounters and reacts with components in the brine and rock in the subsurface19 or they can occur as a result of mixtures created at the surface by mining silicate minerals that are then contacted by water containing dissolved CO2.20 These reactions are relatively slow, and hence any injected CO2 must be contained in the subsurface during the time required for the reaction to occur. A version of mineralization has been considered for basalts,11 which are widely distributed on the Earth and contain more of the reactive Ca, Mg, and Fe bearing minerals than typical sedimentary rocks do. Basalt reaction rates observed in laboratory experiments11 were significantly faster than those envisioned for saline aquifers19 (hundreds to thousands of years).
CO2 can also be immobilized by adsorption on surfaces of organic materials such as coal or shales that are rich in organic material. Fig. 7 compares amounts of CO2, CH4, and N2 that adsorb on a crushed Powder River Basin coal, for example.21 N2 adsorbs weakly, CH4 does so more strongly, and about three times as much CO2 adsorbs as does CH4. Adsorbed CH4 in coals is the source of coal bed methane that now accounts for about 10% of US natural gas production. Because CO2 adsorbs more strongly than CH4, it can replace the CH4, and hence it is possible to recover some CH4 and store CO2 simultaneously. In fact, Seto et al.22 showed that a coal bed can also be used to separate CO2 from N2 (as in a flue gas). The CO2 adsorbs and is retarded, and the N2 flows ahead, recovering CH4 as adsorbed CH4 equilibrates with the flowing N2 with low partial pressure of CH4. The price of that separation, however, is the requirement that large amounts of flue gas be compressed for injection, and because N2 propagates quickly through the coal, CH4 recovered after injected gas reaches production wells would have to be separated from the N2. When large quantities of CO2 adsorb, however, the permeability of the coal decreases. That permeability is mainly associated with natural fractures present in the coal, and as the coal takes up CO2, which still occupies some volume when it is adsorbed, fracture permeability declines. Permeability reductions limit the rate at which CO2 can be injected, a potential difficulty that could limit wide application of storage in coal. Low rates of injection could be increased by drilling more wells, by using horizontal wells, or by fracturing the coal, though that approach would have to be undertaken carefully to avoid creating pathways for leakage. Whether similar issues will arise for storage in shales has not yet been investigated in detail, but shales often have quite low permeability, which will likely limit injection rates.
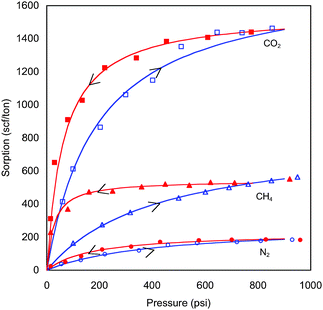 | ||
| Fig. 7 Adsorption of gases on a powdered coal sample (Jessen et al.21). Significantly more CO2 adsorbs than does CH4 or N2 at a given pressure. Hysteresis is also observed. As the pressure increases, the gases adsorb, but upon reduction in pressure more gas remains adsorbed than would adsorb at the same pressure during pressure increases. | ||
The storage mechanisms described above act on a range of time scales. Seals work immediately to limit vertical migration of CO2. Pressure in the subsurface will rise as injection proceeds. How much and how rapidly depends on the overall size and compressibility of the fluids and rock formation. The maximum pressure rise will have to be limited to pressures below which the caprocks do not fracture or faults activate. Dissolution happens quickly where CO2 encounters brine, but the time required to dissolve all of the CO2 is much longer, typically hundreds to thousands of years. Pressure will decline after injection ceases, as gravity redistributes CO2 in the formation and as CO2 dissolves or adsorbs (it occupies less volume in those states). Capillary trapping takes place wherever water invades a zone saturated with CO2. That typically begins at the end of the injection period, and several times the injection time period of 20–30 years will likely be required for most capillary trapping to occur, though it may be possible to reduce this time by injecting water during or after CO2 injection.12Mineralization reactions also act on a range of time scales, but in sedimentary rock systems they are likely to require much longer times than the other mechanisms. Over time, these storage mechanisms reduce the driving forces (buoyancy and pressure) for leakage and the mobility of CO2, and hence storage security should increase with time.4
Thus, several physical mechanisms, acting on a range of time scales, are available to retain CO2 in the subsurface. With careful attention to the combination of physical mechanisms of storage and specific site considerations, it should be possible to store CO2 essentially permanently (on human time scales, at least).
Monitoring of subsurface CO2 injection
Monitoring will be an essential feature of any CO2storage project, just as it is a normal part of operations of a gas injection project for oil recovery. Wells are the most likely leak path for any project,23 though well problems are frequently dealt with by what are known as workovers in the oil industry. Hence measurement of injection rates and injection well pressures will be a routine part of operations. The location of CO2 in the region near a well bore can be detected by logging methods used in the oil industry.4 Those methods can be used to determine whether the injected CO2 is entering and remaining in the target formation near the injection well.A variety of other subsurface monitoring techniques have potential to determine the location of injected CO2 away from the injection well. Seismic methods, which include time-lapse reflection24 or tomographic imaging25 can be used to detect subsurface migration and leaks. Fig. 8 shows an example of time-lapse reflection imaging of the subsurface flow in a CO2 injection project at the Sleipner Field in the North Sea, where CO2 separated from natural gas is injected into a sandstone formation that lies above the formation from which the natural gas is produced. The difference in density and compressibility of the supercritical CO2 phase from that of the resident brine causes reflections of seismic waves that allow detection of the location of the CO2 plume. The sequence of seismic images of the vertical cross section in the upper series and the areal views in the lower series confirm that the injected CO2 has stayed in the intended formation.24,26
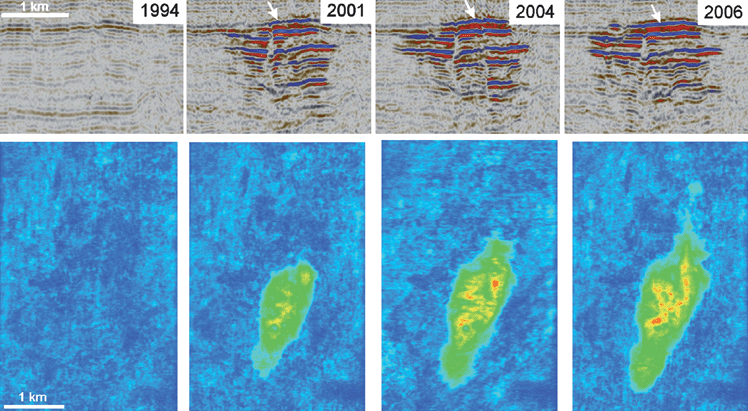 | ||
| Fig. 8 Time-lapse seismic images of the injected CO2 plume at the Sleipner field.22 The upper series shows a cross-sectional image, and the lower series shows an areal view. The 1994 image was taken before the start of CO2 injection. Injection of about 1 Mt CO2 yr−1 began in 1996. Subsequent images show the evolution of the CO2 plume as the CO2 flows through the sandstone formation under the caprock and intermediate shale layers within the sandstone. | ||
Gravity measurements and deformation methods such as synthetic aperture radar or tiltmeter measurements also have potential,4 though they will provide lower resolution indications of fluid movement. Many other methods are also available: injection and sampling of tracers, fluid composition measurements, electromagnetic methods that detect changes in conductivity of fluids, and soil gas and eddy covariance methods that detect CO2 at the surface. For a detailed review and many references see the comprehensive IPCC Special Report on CO2 Capture and Storage.4
More monitoring efforts will be needed early on in the life of a project than will be required later, but appropriate methods will need to be established for the various stages of a project. During the injection period, safe operations will require monitoring for leaks from pipelines, surface facilities, and wells.23 At low concentrations, CO2 is not dangerous. It is a normal constituent of air, and large power plants currently emit millions of tonnes per year directly into the atmosphere. At high concentrations, however, it is an asphyxiant and is toxic. A concentration of 4% CO2 is immediately dangerous to health; the NIOSH and OSHA exposure limits are 5000 ppm (0.5%).27 Because CO2 is more dense than air, making sure that leaking CO2 does not collect in low-lying areas or depressions is essential. After CO2 injection ceases, the potential for leakage declines over time, and hence, the need for monitoring movement of CO2 in the subsurface will also decline over time. The range of monitoring techniques available indicates that a monitoring protocol can be developed to permit safe operation of a project and to detect problems if they occur.
Field experience
Experience with large scale CO2 injection is accumulating around the world. Part of the experience comes from gas injection projects for enhanced oil recovery. For example, CO2 has been injected for about 35 years for the purpose of enhanced oil recovery in west Texas, though most of the CO2 injected is from natural sources of CO2 rather than anthropogenic CO2. Those projects make use of an infrastructure of 5600 km of pipelines23 that deliver CO2 from natural occurrences in New Mexico and Colorado and natural gas separation facilities to 70 oil fields. About 30 Mt CO2 is injected per year in these projects, several of which inject CO2 in quantities that are comparable to or larger than the emissions of a large coal-fired power plant (500 MW).4 The experience gained in transporting and injecting large quantities of CO2 gives reasonable assurance that with careful site selection,4 project design, and operation,21 CO2 can be transported over significant distances and injected into formations that can be expected to retain the CO2 indefinitely.Projects that inject CO2 that would have been emitted to the atmosphere and that are monitored are shown in Fig. 9. The projects shown are at various stages of planning and execution, and some are commercial-scale tests while others are small pilot projects. More than 10 Mt CO2 have been injected (at about 1 Mt CO2yr−1) through a single well into the very large and quite permeable Utsira Formation at Sleipner in the North Sea.4,24,26 At In Salah in Algeria, CO2 separated from natural gas is injected at a rate of about 1 Mt CO2 yr−1 through three long horizontal wells into a much lower permeability zone at the base of the gas reservoir.29 A combined CO2storage and enhanced oil recovery project is being conducted at the Weyburn Field in Saskatchewan, Canada.30 For that project, CO2 containing a small amount of H2S (0.8–2%) is separated at a coal gasification facility in North Dakota and transported by pipeline 320 km to the oil field. The presence of H2S, a very hazardous substance, imposes special safety considerations.23 That project is injecting about 1.8 Mt CO2 yr1, a little over half the rate of CO2 emissions from a 500 MW coal-fired power plant (about 3 Mt CO2yr−1).
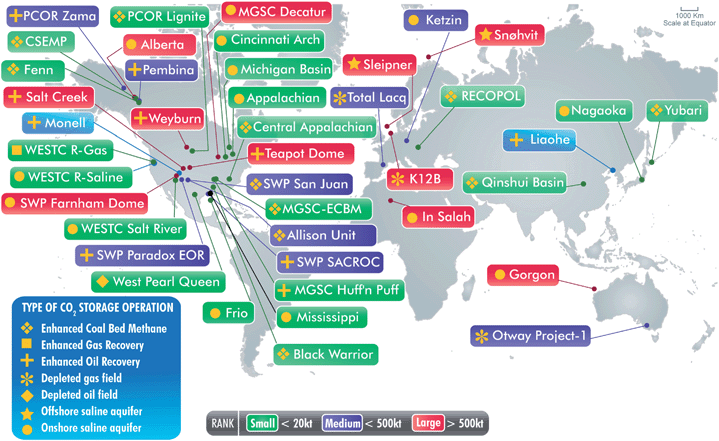 | ||
| Fig. 9 Active CO2storage projects worldwide.26 The largest projects currently injecting CO2 are SACROC in west Texas, Sleipner in the North Sea, In Salah in Algeria, and Weyburn in Canada. Image courtesy of CO2CRC, Australia. | ||
Thus, the large enhanced oil recovery and CO2storage projects have provided significant operating experience that can be applied to guide large-scale CO2storage. What has not yet been done is to demonstrate combined CO2 capture at the scale of a large coal-fired power plant with storage in a variety of geological settings. That will be an important next step if CCS is to be implemented at a scale large enough to have an impact on CO2 emissions. It is worth remembering that large scale is very large indeed. For densities of 500–700 kg/m3, which are typical of pressures and temperatures in the subsurface, 1 GtCO2/yr is equivalent in volume to 25–35 million barrels per day of injection, roughly 50% bigger than current petroleum use in the US. Hence, it will take 330–1000 projects of the 1–3 Mt CO2/yr scale to eliminate about half of the current US emissions of CO2 from electric power generation. It is equally clear that other ways to reduce emissions will also be needed.
Conclusions
This paper has examined whether enough is known about carbon capture and storage (CCS) that implementation can be tested at large scale:Can CO2 be captured efficiently and at an acceptable cost? Technologies to capture CO2 are used widely at commercial scale. They are still relatively expensive in terms of energy costs and therefore in dollar costs for application to power plants. Integration of capture and storage at the scale of a large power plant (500 MW or more) has not yet been demonstrated.
Is there sufficient variety of geologic settings and sufficient volume to store enough CO2 to have an impact on emissions? Estimates of total volumes of potentially suitable pore space indicate that there is sufficient volume available to permit significant impact on emissions. The combination of potential storage in oil and gas reservoirs, deep saline formations, coal beds, basalts, and shales provides potential storage locations within reasonable distance of many large sources of CO2.
Are there sufficient physical mechanisms that will trap the CO2 in the subsurface to allow design of safe storage projects that will not leak?Retention by seal rocks, dissolution, adsorption, capillary trapping, and mineralization reactions are among the mechanisms that can be used to retain CO2 in the subsurface indefinitely. The fact that natural systems store large quantities of buoyant fluids (oil, natural gas, and even CO2) provides confidence that long-term storage is possible.
Can the movement of fluids in the subsurface be monitored and leaks be detected and if necessary, remediated? A wide range of monitoring techniques is available, with differing sensitivities and resolutions. Surface monitoring can detect hazards associated with surface leakage of CO2 and subsurface methods (well logs, pressure measurements, and seismic surveys, etc.) can determine where CO2 migration is taking place. Technology available today is sufficient to allow safe operation of well-sited, well-engineered, and carefully operated subsurface storage projects.
Do we have enough experience with actual operations to undertake storage at a scale large enough to have an impact on emissions? There is sufficient experience with commercial-scale separation and CO2 injection in enhanced oil recovery projects and in specifically designed storage projects to warrant moving to the stage of demonstrating CCS at the scale of large coal-fired power plants.
Progress to date suggests, therefore, that while there is more to be learned about operating at scale and reducing costs, enough is known now about CCS to suggest that it can contribute significantly to reductions in CO2 emissions, even as many other approaches are applied over the same time period.
Acknowledgements
The support of the Global Climate and Energy Project at Stanford University is acknowledged with gratitude.References
- IPCC Climate Change 2007, Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team ed. R. K. Pachauri and A. Reisinger] Intergovernmental Panel on Climate Change, Geneva, Switzerland, 2007.
- J. Deutch and E. J. Moniz, 2007, The Future of Coal: Options for a Carbon-Constrained Word, Massachusetts Institute of Technology Search PubMed.
- EIA Emissions of Greenhouse Gases Report, Energy Information Administration, U.S. Dept. of Energy, Washington, DC , 2008. Seehttp://www.eia.doe.gov/oiaf/1605/ggrpt/carbon.html%23total.
- IPCC, Special Report on Carbon Capture and Storage, 2005, Prepared byWorking Group III of the Intergovernmental Panel on Climate Change, Geneva, Switzerland Search PubMed.
- National Energy Technology Laboratory, 2007, CO2 Sequestration Atlas, U.S. Department of Energy, Washington, DC Search PubMed.
- H. Vidas, R. Hugman, and C. Christa Clapp, Analysis of Geologic Sequestration Costs for the United States and Implications for Climate Change Mitigation, presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- J. J. Dooley, R. T. Dahowski, C. L. Davidson, M. A. Wise, N. Gupta, S. H. Kim and E. L. Malone, 2006, Carbon Dioxide Capture and Storage, Technology Report, Global Energy Technology Strategy Program, Battelle Memorial Institute Search PubMed.
- K. Z. House, D. P. Schrag, C. F. Harvey and K. S. Lackner, Permanent carbon dioxide storage in deep sea sediments, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1291–1295.
- P. G. Brewer, G. Friederich, E. T. Peltzer and F. M. Orr, Jr., Direct Experiments on the Ocean Disposal of Fossil Fuel CO2, Science, 1999, 284, 943–945 CrossRef CAS.
- K. Z. House and D. P. Schrag, The immobility of CO2 in deep sea sediments, presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- B. P. McGrail and et al., Potential for carbon dioxide sequestration in flood basalts, J. Geophys. Res.-Solid Earth, 2006, 111 Search PubMed p. ARTN B12201.
- T. Ide, K. Jessen and F. M. Orr, Jr., Storage of CO2 in saline aquifers: Effects of gravity, viscous,and capillary forces on amount and timing of trapping, Intl. J. Greenhouse Gas Control, 2007, 1, 481–491 Search PubMed.
- J. G. Roof, Snap-Off of Oil Droplets in Water-Wet Pores, Soc. Pet. Eng. J., 1970, 85–90 Search PubMed.
- M. A. Hesse, F. M. Orr, Jr and Tchelepi, Gravity Currents with Residual Trapping, J. Fluid Mech., 2008, 611, 35–60 Search PubMed.
- J. Ennis-King and L. Paterson, 2003, Role of convective mixing in the long-term storage of carbon dioxide in deep saline formations, SPE 84344 pp. 1–12 Search PubMed.
- A. Riaz, M. Hesse, H. Tchelepi and F. M. Orr, Jr., Onset of Convection in a Gravitationally Unstable, Diffusive Boundary Layer in Porous Media, J. Fluid Mech., 2006, 548, 87–111 Search PubMed.
- L. G. H. van der Meer and F. Yavuz, CO2 storage calculations for the Dutch subsurface, presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- L. Chiaramonte, M. D. Zoback, J. Friedmann and V. Stamp, Seal integrity and feasibility of CO2 sequestration in the Teapot Dome EOR pilot: Geomechanical site characterization, Environ. Geol., 2008, 54, 1667–1675 CrossRef CAS.
- J. W. Johnson, J. K. Nitao and K. G. Knauss, 2004, Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms, and sequestration partitioning, in Geological Storage of Carbon Dioxide, ed. S. J. Baines and R. H. Worden, Geol. Soc. Spec. Publ., 233, 107–128 Search PubMed.
- K. S. Lackner, D. P. Butt and C. H. Wendt, Progress on binding CO2 in mineral substrates, Energy Conver. Manage., 1997, 38, S259–S264 Search PubMed.
- K. Jessen, G.-Q. Tang and A. R. Kovscek, Laboratory and Simulation Investigation of Enhanced Coalbed Methane Recovery by Gas Injection, Transport in Porous Media, 2008, 73, 141–159, DOI:10.1007/s11242-007-9165-9.
- C. J. Seto, K. Jessen, and F. M. Orr, Jr., A Multicomponent, Two-Phase Flow Model for CO2 Storage and Enhanced Coal Bed Methane Recovery, SPE 102376, SPE Annual Technical Conference, San Antonio, September 24–27, 2006, in press, Soc. Pet. Eng. J., Sept. 2008. http://www.spe.org/elibrary/servlet/spepreview?id=SPE-102376-MS Search PubMed.
- I. J. Duncan, J.-P. Nicot, and J.-W. Choi, Risk Assessment for future CO2 Sequestration Projects Based CO2 Enhanced Oil Recovery in the U.S., presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- R. A. Chadwick, D. Noy, R. Arts, O. Eiken, Latest time-lapse seismic data from Sleipner yield new insights into CO2 plume development, presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- T. Daley, L. Myer, J. Peterson, E. Majer and G. Hoversten, Time-lapse crosswell seismic and VSP monitoring of injected CO2 in a brine aquifer, Environ. Geol., 2008, 54, 1657–1665 CrossRef CAS.
- C. Hermanrud, Storage of CO2 in saline aquifers – lessons learned from 10 years of injection into the Utsira Formation in the Sleipner area, presented at the Ninth Greenhouse Gas Technology Conference, Washington, D.C, Nov. 16–20, 2008 Search PubMed.
- National Institute of Occupational Safety and Health , Documentation for Immediately Dangerous to Life and Health Concentrations – Carbon Dioxide, U.S. Dept. Health, Education, and Welfare, Center for Disease Control and Prevention, Washington, DC. http://www.cdc.gov/niosh/idlh/124389.html , 1996. Accessed Mar. 10, 2009 Search PubMed.
- Cooperative Research Center for Greenhouse Gas Technologies, Image Library, Australia Dept. of Innovation, Industry, Science, and Research, Canberra. http://www.co2crc.com.au/imagelibrary/. Accessed Feb. 8, 2009.
- I. Wright, CO2 Storage at In Salah, presented at the 2nd International CCS Symposium, Paris, Oct. 4, 2007http://www.colloqueco2.com/presentations2007/ColloqueCO2-2007_Session2_3-Wright.pdf Search PubMed.
- M. Wilson and M. Monea, 2004, IEA GHG CO2 Weyburn Monitoring & Storage Project Summary Report 2000–2004, proceedings 7th Intl. Greenhouse Gas Technology Conference, Vancouver, Sept. 5–9, 2004, http://www.ptrc.ca/siteimages/Summary_Report_2000_2004.pdf. Accessed Dec. 12, 2008 Search PubMed.
- J. Bradshaw , personal communication.
| This journal is © The Royal Society of Chemistry 2009 |
