Exergetic evaluation and improvement of biomass-to-synthetic natural gas conversion
Martin
Juraščík
,
Ana
Sues
and
Krzysztof J.
Ptasinski
*
Laboratory of Environmental Technology, Chemical Engineering Department, Eindhoven University of Technology, Helix, STW 1.22, P.O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: K.J.Ptasinski@tue.nl; Fax: +31 40 244 6653; Tel: +31 40 247 3689
First published on 9th April 2009
Abstract
Synthetic natural gas (SNG) is suggested as an important future energy carrier. The conventional route for SNG production is based on gasification of biomass to synthetic gas and the subsequent methanation of synthetic gas to SNG. This study is aimed to analyze the process units using the concept of exergy. Exergy analysis is a promising method, based on the 2nd law of thermodynamics, to analyze and improve chemical processes. In this work a detailed exergy analysis is performed for the SNG process based on woody biomass gasification. The main elements of the system are gasifier, gas cleaning, synthetic gas compression, methanation and final SNG condition. The above-mentioned process was simulated with a computer model using the flow-sheeting program Aspen Plus. Optimal values of the process conditions, particularly for the methanation reactors, are found. The internal exergy losses of different system units are evaluated. The largest internal exergy losses take place in the gasifier, methanation section and CO2 capture unit. The highest overall exergetic efficiency of 72.6% was found applying the following operating conditions: gasifier 700 °C and 1 bar; 1st methanation reactor 580 °C and 2nd methanation reactor 405 °C.
Broader contextIn many countries, e.g. the Netherlands, the main energy source is natural gas. In order to solve the problems of depletion of fossil fuels and their destructive influence on the environment, synthetic natural gas (SNG) is suggested as an important future energy carrier. Sustainable SNG produced from biomass can provide an attractive option for renewable biofuels. The conventional route for SNG production is based on gasification of biomass to produce synthetic gas and the subsequent methanation of the synthetic gas to SNG. This study is aimed to analyze the biomass-to-SNG conversion using the concept of exergy. Exergy analysis is based on the 2nd law of thermodynamics to analyze and improve chemical processes. In this work a detailed exergy analysis is performed for the SNG process based on woody biomass gasification. The main elements of the system are gasifier, gas cleaning, synthetic gas compression, methanation and final SNG condition. The largest internal exergy losses take place in the gasifier, methanation section and CO2 capture unit. The highest overall exergetic efficiency of 72.6% was found for the following operating conditions: gasifier 700 °C and 1 bar; 1st methanation reactor 580 °C and 2nd methanation reactor 405 °C. |
Introduction
Most of the world energy consumption is supplied by non-renewable energy sources such as oil, coal and natural gas. On the contrary, biomass provides an attractive option for renewable biofuels. Production of biofuels includes numerous combinations of resources, conversion processes and end products. This work is focused on woody biomass-to-synthetic natural gas (SNG) conversion technology. As natural gas is the main energy source in many countries, production of SNG from biomass could be a promising option to substitute the fossil fuels. In literature some studies1–3 are found dealing with the biomass-to-SNG process; e.g. Duret et al.1 reported a thermal efficiency for the wood-to-SNG process of about 58% based on the lower heating value. However, all of these publications are focused on a specific biomass composition from the wide range of available biomass feedstock and most of the published papers express the efficiency of the biomass-to-SNG process by applying thermal efficiencies only. The purpose of this paper is to analyse the biomass-to-SNG process by the exergetic analysis.Basically SNG (methane-rich gas) may be produced via two technological routes; a biological or a thermochemical route. The thermochemical route, which is investigated in this work, is based on the gasification process. Biomass gasifiers typically produce a synthetic gas containing CO, H2, and CH4 as the main components that carry the majority of energy in addition to remaining components such as CO2, H2O and N2, and also a variety of potential contaminants like tars, ammonia, alkalis, etc. First of all, gas cleaning is needed. Secondly, the subsequent chemical processing of the synthetic gas requires specific gas conditions, such as a desired H2/CO ratio, temperature and pressure. Subsequently the gas enters the methanation step. The methanation is a catalytic reaction and there is a substantial risk of catalyst overheating or deactivation due to carbon formation. Finally, the product gas from the methanation is processed to meet the requirements for SNG.
The requirements for the final product are based on the use and distribution infrastructure of SNG. This study refers primarily to the Netherlands, in particular the north part of the country, where the Groningen Natural Gas fields are located. Hence the Groningen Natural Gas specification is taken as a target for the quality of the final SNG product.
Objectives
The objective of this paper is to evaluate and suggest ways to improve the exergetic efficiency of the biomass-to-SNG conversion process. In the investigated technology a woody stream was chosen as a feedstock to produce SNG, which has to meet the Groningen Natural Gas quality requirements. The main requirements for the produced SNG are: gross calorific value (HHV) 31.6–38.7 MJ Nm−3 and Wobbe index 43.4–44.4 MJ Nm−3.3The aim of this work is to study the influence of gasification pressure and methanation temperature on the efficiency of the biomass-to-SNG process.
Process description
A block diagram of a biomass gasification process integrated with methanation is presented in Fig. 1. In this paper woody matter is considered as a feedstock, with the composition listed in Table 1. | ||
| Fig. 1 A block diagram of a biomass gasification process integrated with methanation. | ||
A steam-blown direct gasifier is applied in this study to convert the solid biomass into the synthetic gas. The flow rate for all studied cases was kept constant at a value of 10 kg s−1 wet biomass. Steam at a temperature of 227 °C and at the pressure of the gasifier was used as a gasification agent. An external heat source was used to control the gasification temperature at 700 °C and the pressure range was changed from 1 to 15 bar with a step of 5 bar. The gasifier was operated at the carbon boundary line, which determines the optimal conditions for operating the biomass gasifier from the thermodynamic point of view.4 The flow rate of the gasification agent (steam) was adapted to keep the gasifier at the carbon boundary conditions, as indicated in Table 2.
| Gasification pressure/bar | 1 | 5 | 10 | 15 |
|---|---|---|---|---|
| a MR—methanation reactor. b These methanation reactors worked adiabatically. | ||||
| Flow rate of the gasification agent (steam)/kg s−1 | 3.45 | 5.35 | 6.85 | 6.05 |
| SNG produced per 10 kg s−1 of wet biomass | ||||
| Mass flow rate/kg s−1 | 3.54 | 3.54 | 3.63 | 3.65 |
| Molar flow rate/kmol s−1 | 215.0 | 214.7 | 208.8 | 206.8 |
| Vol. flow rate/Nm3 s−1 | 4.90 | 4.89 | 4.76 | 4.71 |
| Composition of SNG (mol %) | ||||
| H2 | 11.4 | 11.2 | 6.8 | 5.3 |
| CO | 0.5 | 0.5 | 0.2 | 0.1 |
| CO2 | 6.0 | 6.0 | 7.1 | 7.4 |
| CH4 | 79.8 | 79.9 | 83.6 | 84.8 |
| N2 | 2.3 | 2.3 | 2.4 | 2.4 |
| Quality parameters of SNG | ||||
| HHV/MJ Nm−3 | 33.2 | 33.2 | 34.1 | 34.4 |
| Wobbe index/MJ Nm−3 | 44.0 | 44.0 | 44.0 | 44.0 |
| CH4 synthesis temperature/°C | ||||
| 1st MR | 729 | 701b | 652b | 623b |
| 2nd MR | 590 | 578b | 520b | 488b |
| 3rd MR | 428 | 426b | 377b | 356b |
| Percentage of the total amount of CH4 produced in the following parts of the process (%) | ||||
| Gasifier | 13.1 | 39.5 | 52.5 | 59.6 |
| 1st MR | 45.1 | 25.9 | 21.7 | 19.2 |
| 2nd MR | 28.4 | 22.7 | 18.2 | 15.6 |
| 3rd MR | 13.4 | 11.9 | 7.6 | 5.6 |
After gasification the synthetic gas was cooled and eventual condensate was removed at 1 bar. A heat exchanger was applied in this synthetic gas cooling section to produce steam (150 °C, 1 bar). Subsequently, the synthetic gas was pressurized to 28.5 bar using a three-stage compressor with an intercooling heat exchanger. The compressed synthetic gas was heated to a temperature of 398 °C and then passed through a gas cleaning section. It was decided to use dry high-temperature adsorption methods for gas cleaning since the synthetic gas from woody biomass gasification contains low amounts of chlorine and sulfur components.5 However, this section was not simulated in detail; to calculate the exergy losses a pressure drop of 0.5 bar was assumed.
Subsequently, the synthetic gas entered the methane synthesis section at a pressure of 28 bar. The methane synthesis is a catalytic exothermal process. In this study a nickel based catalyst was applied. It was assumed that the catalyst has a water–gas-shift activity. In the methanation reactors CO, CO2 and H2 are converted into CH4via reversible reactions:
| CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) ΔH = −206 kJ mol−1 | (1) |
| CO2(g) + 4H2(g) ↔ CH4(g) + 2H2O(g) ΔH = −165 kJ mol−1 | (2) |
In the reactor, the catalyzed water–gas-shift reaction is active:
| CO(g) + H2O(g) ↔ H2(g) + CO2(g) ΔH = −41 kJ mol−1 | (3) |
Besides the above mentioned reaction, carbon formation may also occur:
| 2CO(g) ↔ CO2(g) + C(s) ΔH = −172 kJ mol−1 | (4) |
| CO(g) + H2(g) ↔ C(s) + H2O(g) ΔH = −131 kJ mol−1 | (5) |
To simulate the methane synthesis the steam-moderated ICI high-temperature once-through methanation process was chosen.6 A detailed scheme of the methanation section is shown in Fig. 2. This section consists of three methanation rectors and two heat exchangers placed between the methanation rectors in order to control the temperature of gas entering the 2nd and 3rd methanation reactor. The inlet gas temperatures of the methanation reactors were kept constant according to the ICI process (1st 398 °C, 2nd 325 °C and 3rd 300 °C). Steam (398 °C, 28 bar) was added to the methanation section to avoid carbon formation. Since a low temperature has a positive influence on the methanation process and there is a risk of overheating of the catalyst, the temperature in methanation reactors was controlled in order not to exceed the desired temperatures (729 °C for the 1st reactor, 590 °C for the 2nd reactor, 425 °C for the 3rd reactor). Hence, according to the synthetic gas composition (influenced by gasification conditions) methanation reactors were operated adiabatically or cooled. This is discussed later in the results section of this paper. As it is indicated in Fig. 2 steam (212.5 °C, 20 bar) was produced in two heat exchangers placed between the methanation rectors. The produced steam can be considered as an additional product of the biomass-to-SNG process.
 | ||
| Fig. 2 A detailed scheme of the methanation section according to the steam-moderated ICI high-temperature once-through methanation process. The indicated temperatures of streams entering and leaving the methanation reactors (MR) are the original temperatures of the ICI technology.6 | ||
In addition to investigation of the influence of gasification pressure on the efficiency of the biomass-to-SNG process, methane synthesis temperature in the 1st and 2nd methanation reactors was also decreased for fixed gasification conditions (gasification temperature at 700 °C and pressure at 1 bar).
After the methanation section the raw product gas (raw SNG) was cooled in a heat exchanger to produce a steam (150 °C, 1 bar). The temperature of cooled raw SNG was set to 40 °C. After cooling of the raw SNG condensed liquid was separated from the gas at a pressure of 28 bar.
The cooled raw SNG was compressed to a pressure of 40 bar and subsequently entered a CO2 removal section. Also the condensed liquid, which contains dissolved CH4, entered the CO2 removal section where the dissolved gas was released. A physical absorption method based on dimethylether of polyethylene-glycol solvent (commercially called SELEXOL method) was chosen as a CO2 removal technology. A detailed description of the physical absorption CO2 removal section can be found in the work of Lampert and Ziebik,7 whose concept was adopted for this work. The CO2 removal absorption column worked at the temperature of 30 °C and the pressure of 40 bar. A CO2 rich gas stream leaving the CO2 removal section was considered in this study as waste. Finally, the produced SNG was compressed to 66 bar and cooled to 25 °C.
Methods
The biomass-to-SNG process was simulated with a computer model using the flow-sheeting program Aspen Plus. Process simulations were carried out for various gasification pressures and methane synthesis temperatures. The equilibrium (Gibbs) model was used to simulate the gasifier and methane synthesis.The mass and energy balances obtained in Aspen Plus formed the basis for exergy calculations in a separate spreadsheet. The exergy method was applied recently to analyze many chemical and energy processes based on the 1st and 2nd law of thermodynamic. The aim of applying exergy analysis is to identify units in a system with the largest exergy losses. The exergy balance of a process can be represented in the following form using exergy values of all streams entering and leaving the process:
 | (6) |
 and
and  are exergy flow of all entering and leaving material streams, respectively, EQ and EW are the sums of all thermal exergy and work interactions involved in a process. The difference between the concept of exergy and those of mass and energy is that exergy is not conserved but subjected to dissipation. It means that the exergy leaving any process step will always be less than the exergy in. The difference between all entering exergy streams and that of leaving streams is called irreversibility I. Irreversibility represents the exergy destruction in the process by irreversible effects as the loss of quality of materials and energy due to dissipation. Irreversibility is often called the internal exergy loss. In addition to internal exergy losses also external exergy losses can find place in the processes which correspond to a waste heat of waste product stream and are rejected into the environment.
are exergy flow of all entering and leaving material streams, respectively, EQ and EW are the sums of all thermal exergy and work interactions involved in a process. The difference between the concept of exergy and those of mass and energy is that exergy is not conserved but subjected to dissipation. It means that the exergy leaving any process step will always be less than the exergy in. The difference between all entering exergy streams and that of leaving streams is called irreversibility I. Irreversibility represents the exergy destruction in the process by irreversible effects as the loss of quality of materials and energy due to dissipation. Irreversibility is often called the internal exergy loss. In addition to internal exergy losses also external exergy losses can find place in the processes which correspond to a waste heat of waste product stream and are rejected into the environment.
Exergy of streams (biomass, gases, liquid, heat and work) were calculated using the concept by Szargut et al.8 For each section in the process an exergy balance was made and an internal exergy loss (process irreversibility) was calculated. The exergetic efficiency is defined as the ratio between useful exergy output from the process to the necessary exergy input to this process. In this study three definitions of rational exergetic efficiencies were applied as follows:
 | (7) |
 | (8) |
 | (9) |
 is the sum of exergy flow rate of product steam streams,
is the sum of exergy flow rate of product steam streams,  is the sum of all product thermal exergy of a process,
is the sum of all product thermal exergy of a process, 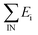 is the exergy flow rate of all entering material streams,
is the exergy flow rate of all entering material streams,  and
and  are the sums of all thermal exergy and work entering a process, respectively.
are the sums of all thermal exergy and work entering a process, respectively.
Hence, efficiency ψ1 expressed by eqn (7) considers only produced SNG as the only product of the biomass-to-SNG technology. Moreover, if produced steam within the process (cooling synthetic gas and raw SNG, and in the methanation section) is considered as the additional product of the biomass-to-SNG process then the efficiency of the process ψ2 is expressed by eqn (8). Finally, in this paper the overall exergetic efficiency ψ3 is given by eqn (9), where heat removed from the methanation reactors was treated also as an additional product to the remaining main products (SNG and steam) of the process. Generally, the efficiency ψ3 can be considered as the real ‘exergy efficiency’; however it refers only to process cases when high temperature heat is produced in cooled methanation reactors. In the case when methanation reactors are not cooled and work as adiabatic reactors the efficiency ψ2 can be considered as the real ‘exergy efficiency’.
Results and discussion
Influence of gasification condition
Fig. 3 shows a comparison of mass and volumetric SNG product rates, SNG composition, efficiencies of the process and internal exergy losses of the technological units for the gasification temperature of 700 °C and four different gasification pressures in the range from 1 to 15 bar based on the 10 kg s−1 wet biomass input. Moreover, Table 2 shows the main characteristics of the biomass-to-SNG process for the same gasification operating condition. Table 3 summarizes exergy flow rates of the main streams of the biomass-to-SNG process for the gasification temperature 700 °C and gasification pressure 1 bar.| No. | Stream | Exergy flow rate/MW |
|---|---|---|
| 1 | Biomass feed | 178.5 |
| 2 | Steam (gasifying agent) | 2.13 |
| 3 | Gasifier heat | 32.42 |
| 4 | Steam produced in gas cooler | 3.91 |
| 5 | Syngas compressor work | 11.84 |
| 6 | Syngas heat | 2.35 |
| 7 | Steam for methanation section | 4.02 |
| 8 | Steam from methanation reactors | 9.46 |
| 9 | Heat from methanation reactors | 7.25 |
| 10 | Steam from gas cooling | 6.35 |
| 11 | Compression work raw SNG | 0.41 |
| 12 | Work for CO2 removal | 10.83 |
| 13 | Work SNG compressor | 0.40 |
| 14 | SNG product | 150.8 |
 | ||
| Fig. 3 Influence of gasification pressure on the performance of the biomass-to-SNG process; (a) product flow rates, (b) exergetic efficiencies of the process, (c) SNG composition, (d) internal exergy losses of the technological units of the process. | ||
Fig. 3 shows the opposite influence of gasification pressure on SNG production in terms of product mass and volumetric flow rate. Increasing gasification pressure has a negative influence on SNG volumetric flow rate. On the contrary, increasing gasification pressure from 1 to 15 bar has a positive effect on the SNG mass flow rate. The difference in these tendencies is caused by changing composition of the final SNG for different gasification pressures. However, the exergy flow rate of the final SNG product is about 151 MW and is not influenced by the pressure change in the gasifier.
Fig. 3 also shows a comparison of the exergetic efficiencies ψ1, ψ2 and ψ3 according to eqn (7–9) of the biomass-to-SNG process for different gasification pressures. Gasification pressure has a positive influence on the exergetic efficiency to SNG produced in the process. The exergetic efficiency ψ1 (see eqn (7), Fig. 3b), considering only SNG as the process product, ranges between 60 to 63%. If the produced steam is considered as the additional product, then the efficiency of the process ψ2 (eqn 8, Fig. 3b) increases by adding on average about 8%. Moreover, if the heat released from cooled methanation reactors is considered also as a product then the efficiency of the process ψ3 (eqn (9), Fig. 3b) increases. Obviously, this increase occurs only when the methanation reactors are cooled (operate non-adiabatically, at the gasification pressure of 1 bar). In Table 2 it is indicated when the methanation reactors operated adiabatically or were cooled.
As it can be seen from a comparison of SNG volumetric productivity (see Fig. 3a) and exergy efficiencies of the biomass-to-SNG process (see Fig. 3b), they have opposite relation to gasification pressure. To understand these effects, it is necessary to identify exergy of all input and output streams. As it was mentioned above, the exergy flow rate of the final SNG product has approximately the same value (151 MW) for all gasification pressures used. On the other hand, exergy input of the biomass-to-SNG process decreases with pressure increasing in gasifier. Hence, ψ1 (Fig. 3b) increases with gasification pressure according to eqn (7).
Internal exergy losses (I) of the technological units for the biomass-to-SNG process, for the gasifier operating at the temperature of 700 °C and at four different gasification pressures (1, 5, 10 and 15 bar, respectively) and based on the wet biomass flow rate of 10 kg s−1 are shown in Fig. 3d. As it can be seen the largest process irreversibilities take place in the gasifier, methanation section and CO2 capture unit. On the other hand, process irreversibility of gas cleaning, product gas compression and final SNG compression step (0.06, 0.14 and 0.12 MW, respectively) are rather small.
After the methanation section, about 92% of CO2 present in the raw SNG (gas stream leaving methanation section) has to be removed to reach the quality requirements for the SNG. The final composition as well as the gross calorific value (HHV) and the Wobbe index of the produced SNG are listed in Table 2 and reach the specification requirements for the final SNG product.
All three methanation reactors were cooled when the gasifier was operated at the lowest applied pressure (1 bar). When applying the higher gasification pressures (5, 10 and 15 bar, respectively)—the methanation reactors operate adiabatically. The temperatures of the rectors are indicated in Table 2. The explanation of this fact could be that at higher gasification pressures a significant amount of CH4 was already produced in the gasifier, resulting in less production of CH4 by the exothermic methane synthesis in the methanation reactors. Hence, the reactors may work adiabatically without overheating.
Influence of methanation reactor conditions
To improve the efficiency of the process an effort should be paid to maximize the products in terms not only of their quantities but also in terms of quality by using minimal possible input to the system. One of the ways to improve the exergetic efficiency of the process is to minimize the internal exergy losses (irreversibilities) of the whole system or separate units by applying technologically suitable operating conditions. As it was mentioned earlier the majority of the total internal exergy loss of the biomass-to-SNG process is represented by the irreversibilities of gasifier, methanation section and CO2 removal section. Since the effect of various gasification conditions on the efficiency was presented in more details previously9 the present paper pays attention to the methanation reactors conditions in terms of the temperature of methane synthesis.In order to study the influence of the methanation condition, the effect of temperature in the 1st and 2nd methanation reactors was investigated. An overview of the temperature range used in this paper for the methanation reactors is given in Fig. 4. In this part of the study gasification conditions were fixed at 700 °C and 1 bar. These gasification parameters were chosen due to the fact that at these conditions the irreversibility in gasifier is the largest from all above observed. However, gasifier conditions also influence the irreversibilities in subsequent units (SynG cooling, SynG flash, SynG compression, SynG cleaning-up) and the sum of internal exergy losses of these units is the smallest one for gasification conditions of 700 °C and 1 bar from all gasification conditions studied. On the contrary, applying these gasification conditions, the highest internal exergy loss was found also for the methane synthesis section. Therefore, the values of ψ1 and ψ2 are the lowest for these gasification conditions. Moreover, at the above-mentioned gasification conditions the methanation reactors operate at the highest temperatures (see Table 2), what leads to relatively low conversion to CH4, as this process is exothermic (eqn (1–2)). Lowering temperature in methanation reactors will lead to increased conversion and possibly to improved efficiency of the whole process.
 | ||
| Fig. 4 An overview of the temperature range in methanation reactors (MR) used in this paper; (a) the temperature range of the 1st–3rd MR when studying the influence of the 1st MR, (b) the temperature range of the 2nd–3rd MR when studying the influence of the 2nd MR for the temperature of the 1st MR fixed at 729 °C and (c) the temperature range of the 2nd–3rd MR when studying the influence of the 2nd MR for temperature of the 1st MR fixed at 580 °C. | ||
Influence of the temperature in the 1st methanation reactor
The studied temperature range for the 1st methanation reactor was 580 to 729 °C, see Fig. 4a. The 1st reactor was cooled (heat was removed) to reach the desired temperature and the 2nd and the 3rd reactors operated adiabatically or were cooled to keep the reactors bellow the original ICI process temperatures.6 The effect of temperature in the 1st methanation reactor on the exergetic efficiency of the whole plant was studied for fixed gasification conditions that is gasification temperature 700 °C and pressure 1 bar. Table 4 shows whenever the reactors operated adiabatically or were cooled together with the operating temperatures of the reactors.| CH4 synthesis temperature/°C | ||||||
|---|---|---|---|---|---|---|
| a These methanation reactors worked adiabatically. | ||||||
| 1st MR | 729 | 700 | 670 | 640 | 610 | 580 |
| 2nd MR | 590 | 577a | 541a | 507a | 474a | 445a |
| 3rd MR | 428 | 425a | 393a | 368a | 348a | 335a |
| SNG produced per 10 kg s−1 of wet biomass | ||||||
| Mass flow rate/kg s−1 | 3.54 | 3.55 | 3.60 | 3.64 | 3.66 | 3.67 |
| Molar flow rate/kmol s−1 | 215 | 215 | 211 | 208 | 206 | 205 |
| Vol. flow rate/Nm3 s−1 | 4.82 | 4.81 | 4.72 | 4.66 | 4.63 | 4.60 |
| Composition of SNG (mol %) | ||||||
| H2 | 11.4 | 11.1 | 8.1 | 6.1 | 4.9 | 4.1 |
| CO | 0.5 | 0.5 | 0.2 | 0.1 | 0.1 | 0.1 |
| CO2 | 6.0 | 6.1 | 6.8 | 7.2 | 7.5 | 7.6 |
| CH4 | 79.8 | 80.0 | 82.5 | 84.2 | 85.2 | 85.8 |
| N2 | 2.3 | 2.3 | 2.4 | 2.4 | 2.4 | 2.4 |
| Quality parameters of SNG | ||||||
| HHV/MJ Nm−3 | 33.2 | 33.3 | 33.9 | 34.2 | 34.5 | 34.6 |
| Wobbe index/MJ Nm−3 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 |
| Percentage of the total amount of CH4 produced in the following parts of the process (%) | ||||||
| Gasifier | 13.1 | 13.1 | 13.0 | 12.9 | 12.8 | 12.8 |
| 1st MR | 45.1 | 52.6 | 58.2 | 63.2 | 67.8 | 71.7 |
| 2nd MR | 28.4 | 22.6 | 19.9 | 17.2 | 14.5 | 11.9 |
| 3rd MR | 13.4 | 11.8 | 9.0 | 6.7 | 4.9 | 3.5 |
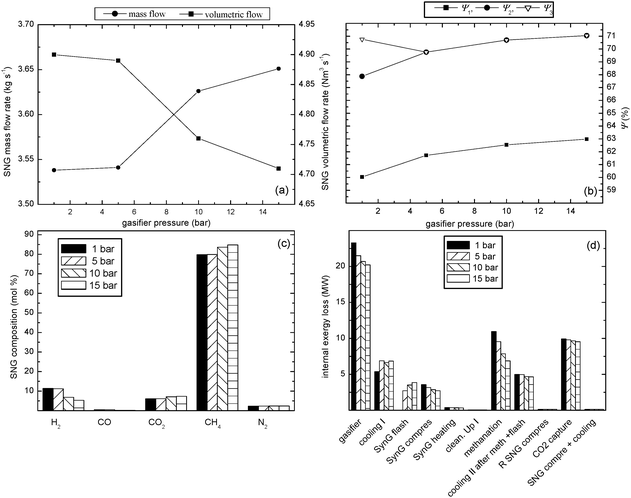
Fig. 5 shows mass and volumetric SNG product flow rates, SNG composition, efficiencies of the process and internal exergy losses of the technological units for the biomass-to-SNG process carried out at six different temperatures in the 1st methanation reactor (580, 610, 640, 670, 700, 729 °C, respectively) based on the 10 kg s−1 wet biomass input. Moreover, Table 4 shows the main characteristics of the biomass-to-SNG process evaluated at these operating conditions.
 | ||
| Fig. 5 Influence of the temperature in the 1st methanation reactor on the performance of the biomass-to-SNG process; (a) product flow rates, (b) exergetic efficiencies of the process, (c) SNG composition, (d) internal exergy losses of the technological units of the process. | ||
Fig. 5a shows the opposite influence of the temperature in the 1st methanation reactor on SNG production in terms of product mass and volumetric flow rate. Decreasing temperature in the 1st methanation reactor has a negative influence on SNG volumetric flow rate and on the contrary a positive influence on SNG mass flow rate. The difference in these tendencies is caused by different composition of the final SNG, see Table 4 and Fig. 5c. The exergy flow rate of the final SNG product is approximately the same, about the value of 151 MW, for all methanation temperatures used in the 1st reactor.
Fig. 5b also shows a comparison of the exergetic efficiencies ψ1, ψ2 and ψ3 according to eqn (7–9) of the biomass-to-SNG process at gasification conditions (700 °C and 1 bar) and different temperatures of the 1st methanation reactor. Decreasing temperature in the 1st methanation reactor does not influence the exergetic efficiency considering only SNG as a process product ψ1 (see eqn (7), Fig. 5b) since the exergy input of the system and the exergy content of the final SNG stream do not change. On the contrary, decreasing temperature in the 1st methanation reactor has a negative effect on the exergetic efficiency concerning SNG and produced steam as process products ψ2 (see eqn (8), Fig. 5b). As it was mentioned before, the exergy input into the system and the exergy content of the final SNG stream do not change, but lowering temperatures of the gas streams leaving the methanation reactors (see Table 4 for the reactor temperatures) allows it to produce less steam. Hence exergy content of the produced steam streams decreases with decreasing the temperature in the reactors. It results in decline of ψ2 with lowering methanation temperature. On the other hand, decreasing temperature in the 1st methanation reactor requires more heat to be removed from the reactor. Hence overall process exergetic efficiency concerning SNG, produced steam and also removed heat as process products, ψ3 (see eqn (9), Fig. 5b), increases with decline of temperature in the 1st methanation reactor.
Fig. 5d shows internal exergy losses (I) of the technological units for the biomass-to-SNG process operating the gasifier at the temperature of 700 °C and gasification pressures of 1 bar and for six different temperatures in the 1st methanation reactor based on the wet biomass flow rate of 10 kg s−1. It can be seen, that lowering the temperature of the 1st methanation reactor reduces the internal exergy losses in methanation section, cooling and flash section of the raw SNG and CO2 removal units.
After the methanation section, about 91–92% of CO2 present in the raw SNG (gas stream leaving methanation section) has to be removed to reach the quality requirements for the SNG. The final composition as well as the gross calorific value (HHV) and the Wobbe index of the produced SNG are listed in Table 4.
Influence of the temperature in the 2nd methanation reactor
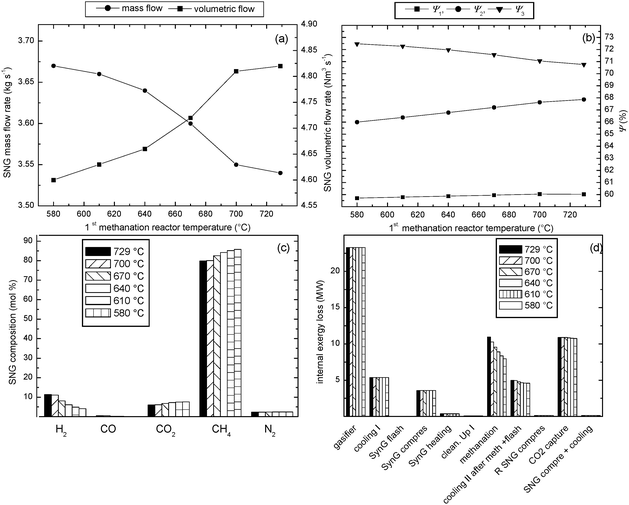
To investigate the effect of the temperature in the 2nd methanation reactor two boundary temperatures of the 1st methanation reactor from the previous section (mentioned before) were chosen (729 and 580 °C). The studied range applied in a case of the highest temperature in the 1st methanation reactor (729 °C, see Fig. 4b) was 470 °C to 590 °C with a step of 20 °C and in a case of the lowest temperature in the 1st methanation reactor (580 °C, see Fig. 4c) was 405 °C to 445 °C with a step of 10 °C. The effect of temperature in the 2nd methanation reactor on the exergetic efficiency of the whole plant was studied for fixed gasification conditions that is a gasification temperature 700 °C and a pressure of 1 bar.The results of this study are shown in Fig. 6, 7 and also in Table 5, 6. It can be noticed for both studied series of the temperature in the 2nd methanation reactor that decreasing the temperature has a similar effect. It has a negative influence on the SNG volumetric product flow rate, a slightly negative influence on exergetic efficiency to SNG (ψ1, Fig. 6b, 7b) and also negative influence on the efficiency when SNG and produced steam are considered as products (ψ2, Fig. 6b, 7b). On the contrary, decreasing temperature in the 2nd methanation reactor has a positive influence on the overall exergetic efficiency (ψ3, Fig. 6b, 7b). The reason why ψ2 decreases with the temperature in the methanation reactor is that lower temperature of the gas stream leaving the reactor allows production of less steam, which affects the exergy output of the process in term of SNG and steam product streams. However, the amount of the heat removed from the temperature controlled (cooled) reactors increases with the temperature decrease in the methanation reactor. Due to a more dominant increase in exergy content in heat streams leaving the cooled reactors than the decrease in exergy content in both SNG and steam product streams the overall exergetic efficiency (ψ3) increases with lowering the temperature in methanation reactors.
| CH4 synthesis temperature/°C | |||||||
|---|---|---|---|---|---|---|---|
| a These methanation reactors worked adiabatically. | |||||||
| 1st MR | 729 | ||||||
| 2nd MR | 590 | 570 | 550 | 530 | 510 | 490 | 470 |
| 3rd MR | 428 | 419a | 401a | 384a | 370a | 357a | 346a |
| SNG produced per 10 kg s−1 of wet biomass | |||||||
| Mass flow rate/kg s−1 | 3.54 | 3.56 | 3.59 | 3.62 | 3.64 | 3.65 | 3.66 |
| Molar flow rate/kmol s−1 | 215 | 214 | 211 | 210 | 208 | 207 | 206 |
| Vol. flow rate/Nm3 s−1 | 4.82 | 4.79 | 4.74 | 4.70 | 4.67 | 4.64 | 4.62 |
| Composition of SNG (mol %) | |||||||
| H2 | 11.4 | 10.4 | 8.7 | 7.3 | 6.2 | 5.4 | 4.7 |
| CO | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 |
| CO2 | 6.0 | 6.2 | 6.6 | 6.9 | 7.2 | 7.4 | 7.5 |
| CH4 | 79.8 | 80.6 | 82.0 | 83.2 | 84.1 | 84.7 | 85.3 |
| N2 | 2.3 | 2.3 | 2.3 | 2.4 | 2.4 | 2.4 | 2.4 |
| Quality parameters of SNG | |||||||
| HHV/MJ Nm−3 | 33.2 | 33.4 | 33.7 | 34.0 | 34.2 | 34.4 | 34.5 |
| Wobbe index/MJ Nm−3 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 |
| Percentage of the total CH4 produced in the following parts of the process (%) | |||||||
| Gasifier | 13.1 | 13.1 | 13.0 | 12.9 | 12.9 | 12.9 | 12.8 |
| 1st MR | 45.1 | 44.9 | 44.1 | 43.4 | 42.9 | 42.3 | 41.9 |
| 2nd MR | 28.4 | 30.9 | 33.3 | 35.4 | 37.3 | 39.1 | 40.6 |
| 3rd MR | 13.4 | 11.2 | 9.6 | 8.2 | 6.9 | 5.7 | 4.7 |
| CH4 synthesis temperature/°C | |||||
|---|---|---|---|---|---|
| a These methanation reactors worked adiabatically. | |||||
| 1st MR | 580 | ||||
| 2nd MR | 445a | 435 | 425 | 415 | 405 |
| 3rd MR | 335a | 331a | 327a | 323a | 320a |
| SNG produced per 10 kg s−1 of wet biomass | |||||
| Mass flow rate/kg s−1 | 3.67 | 3.68 | 3.68 | 3.68 | 3.69 |
| Molar flow rate/kmol s−1 | 205 | 205 | 205 | 205 | 204 |
| Vol. flow rate/Nm3 s−1 | 4.60 | 4.60 | 4.59 | 4.59 | 4.58 |
| Composition of SNG (mol %) | |||||
| H2 | 4.1 | 3.9 | 3.7 | 3.5 | 3.4 |
| CO | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| CO2 | 7.6 | 7.7 | 7.7 | 7.7 | 7.8 |
| CH4 | 85.8 | 86.0 | 86.2 | 86.3 | 86.4 |
| N2 | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 |
| Quality parameters of SNG | |||||
| HHV/MJ Nm−3 | 34.6 | 34.7 | 34.7 | 34.7 | 34.8 |
| Wobbe index/MJ Nm−3 | 44.0 | 44.0 | 44.0 | 44.0 | 44.0 |
| Percentage of the total CH4 produced In the following parts of the process (%) | |||||
| Gasifier | 12.8 | 12.8 | 12.8 | 12.8 | 12.8 |
| 1st MR | 71.7 | 71.6 | 71.5 | 71.4 | 71.3 |
| 2nd MR | 11.9 | 12.5 | 12.9 | 13.4 | 13.9 |
| 3rd MR | 3.5 | 3.1 | 2.8 | 2.4 | 2.1 |
 | ||
| Fig. 6 Influence of temperature in the 2nd methanation reactor on the performance of the biomass-to-SNG process for the methanation temperature of the 1st reactor of 729 °C and gasification condition of 700 °C and 1 bar; (a) product flow rates, (b) exergetic efficiencies of the process, (c) SNG composition, (d) internal exergy losses of the technological units of the process. | ||
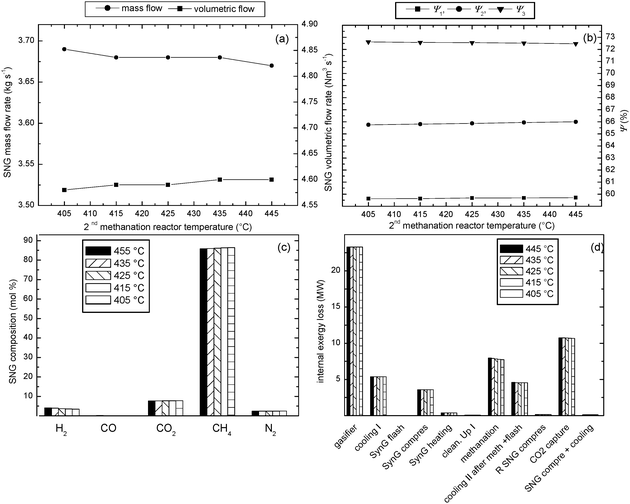 | ||
| Fig. 7 Influence of temperature in the 2nd methanation reactor on the performance of the biomass-to-SNG process for the methanation temperature of the 1st reactor of 580 °C and gasification condition of 700 °C and 1 bar; (a) product rates, (b) exergetic efficiencies of the process, (c) SNG composition, (d) internal exergy losses of the technological units of the process. | ||
The highest overall efficiency of 72.6% was found applying the following operating conditions of: gasifier 700 °C and 1 bar; 1st methanation reactor 580 °C and 2nd methanation reactor 405 °C, see Fig. 7b. It should be noted that the process flowsheet shown in Fig. 1 can be subjected to a more detailed heat integration, e.g. by using the hot water discharged by gas compressors cooling systems as a cooling medium in the methanation section or to use the waste heat from the gas and SNG cooling section to pre-heat the feed water for streams 4, 8, and 10. However, such a heat integration is out of the scope of the present paper but it could possibly lead to an additional exergy recovery and subsequently to a further increase of the overall exergy efficiency.
Decreasing the temperature in the 2nd methanation reactor leads to a higher CH4 content in the final SNG product stream. On the contrary, H2 concentration decreases as H2 is converted to CH4. The explanation of this effect is that lower temperature in methane synthesis reactors shifts the reversible reactions (eqn (1–3)) more to the product side.
Also decreasing the temperature in the 2nd methanation reactor reduces the internal exergy losses in the methanation section. However, this section of the process still remains, together with gasifier and CO2 removal section, the main contributor to the total internal exergy loss of the process.
All the above discussed effects of the temperature in the 2nd methanation rector are more dominant in the studied case when applying the higher methanation temperature in the 1st reactor (729 °C). At this temperature a significant part of CH4 is produced in the 2nd and 3rd methanation reactors. On the other hand, using the lower methanation temperature in the 1st reactor (580 °C), the majority of CH4 is produced in the 1st methanation reactor. Hence, a change of the temperature in the 2nd methanation reactor has a slight effect on the process in terms of product rates, efficiency or SNG product composition.
Moreover, lower temperature range in the 1st methanation reactor, together with high CH4 production, requires sufficient reactor design for reactor cooling (heat transfer) due to a substantial risk of local overheating of the catalysis.
Considering eqn (1–2) as the main stoichiometric routes to produce CH4 in the gasifier and methanation reactors together with the water–gas-shift reaction (eqn (3)) in order to evaluate the complete theoretical conversion of biomass to CH4 results in the conversion degree of 52.5% to CH4 and 47.5% to CO2, respectively. In all studied cases in this paper the conversion degree of the biomass (carbon) to CH4 reached about 51% as in the final SNG stream some H2 is still present as possible reactant to produce CH4. However, the produced SNG at the composition listed in Tables 2 and 4–6 meets the requirements for the final product in terms of maximal allowed contained H2 and also in terms of energy content (HHV, Wobbe index).
As it can be noticed from the explanation above, significant amounts of CO2 are produced in the system as a consequence of H2 production (methanation reactant) via the water–gas-shift reaction (eqn (3)). By removing of about 91–92% of CO2 contained in the raw SNG stream leaving the methanation section it is possible to reach the energy requirement of the final SNG product. Taking into account the internal exergy loss of the CO2 removal unit together with its impact on the quality of the final product this unit plays an important role in the whole process. However, this study focuses only on the effect of gasification and methanation operating condition on the process efficiency.
Conclusion
This paper presents the results of the exergetic evaluation of the biomass-to-SNG process. The main process units of this technology are gasifier, gas cleaning, synthetic gas compression, methane synthesis and final SNG condition. The study was focused on the influence of the gasification conditions (pressure) and the temperature of the methanation section on the whole process performance. The analyzed temperature of gasifier was 700 °C and the pressure in the gasifier range was changed from 1 to 15 bar. Moreover, the effect of temperature changes in the 1st and 2nd methanation rectors was investigated for the fixed gasification conditions of 700 °C and 1 bar.The results showed that the largest internal exergy losses take place in the biomass gasifier, methane synthesis part and CO2 capture unit. It was demonstrated that increasing gasification pressure has a positive influence on the exergetic efficiency to SNG and produced steam in the biomass-to-SNG process. Also, decreasing the temperature in the methanation rectors leads to increasing the overall exergetic efficiency of the process.
The highest overall efficiency of 72.6% was found applying the following operating conditions: gasifier 700 °C and 1 bar; 1st methanation reactor 580 °C and 2nd methanation reactor 405 °C.
Finally, exergy analysis principally gives results on thermodynamic efficiency and subsequent economical analysis can provide final judgment on possible applications.
Acknowledgements
The project is sponsored by the Cartesius Institute, in Leeuwarden, Friesland, The Netherlands.References
- A. Duret, C. Friedli and F. Maréchal, J. Cleaner Production, 2005, 13, 1434–1446 Search PubMed.
- F. Müller-Langer, A. Vogel, M. Kaltschmitt and D. Thrän, 15th European Biomass Conference & Exhibition, Berlin, 2007, 1908–1911 Search PubMed.
- R. W. R. Zwart, H. Boerrigter, E. P. Deurwaarder and C. M. van der Meijden, S. V. B. van Paasen, Production of Synthetic Natural Gas (SNG) from Biomass. ECN Report No. ECN-C-06-032, Petten, The Netherlands, 2006 Search PubMed.
- J. J. Prins, K. J. Ptasinski and F. J. J. G. Janssen, Chem. Eng. Science, 2003, 58, 1003–1011 CrossRef.
- S. V. B. van Paasen, M. K. Cieplik and N. P. Phokawat, Gasification of Non-woody Biomass. ECN Report No. ECN-C-06-032, Petten, The Netherlands, 2006 Search PubMed.
- B. B. Pearce, M. V. Twigg and C. Woodward, in Catalyst Handbook, ed. M. V. Twigg. Wolfe Publishing Ltd.London 2nd edn, 1989, ch. 7, pp. 376–378 Search PubMed.
- K. Lampert and A. Ziebik, Energy, 2007, 32, 521–527 CrossRef CAS.
- J. Szargut, D. R. Morris and F. R. Steward, in Exergy analysis of thermal, chemical and metallurgical processes, Hemisphere Publishing Corporation, New York, 1st edn, 1988, pp 1–332 Search PubMed.
- M. Jurascik, A. Sues and K. J. Ptasinski, Energy, submitted Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |
