An efficient one-pot procedure for asymmetric bifunctionalization of 5,15-disubstituted porphyrins: a simple preparation of mesoacyl-, alkoxycarbonyl-, and carbamoyl-substituted meso-formylporphyrins†
Toshikatsu
Takanami
*,
Atsushi
Wakita
,
Jun
Matsumoto
,
Sadashige
Sekine
and
Kohji
Suda
*
Meiji Pharmaceutical University, 2-522-1, Noshio, Kiyose, Tokyo 204-8588, Japan. E-mail: takanami@my-pharm.ac.jp; suda@my-pharm.ac.jp; Fax: +81-42-495-8779
First published on 19th November 2008
Abstract
An efficient one-pot procedure which converts 5,15-disubstituted porphyrins into their corresponding mesoacyl-, alkoxycarbonyl-, and carbamoyl-substituted meso-formylporphyrins has been developed, where the procedure involves a sequential SNAr reaction of porphyrins with PyMe2SiCH2Li, followed by acylation or related reactions and oxidation.
There has been continuous interest in the synthesis of porphyrins and their derivatives due to their ubiquitous nature and broad spectrum of applications in different fields such as catalysis, medicine, molecular recognition/sensing, and materials science.1,2 It is also well documented that the physical, chemical and biological properties of porphyrins can be precisely controlled through the introduction of peripheral substituents with diverse electronic and steric environments.1 As a consequence, intensive efforts have been dedicated to the discovery of new synthetic intermediates and strategies for preparing porphyrin derivatives with a variety of peripheral substituents.3–5 Asymmetric porphyrins bearing two distinct reactive carbonyl groups at the meso positions (their generalized structure is illustrated in Fig. 1) are regarded as valuable building blocks for more complex porphyrin systems, as each of these carbonyl groups directly attached on the porphyrin core can be individually replaced with other functionalities. However, to our knowledge, there are as yet no reports on the preparation of such asymmetric porphyrins that bear two carbonyl groups with distinct chemical reactivities.6
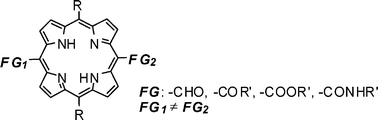 | ||
| Fig. 1 Asymmetric bifunctionalized porphyrins bearing two different carbonyl groups at the meso-positions. | ||
Herein, we report our studies on the development of an efficient, general one-pot procedure for the direct conversion of the 5,15-disubstituted porphyrin1 into the meso-acyl-, meso-alkoxycarbonyl-, and meso-carbamoyl-substituted meso-formylporphyrins 3, 5, and 7, respectively. The process involves a sequential nucleophilic substitution (SNAr reaction)7 with (2-pyridyldimethylsilyl)methyllithium (PyMe2SiCH2Li)8 followed by acylation or related reactions and oxidation. This simple one-pot procedure can be carried out under mild conditions with a wide range of 5,15-diaryl- and 5,15-dialkyl-substituted porphyrins, allowing direct access to a variety of asymmetric porphyrins with formyl groups and other chemically reactive functionalities directly attached at the meso positions in good yields.
Recently, our research group disclosed a novel direct meso formylation of the 5,15-disubstituted porphyrins1 based on a one-pot three-step procedure via the SNAr reaction of porphyrins with PyMe2SiCH2Li followed by hydrolysis and oxidation with DDQ, where the PyMe2SiCH2 group works as a latent formyl functionality in the reaction.5c As shown in Scheme 1, the reaction proceeds through the anionic intermediate I generated from the SNAr reaction of porphyrins with PyMe2SiCH2Li, which is subsequently hydrolyzed to form the dihydroporphyrin II and that in turn is oxidized to the meso-formylporphyrin III. Thus, we envisaged that the anionic intermediate I would be trapped by electrophiles (FG-X) such as acyl chlorides to form the asymmetric porphyrinIV with a formyl group and other chemically reactive functionalities.
 | ||
| Scheme 1 Reaction pathway for one-pot formylation of 5,15-disubstituted porphyrins using SNAr reaction with PyMe2SiCH2Li, and our hypothesis for their direct asymmetric bifunctionalization. | ||
In order to confirm our hypothesis, we initially examined the asymmetric bifunctionalization of 5,15-diphenylporphyrin 1a by using a one-pot procedure involving a SNAr reaction with PyMe2SiCH2Li followed by the trapping of the resulting anion with benzoylchloride2a as an electrophile and oxidation. Thus, a solution of 1a in THF was treated in the following order with: 10 equiv. of PyMe2SiCH2Li at −78 °C to room temperature, 10 equiv. of 2a at −40 °C to room temperature, aqueous HCl at 0 °C, and then 10 equiv. of DDQ at 65 °C. The one-pot sequential reaction proceeded smoothly, enabling the direct conversion of 1a into the desired asymmetric bifunctionalized product 5-benzoyl-15-formyl-10,20-diphenylporphyrin3aa in 85% yield (Table 1, entry 1).
|
|
||||
|---|---|---|---|---|
| Entry | Porphyrin | Acylchloride | Product | Yielda (%) |
| a Isolated yield. | ||||
| 1 | 1a (R1 = Ph) | 2a (R2 = Ph) | 3aa | 85 |
| 2 | 1a (R1 = Ph) | 2b (R2 = 4-MeOPh) | 3ab | 70 |
| 3 | 1a (R1 = Ph) | 2c (R2 = 4-CF3Ph) | 3ac | 77 |
| 4 | 1a (R1 = Ph) | 2d (R2 = Me) | 3ad | 68 |
| 5 | 1a (R1 = Ph) | 2e (R2 = iPr) | 3ae | 61 |
| 6 | 1b (R1 = p-Tol) | 2a (R2 = Ph) | 3ba | 70 |
| 7 | 1c (R1 = 3-MeOPh) | 2a (R2 = Ph) | 3ca | 74 |
| 8 | 1d (R1 = 3-CF3Ph) | 2a (R2 = Ph) | 3da | 72 |
| 9 | 1e (R1 = iBu) | 2a (R2 = Ph) | 3ea | 71 |
To determine the scope of the above procedure, different 5,15-substituted free-base porphyrins1a–e and acylchlorides2a–e were employed as substrates (Table 1). The benzoylchloride derivatives 2b and 2c with methoxy and trifluoromethyl substituents on their aromatic ring are compatible with the asymmetric bifunctionalization of 1a to afford the corresponding mesoacyl-substituted formylporphyrins 3ab and 3ac in good yields (entries 2 and 3). Both the primary and the secondary aliphatic acylchlorides could also participate as substrates in the transformation (entries 4 and 5). With regard to 5,15-diarylporphyrins, substrates with electron-rich, electron-neutral, and electron-poor aromatic moieties on the porphyrin core are all compatible with the asymmetric bifunctionalization conditions (entries 6–8). The 5,15-dialkyl-substituted porphyrin1e also yielded a favorable reaction (entry 9).
With the use of the chloroformate 4 as an electrophile , the meso-alkoxycarbonyl-substituted formylporphyrins 5 can also be prepared similarly (Table 2). For example, the sequential treatment of 1a with PyMe2SiCH2Li (10 equiv.) followed by 4a (10 equiv.), aqueous HCl, and DDQ (10 equiv.) led to the formation of 5-formyl-15-methoxycarbonyl-10,20-diphenylporphyrin5aa in 76% yield (entry 1). Likewise, other porphyrins1b–e and chloroformates including isopropylchloroformate4b and benzylchloroformate4c readily participated in the transformation as substrates, furnishing the corresponding meso-alkoxycarbonyl-substituted formylporpyrins in good to moderate yields (entries 2–7).
|
|
||||
|---|---|---|---|---|
| Entry | Porphyrin | Acylchloride | Product | Yielda (%) |
| a Isolated yield. | ||||
| 1 | 1a (R1 = Ph) | 4a (R2 = Me) | 5aa | 76 |
| 2 | 1a (R1 = Ph) | 4b (R2 = iPr) | 5ab | 70 |
| 3 | 1a (R1 = Ph) | 4c (R2 = CH2Ph) | 5ac | 46 |
| 4 | 1b (R1 = p-Tol) | 4a (R2 = Me) | 5ba | 65 |
| 5 | 1c (R1 = 3-MeOPh) | 4a (R2 = Me) | 5ca | 62 |
| 6 | 1d (R1 = 3-CF3Ph) | 4a (R2 = Me) | 5da | 60 |
| 7 | 1e (R1 = iBu) | 4a (R2 = Me) | 5ea | 60 |
The one-pot procedure can also be applied to the synthesis of the meso-carbamoyl-substituted formylporphyrins 7 by using isocyanates 6 as an electrophile (Table 3). For example, subjecting the diphenylporphyrin 1a and phenylisocyanates 6a–c substituted with electron-rich, electron-neutral, and electron-poor arenes to the asymmetric bifunctionalization conditions led to the desired meso-carbamoyl-substituted formylporphyrins in 73–60% yields (entries 1–3). The process is not limited to arylisocyanates. Nonbranched, branched, and cyclic alkylisocyanates were all compatible with the asymmetric bifunctionalization conditions, and gave the corresponding asymmetric porphyrins in good yields (entries 4–6). As expected, the reactions of other porphyrins1c–e with ethylisocyanate 6e proceeded smoothly to furnish the corresponding meso-carbamoyl-substituted formylporphyrins 7cd, 7dd, and 7ed in 68–66% yields (entry 7–9).
|
|
||||
|---|---|---|---|---|
| Entry | Porphyrin | Isocyanate | Product | Yielda (%) |
| a Isolated yield. | ||||
| 1 | 1a (R1 = Ph) | 6a (R2 = Ph) | 7aa | 73 |
| 2 | 1a (R1 = Ph) | 6b (R2 = 4-MeOPh) | 7ab | 65 |
| 3 | 1a (R1 = Ph) | 6c (R2 = 4-BrPh) | 7ac | 60 |
| 4 | 1a (R1 = Ph) | 6d (R2 = Et) | 7ad | 85 |
| 5 | 1a (R1 = Ph) | 6e (R2 = iPr) | 7ae | 74 |
| 6 | 1a (R1 = Ph) | 6f (R2 = c-C6H11) | 7af | 71 |
| 7 | 1c (R1 = 3-MeOPh) | 6d (R2 = Et) | 7cd | 66 |
| 8 | 1d (R1 = 3-CF3Ph) | 6d (R2 = Et) | 7dd | 68 |
| 9 | 1e (R1 = iBu) | 6d (R2 = Et) | 7ed | 67 |
Although most of the reactions described above were performed on a 0.1 mmol scale (see ESI† ), they can easily be scaled up if needed. For example, the reaction employing the porphyrin1a and the ethylisocyanate 6e as substrates can be carried out on a 1 mmol scale under similar reaction conditions, furnishing the desired porphyrin7ae at 515 mg and in 92% yield, which is virtually the same yield as that on a 0.1 mmol scale (cf. Table 3, entry 4).
In summary, we have developed a novel and facile one-pot procedure for the direct asymmetric bifunctionalization of 5,15-disubstituted free base porphyrinsvia a sequential SNAr reaction with PyMe2SiCH2Li followed by acylation or related reactions and oxidation. This simple one-pot procedure provides an efficient approach to the synthesis of a variety of asymmetric free base porphyrins which bear a formyl group and other chemically reactive functional groups, such as acyl, alkoxycarbonyl, and carbamoyl functionalities, at the meso positions in good yields. The operational simplicity as well as the mild reaction conditions and the broad substrate scope render this method attractive for the synthesis of more complicated porphyrin derivatives, which could find potential applications in areas such as catalysis, medicine, and molecular recognition/sensing. Such studies are currently under way in our laboratory, the results from which will be reported in due course.
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI) from JSPS and a Special Grant (GAKUCHO-GRANT) from Meiji Pharmaceutical University.
Notes and references
- The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 1999–2003, vol. 1–20 Search PubMed.
- We have developed porphyrin-based Lewis acid catalysts that can promote regio- and stereoselective isomerization of epoxides to carbonyl compounds and Claisen rearrangement of allylvinyl ethers, see: (a) K. Suda, K. Baba, S. Nakajima and T. Takanami, Chem. Commun., 2002, 2570 RSC; (b) K. Suda, T. Kikkawa, S. Nakajima and T. Takanami, J. Am. Chem. Soc., 2004, 126, 9554 CrossRef CAS; (c) T. Takanami, M. Hayashi, F. Hino and K. Suda, Tetrahedron Lett., 2005, 46, 2893 CrossRef CAS; (d) T. Takanami, M. Hayashi, K. Iso, H. Nakamoto and K. Suda, Tetrahedron, 2006, 62, 9467 CrossRef CAS.
- For some examples of leading works on functionalization reactions of porphyrins, see: (a) S. G. DiMagno, V. S.-Y. Lin and M. J. Therien, J. Am. Chem. Soc., 1993, 115, 2513 CrossRef CAS; (b) S. G. DiMagno, V. S.-Y. Lin and M. J. Therien, J. Org. Chem., 1993, 58, 5983 CrossRef CAS; (c) R. W. Boyle, C. K. Johnson and D. Dolphin, J. Chem. Soc., Chem. Commun., 1995, 527 RSC; (d) Y. Chen and X. P. Zhang, J. Org. Chem., 2003, 68, 4432 CrossRef CAS; (e) G. Y. Gao, A. J. Colvin, Y. Chen and X. P. Zhang, Org. Lett., 2003, 5, 3261 CrossRef CAS; (f) G. Y. Gao, Y. Chen and X. P. Zhang, Org. Lett., 2004, 6, 1837 CrossRef CAS; (g) G. Y. Gao, A. J. Colvin, Y. Chen and X. P. Zhang, J. Org. Chem., 2004, 69, 8886 CrossRef CAS; (h) H. Hata, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2005, 127, 8264 CrossRef CAS; (i) C. Liu, D.-M. Shen and Q.-Y. Chen, J. Org. Chem., 2007, 72, 2732 CrossRef CAS; (j) G.-Y. Gao, J. V. Ruppel, D. B. Allen, Y. Chen and X. P. Zhang, J. Org. Chem., 2007, 72, 9060 CrossRef CAS; (k) Y. Matano, T. Shinokura, K. Matsumoto, H. Imahori and H. Nakano, Chem.–Asian J., 2007, 2, 1417 CrossRef CAS; (l) S. Horn, N. N. Sergeeva and M. O. Senge, J. Org. Chem., 2007, 72, 5414 CrossRef CAS; (m) Y. Matano, K. Matsumoto, Y. Nakao, H. Uno, S. Sakaki and H. Imahori, J. Am. Chem. Soc., 2008, 130, 4588 CrossRef CAS.
- (a) M. O. Senge, Acc. Chem. Res., 2005, 38, 733 CrossRef CAS; (b) M. O. Senge, S. S. Hatscher, A. Wiehe, K. Dahms and A. Kelling, J. Am. Chem. Soc., 2004, 126, 13634 CrossRef CAS; (c) K. Dahms, M. O. Senge and M. B. Bakar, Eur. J. Org. Chem., 2007, 3833 CrossRef CAS; (d) X. Feng and M. O. Senge, Tetrahedron, 2000, 56, 587 CrossRef CAS; (e) Y. M. Shaker and M. O. Senge, Heterocycles, 2005, 65, 2441 Search PubMed ; and references cited therein.
- We have reported several functionalization reactions of porphyrins: (a) T. Takanami, M. Hayashi, F. Hino and K. Suda, Tetrahedron Lett., 2003, 44, 7353 CrossRef CAS; (b) T. Takanami, M. Hayashi, H. Chijimatsu, W. Inoue and K. Suda, Org. Lett., 2005, 7, 3937 CrossRef CAS; (c) T. Takanami, A. Wakita, A. Sawaizumi, K. Iso, H. Onodera and K. Suda, Org. Lett., 2008, 10, 685 CrossRef CAS; (d) T. Takanami, M. Yotsukura, W. Inoue, N. Inoue, F. Hino and K. Suda, Heterocycles, 2008, 76, 439 CAS.
- Multi-step total syntheses of asymmetric bifunctionalized porphyrins that bear two different functional groups, such as acyl and hydroxymethyl, borolanyl and alkynyl, vinyl and alkynyl, and so on, at the meso-positions have been reported, see: (a) T.-G. Zhang, Y. Zhao, I. Asselberghs, A. Persoons, K. Clays and M. J. Therien, J. Am. Chem. Soc., 2005, 127, 9710 CrossRef CAS; (b) S. Shanmugathasan, C. K. Johnson, C. Edwards, E. K. Matthews, D. Dolphin and R. W. Boyle, J. Porphyrins Phthalocyanines, 2000, 4, 228 CrossRef CAS; (c) M. Yeung, A. C. H. Ng, M. G. B. Drew, E. Vorpagel, E. M. Breitung, R. J. McMahon and D. K. P. Ng, J. Org. Chem., 1998, 63, 7143 CrossRef CAS; (d) T. S. Balaban, A. D. Bhise, M. Fischer, M. Linke-Schaetzel, C. Roussel and N. Vanthuyne, Angew. Chem., Int. Ed., 2003, 42, 2140 CrossRef CAS; (e) Z. Yao, J. Bhaumik, S. Dhanalekshmi, M. Ptaszek, P. A. Rodriguez and J. S. Lindsey, Tetrahedron, 2007, 63, 10657 CrossRef CAS.
- Porphyrin modification based on the SNAr strategy with organolithium reagents was pioneered by the research group of Senge. They have widely applied the strategy to the preparation of various asymmetric porphyrins bearing different alkyl and/or aryl substituents at the meso positions, see ref. 4.
- (a) K. Itami, K. Mitsudo and J. Yoshida, Tetrahedron Lett., 1999, 40, 5533 CrossRef CAS; (b) K. Itami, K. Mitsudo and J. Yoshida, Tetrahedron Lett., 1999, 40, 5537 CrossRef CAS; (c) K. Itami, T. Kamei, K. Mitsudo, T. Nokami and J. Yoshida, J. Org. Chem., 2001, 66, 3970 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b817551a |
| This journal is © The Royal Society of Chemistry 2009 |



