Cucurbituril-based nanoparticles: a new efficient vehicle for targeted intracellular delivery of hydrophobic drugs†
Kyeng Min
Park
a,
Kyungwon
Suh
a,
Hyuntae
Jung
b,
Don-Wook
Lee
b,
Youngjoo
Ahn
a,
Jeeyeon
Kim
a,
Kangkyun
Baek
a and
Kimoon
Kim
*ab
aNational Creative Research Initiative Center for Smart Supramolecules (CSS) and Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 790-784, Korea. E-mail: kkim@postech.ac.kr; Fax: +82 54-279-8129; Tel: +82 54-279-2113
bSchool of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology (POSTECH), Pohang, 790-784, Korea
First published on 13th November 2008
Abstract
Cucurbituril-based nanoparticles (CB[6]NPs) serve as new efficient vehicles for delivery of hydrophobic drugs, which have unique features including (1) a high drug loading capacity and efficiency, (2) noncovalently tunable surfaces, (3) efficient delivery of hydrophobic drugs into a cancer cell by receptor-mediated endocytosis, and (4) facile release of drugs into cytoplasm, which enhances the pharmaceutical effects of the drugs.
One of the major concerns regarding cancer treatment with clinically used hydrophobic drugs such as paclitaxel (PTX) is their low therapeutic effects due to poor aqueous solubility and lack of specificity.1 This limitation can be overcome at least in part by targeted delivery of such drugs using nanomaterials2 such as liposomes,2b micelles2c and nanoparticles (NPs).2d,e Although a wide range of nanomaterials made of various building blocks ranging from conventional lipids to polymer amphiphiles have been explored to date, the search for safe, efficient nano-sized vehicles for drug delivery continues.2 Recently, amphiphilic macrocyclic molecules and nanomaterials therefrom have been investigated as drug delivery vehicles by harnessing their hydrophobic cavities to encapsulate hydrophobic drugs, but most efforts have been focused on NPs made of cyclodextrins (CDs) or their derivatives.3
Cucurbit[6]uril (CB[6]), a member of the macrocyclic host family cucurbit[n]uril (CB[n]) comprising six glycoluril units has a hydrophobic cavity similar to that of α-CD. However, unlike CDs, it has two identical, carbonyl-fringed entrances to the cavity. Most importantly, CB[6] forms stable host–guest complexes with a wide range of small molecules, especially polyamines with extremely high binding affinity (K > 106 M−1) in aqueous solution.4 Recent development of a direct functionalization method for CB[n] allowed us to synthesize a wide range of tailor-made CB[n] derivatives,5a and to explore their applications5b including the synthesis of CB[6]-based vesicles5c and polymer nanocapsules.5d Herein, we report novel CB[6]-based NPs (CB[6]NPs) formed by a new CB[6] derivative, which may serve as effective vehicles for targeted intracellular delivery of hydrophobic drugs. The unique features of CB[6]NPs include (1) hydrophobic drugs can be loaded inside the NPs with a high loading capacity and efficiency, (2) the surface of hydrophobic drug-loaded CB[6]NPs can be easily modified with a targeting ligand and/or fluorescent probe in a noncovalent manner by taking advantage of the strong host–guest interactions between CB[6] and polyamine derivatives, (3) drug-loaded CB[6]NPs can be efficiently delivered to a cancer cell and internalized by receptor-mediated endocytosis, and (4) hydrophobic drugs can be released into cytoplasm after receptor-mediated endocytosis, which enhances the pharmaceutical effects of the drugs.
The new CB[6] derivative, (3-(6-hydroxyhexanethio)propan-1-oxy)nCB[6] (average n = 11.4) (1) (Scheme 1), was synthesized by photoreaction between (allyloxy)12CB[6]5a and 6-mercaptohexanol in methanol. CB[6]NPs were prepared by adding water to 1 dissolved in a minimum volume of ethanol and sonicating it for 30 min to allow formation of NPs. The NPs have been characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). A characteristic morphology of solid spherical NPs was observed by TEM (Fig. S1, ESI†). In the concentration range of 1 × 10−3–1 × 10−6 M, the average size of the spherical NPs determined by TEM is 190 ± 50 nm, which is consistent with 160 ± 40 nm determined by dynamic light scattering with samples dispersed in water.
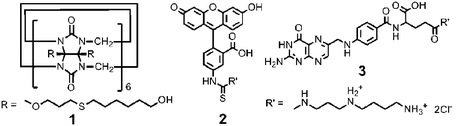 | ||
| Scheme 1 | ||
Having established the nanometre-sized solid particle nature of 1, we decided to investigate whether the water-dispersed CB[6]NPs can encapsulate hydrophobic molecules inside similar to conventional polymer NPs.2d,e A representative hydrophobic dye, Nile Red was chosen to investigate the loading properties of CB[6]NPs. Nile Red does not fluoresce in water, but emits red fluorescence in a hydrophobic environment.6 Nile Red loaded CB[6]NPs (CB[6]NPs⊃NR) were prepared by the same procedure as before, except using an ethanolic solution of Nile Red instead of pure ethanol. Confocal laser scanning microscopy showed strong red fluorescent dots dispersed in water, corresponding to the emission of Nile Red with λmax at 609 nm as independently determined by emission spectroscopy (Fig. S2a,c, ESI†). TEM and light scattering studies revealed little change in morphology and hydrodynamic radius of CB[6]NPs after loading of Nile Red. Taken together, we conclude that the hydrophobic molecules are successfully entrapped inside the CB[6]NPs.
The NPs are made of a CB[6] derivative with a cavity which is known to bind strongly with various guest molecules, in particular, polyamines including spermine (K > 1010 M−1) and spermidine (K > 108 M−1).4f Taking advantage of the exceptional host–guest chemistry, a “tag” can be easily introduced to the surface of the CB[6]NPs in a noncovalent manner simply by treating with a tag-attached polyamine as we demonstrated with CB[6]-based vesicles5c and polymer nanocapsules.5d For example, we can decorate the surface of CB[6]NPs with the fluorescent probe fluorescein isothiocyanate (FITC) (2@CB[6]NPs), simply by adding 1 equiv. of FITC-spermidine conjugate (2) to a solution of CB[6]NPs, stirring the mixture for 1 h, and then dialyzing it against water to remove unbound 2. The number of accessible CB[6] cavities of CB[6]NPs was quantified by measuring the amount of unbound 2 recovered during the dialysis by fluorometry, which turned out to be 26 ± 4% of all the CB[6] derivative 1 constituting CB[6]NPs.
The surface of the CB[6]NPs⊃NR can also be decorated with 2 using the same procedure (2@CB[6]NPs⊃NR). Confocal laser scanning microscopy (Fig. S3a,b, ESI†) showed green and red fluorescent dots corresponding to emission of 2 and Nile Red with λmax at 521 and 609 nm, respectively. The emission band of 2 decorated on CB[6]NPs⊃NR was red-shifted by 6 nm with respect to that of free 2 (Fig. S3d, ESI†). The dots in the FITC and Nile Red channels were well overlapped (Fig. S3c, ESI†), indicating that the surface of CB[6]NPs was successfully decorated with 2 while Nile Red was loaded inside CB[6]NPs. The TEM image (Fig. S3e, ESI†) showed that 2@CB[6]NPs⊃NR maintained its spherical morphology and size even after the noncovalent surface modification using host–guest chemistry. Taken together, CB[6]NPs can entrap hydrophobic guest molecules inside the NPs, and at the same time, various “tag” units can be introduced on the surface of CB[6]NPs in a noncovalent manner without significant morphological changes.
To demonstrate the potential utility of CB[6]NPs as new vehicles for targeted drug delivery to tumors, an in vitro study was carried out using Nile Red as a model hydrophobic drug, and human ovarian carcinoma HeLa cells that have over-expressed folate receptors on the surface as a target cell. The cancer cells require excessive folic acid, a high affinity ligand to the folate receptors, for their rapid proliferation. Conjugates of folic acid have been previously studied for targeted delivery of oligonucleotides,7cdrugs7d and nanomaterials7e to cancer cells, wherein these conjugates are internalized through folate receptor-mediated endocytosis.7 We prepared folate-spermidine conjugate (3)5d decorated CB[6]NPs⊃NR (3@CB[6]NPs⊃NR), using the same procedure as described above. (Fig. S4, ESI†). The intracellular uptake of 3@CB[6]NPs⊃NR and CB[6]NPs⊃NR into HeLa cells was examined by confocal laser scanning microscopy using 543 nm light for excitation of Nile Red.
As illustrated in Fig. S5, ESI†, only 3@CB[6]NPs⊃NR showed facile internalization into the cell after 1 h incubation at 37 °C. No or only small translocation of CB[6]NPs⊃NR was observed without the targeting ligand (3) decorating the CB[6]NPs surface (Fig. S5b, ESI†), in the presence of an excess amount of extra folic acid in the culture medium (Fig. S5d, ESI†), or incubation at 4 °C (Fig. S5e, ESI†), which suggested that the mechanism of the cellular uptake is most likely folate receptor-mediated endocytosis. The result was also confirmed by flow cytometry (Fig. S6, ESI†).
To investigate the intracellular location of CB[6]NPs after endocytosis, we incubated HeLa cells with 2 (fluorescence probe) and 3 (targeting ligand) decorated CB[6]NPs ((2 + 3)@CB[6]NPs) and LysoTracker Red, a probe highly selective for cellular organelles with low internal pHs such as endosomes. As shown in Fig. S7, ESI†, a good overlap between the green dots and red dots independently observed by the FITC channel and LysoTracker Red channel, respectively, of confocal laser scanning microscopy, indicated that CB[6]NPs were located predominantly at endosomes after endocytosis in the HeLa cells.
Similarly, to monitor the behavior of Nile Red loaded in CB[6]NPs after endocytosis by confocal laser scanning microscopy, we incubated HeLa cells with (2 + 3)@CB[6]NPs⊃NR for 1 h at 37 °C, the surface of which was decorated with 2 and 3. Interestingly, as seen in Fig. 1, Nile Red was distributed over the whole cell except the nucleus after 1 h incubation, while the green fluorescence from 3 decorating the surface of CB[6]NPs was localized, which indicated that after endocytosis, Nile Red was released into cytoplasm whereas CB[6]NPs appeared to remain in endosomes while maintaining their nanoparticle nature. At the moment, it is not clear how Nile Red is released from CB[6]NPs trapped in endosomes into cytoplasm.8 Nevertheless, considering that any drug loaded in nanomaterials must be released into the cytoplasm after endocytosis for efficient delivery, the facile release of the hydrophobic dye into cytoplasm increases the applicability of CB[6]NPs in targeted intracellular drug delivery.
![Confocal laser scanning microscopy images of HeLa cells incubated with (2 + 3)@CB[6]NPs⊃NR for 1 h at 37 °C: (a) FITC channel, (b) Nile Red channel, (c) overlay of (a) and (b) (scale bar = 10 μm), and (d) taken with different focal depths along the view axis.](/image/article/2009/CC/b815009e/b815009e-f1.gif) | ||
| Fig. 1 Confocal laser scanning microscopy images of HeLa cells incubated with (2 + 3)@CB[6]NPs⊃NR for 1 h at 37 °C: (a) FITC channel, (b) Nile Red channel, (c) overlay of (a) and (b) (scale bar = 10 μm), and (d) taken with different focal depths along the view axis. | ||
Encouraged by the series of observations, we then examined the delivery of hydrophobic drug PTX to HeLa cells using CB[6]NPs as a carrier and cell growth inhibition induced by cytotoxicity of PTX delivered by CB[6]NPs. We first determined the PTX loading efficiency of CB[6]NPs. In the case of CB[6]NPs⊃PTX prepared with 2.00 mg of CB[6] derivative 1 and 0.20 mg of PTX, PTX was loaded in CB[6]NPs with a loading capacity of 8.6 ± 0.6% (w/w) and a loading efficiency of 94 ± 6% (w/w), which was found to be the best condition for the preparation of CB[6]NPs⊃PTX for cell growth inhibition assay (Table S1, ESI†). The PTX loading capacity and efficiency of CB[6]NPs are similar or higher than those reported for polymer based NPs such as hydrophobically modified glycol chitosan NPs9 (10% (w/w) loading capacity with 90% (w/w) efficiency) and stearate-grafted chitosan oligosaccharides NPs10 (5.6% (w/w) loading capacity with 89.5% (w/w) efficiency).11
The cell growth inhibition induced by the cytotoxicity of PTX delivered by CB[6]NPs with and without targeting ligands was evaluated by a standard MTT assay. First of all, the cytotoxicity of CB[6]NPs to HeLa cells is negligible at a concentration below 40 μg mL−1 (Fig. S8, ESI†). Therefore, all the experiments were done with CB[6]NPs at a concentration of 40 μg mL−1 or below. HeLa cells were incubated with various concentrations of free PTX, CB[6]NPs⊃PTX, or 3@CB[6]NPs⊃PTX in the cell culture medium for 1 h at 37 °C, and were then incubated for another 3 days after replacing the culture medium with a fresh one. IC50 (50% of cell growth inhibition concentration) values after treatments were measured and are compared in Table S2, ESI†. The IC50 value of CB[6]NPs⊃PTX was 0.33 ± 0.10 μg mL−1, which means that the PTX loaded in CB[6]NPs was approximately 3.8-fold more effective than free PTX (IC50 = 1.24 ± 0.20 μg mL−1) presumably due to internalization of CB[6]NPs⊃PTX into cells though nonspecific interactions, which was consistent with the nonspecific internalization of CB[6]NPs⊃NR into HeLa cells observed by confocal laser scanning microscopy and flow cytometry (Fig. S5b,d,e, Fig. S6b,d,e, ESI†). Most importantly, however, the IC50 value of 3@CB[6]NPs⊃PTX was 0.08 ± 0.02 μg mL−1, approximately 15.5-fold smaller than that of free PTX. The significantly enhanced cytotoxicity of 3@CB[6]NPs⊃PTX to HeLa cells may be attributed to facile internalization of 3@CB[6]NPs⊃PTX by folate receptor-mediated endocytosis followed by release of PTX into cytoplasm.
In summary, we report novel nanoparticles CB[6]NPs made of a new CB[6] derivative, in which hydrophobic drugs can be loaded in a high loading capacity and efficiency. Their surfaces can be easily decorated in a nondestructive, noncovalent, and modular manner simply by mixing the NPs with tag-polyamine conjugates which can be used as a fluorescent probe or targeting ligand, by taking advantage of the exceptional ability of CB[6] to bind polyamines tightly. Moreover, we demonstrated the selective cellular uptake of targeting ligand-decorated CB[6]NPs by receptor-mediated endocytosis, and facile release of loaded hydrophobic dye molecules into cytoplasm after endocytosis. A significantly increased cytotoxicity of PTX using targeting ligand-decorated CB[6]NPs has also been demonstrated. Taken together, the remarkable abilities of CB[6]NPs make them a novel platform for efficient intracellular and cytoplasmic delivery systems for clinically available hydrophobic drugs such as PTX, which opens the possibility of practical applications of CB[6]-based nanomaterials as targeted drug delivery systems. Further work using animals is now in progress.
We gratefully acknowledge the CRI Program and the BK21 program of the Korean Ministry of Education, Science and Technology and POSTECH Biotech Center for support of this work.
Notes and references
- V. Martin, Semin. Oncol. Nurs., 1993, 9, 2 CAS.
- (a) D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotech., 2007, 2, 751 CrossRef CAS; (b) V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145 CrossRef CAS; (c) V. P. Torchilin, Cell. Mol. Life Sci., 2004, 61, 2549 CrossRef CAS; (d) R. Sinha, G. J. Kim, S. Nie and D. M. Shin, Mol. Cancer Ther., 2006, 5, 1909 CrossRef CAS; (e) L. Brannon-Peppas and J. O. Blanchette, Adv. Drug Delivery Rev., 2004, 56, 1659.
- (a) D. Duchêne, G. Ponchel and D. Wouessidjewe, Adv. Drug Delivery Rev., 1999, 36, 29 CrossRef CAS; (b) E. Memisoglu, A. Bochot, M. Şen, D. Duchene and A. A. Hincal, Pharm. Res., 2003, 20, 117 CrossRef CAS; (c) E. Bilensoy, O. Grürkaynak, M. Ertan, M. Şen and A. A. Hincal, J. Pharm. Sci., 2008, 97, 1519 CrossRef CAS.
- (a) W. L. Mock, Top. Curr. Chem., 1995, 175, 1000; (b) J. W. Lee, S. Samal, N. Selvapalam, H.-J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS; (c) K. Kim and H.-J. Kim, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, Marcel Dekker, New York, 2004, pp. 390–397 Search PubMed; (d) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS; (e) Y. J. Jeon, S. -Y. Kim, Y. H. Ko, S. Sakamoto, K. Yamaguchi and K. Kim, Org. Biomol. Chem., 2005, 3, 2122 RSC; (f) M. V. Rekharsky, Y. K. Ko, N. Selvapalam, K. Kim and Y. Inoue, Supramol. Chem., 2007, 19, 39 CrossRef CAS.
- (a) S. Y. Jon, N. Selvapalam, D. H. Oh, J.-K. Kang, S.-Y. Kim, Y. J. Jeon, J. W. Lee and K. Kim, J. Am. Chem. Soc., 2003, 125, 10186 CrossRef CAS; (b) K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 26 Search PubMed; (c) H.-K. Lee, K. M. Park, Y. J. Jeon, D. Kim, D. H. Oh, H. S. Kim, C. K. Park and K. Kim, J. Am. Chem. Soc., 2005, 127, 5006 CrossRef CAS; (d) D. Kim, E. Kim, J. Kim, K. M. Park, K. Baek, M. Jung, Y. H. Ko, W. Sung, H. S. Kim, J. H. Suh, C. G. Park, O. S. Na, D. -k. Lee, K. E. Lee, S. S. Han and K. Kim, Angew. Chem., Int. Ed., 2007, 46, 3471 CrossRef.
- (a) E. R. Gillies and J. M. J. Fréchet, Chem. Commun., 2003, 1640 RSC; (b) Y. b. Lim, E. Lee and M. Lee, Angew. Chem., Int. Ed., 2007, 46, 3475 CrossRef CAS.
- (a) A. R. Hilgenbrink and P. S. Low, J. Pharm. Sci., 2005, 94, 2135 CrossRef CAS; (b) C. P. Leamon and P. S. Low, Adv. Drug Delivery Rev., 2002, 54, 675 CrossRef CAS; (c) X. B. Zhao and R. J. Lee, Adv. Drug Delivery Rev., 2004, 56, 1193 CrossRef CAS; (d) C. P. Leamon and J. A. Reddy, Adv. Drug Delivery Rev., 2004, 56, 1127 CrossRef CAS; (e) J. Kim, J. E. Lee, S. H. Lee, J. H. Yu, J. H. Lee, T. G. Park and T. Hyeon, Adv. Mater., 2008, 20, 478 CrossRef CAS.
- Release of hydrophobic dyes from micelles made of block copolymers in cells has been reported. However, the dye release mechanism seems to be different from that in this study: R. Savic, L. Luo, A. Eisenberg and D. Maysinger, Science, 2003, 300, 615 Search PubMed.
- J. -H. Kim, Y. -S. Kim, S. Kim, J. H. Park, K. Kim, K. Choi, H. Chung, S. Y. Jeong, R. -W. Park, I. -S. Kim and I. C. Kwon, J. Controlled Release, 2006, 111, 228 CrossRef CAS.
- J. You, F.-Q. Hu, Y.-Z. Du and H. Yuan, Biomacromolecules, 2007, 8, 2450 CrossRef CAS.
- An exceptionally high PTX loading capacity (28.3% (w/w)) was recently reported for PTX-attached polymer-based NPs. In this case, however, PTX was covalently attached to polylactide (PLA) in preparation of NPs: R. Tong and J. Cheng, Angew. Chem., Int. Ed., 2008, 47, 4830 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, synthesis and characterization of 1 and 2, characterization of nanoparticles and additional images. See DOI: 10.1039/b815009e |
| This journal is © The Royal Society of Chemistry 2009 |
