Diazaphospholidine terminated polyhedral oligomeric silsesquioxanes in the hydroformylation of vinyl acetate†
Nicolas R.
Vautravers
and
David J.
Cole-Hamilton
*
EaStCHEM, School of Chemistry, University of St. Andrews, St. Andrews, Fife, UK KY16 9ST. E-mail: djc@st-and.ac.uk; Fax: +44-1334-463808; Tel: +44-1334-463805
First published on 21st November 2008
Abstract
The poorly active, monodentate SemiEsphos phosphine has been turned into an active ligand for rhodium catalysed vinyl acetate hydroformylation by attachment to the periphery of a polyhedral oligomeric silsesquioxane.
Dendrimers are tree-like macromolecules with a high degree of symmetry and a precise hyperbranched structure. They have been used in various applications ranging from biology to materials science.1 Their utilisation in catalysis is also well documented because there is the possibility of their use in catalyst–product separation by ultrafiltration techniques2 or because complexes of dendrimeric ligands can sometimes outperform their parent models (so called “dendritic effects”).3 For instance, a Polyhedral Oligomeric SilSesquioxane (POSS) with 16 terminal diphenylphosphines has been shown to give much higher selectivity to the desired linear product (linear : branched ratio = 13.9) in an oct-1-ene hydroformylation reaction than any of its small molecule analogues (l : b typically 2–5).4 This was one of the very first positive dendritic effects to be discovered and it was attributed to the high steric crowding on the periphery of the dendrimer forcing the phosphines into the ideal equatorial–equatorial coordination at rhodium leading to such a high selectivity.5,6 Bearing in mind that these peripheral constraints can change the selectivity of catalytic reactions so that the selectivities normally associated with bidentate ligands can be obtained with unidentate analogues, we postulated that an inactive monodentate ligand might be turned into an active bidentate one upon attachment to the periphery of POSS cored dendrimers.
The bis(diazaphospholidine) ligand, ESPHOS (Scheme 1), is a bidentate C2 symmetric ligand, capable of acting as a ligand to rhodium for the asymmetric hydroformylation of vinyl acetate with regio- and stereo-selectivities that are amongst the highest yet reported (Scheme 1).7 The unidentate analogue, SemiEsphos, is a very poor ligand for this reaction giving very low conversions (Scheme 1).7 We report in this paper that the attachment of this unidentate ligand onto the periphery of a POSS centred macromolecule allows it to become active for vinyl acetate hydroformylation, but that the degree of crowding seems to be important in determining the outcome of the reaction.
 | ||
| Scheme 1 Asymmetric hydroformylation of vinyl acetate catalysed by rhodium complexes of ESPHOS. SemiEsphos gives very poor results (yield <15%, b : l 70 : 30, ee <2%).7 | ||
In order to try to force SemiEsphos to behave like ESPHOS, we designed and synthesized three new first generation SemiEsphos decorated POSS cored macromolecules by the sequence of reactions depicted in Scheme 2 (see ESI for details† ). Lithiation of 3-bis(dimethylamino)phosphinobromobenzene, prepared by a literature method,8 followed by reaction with readily available chlorosilane terminated POSS (1)9 led to bis(dimethylamino)phosphine terminated dendrimers (2). The synthesis was completed by amine exchange with (S)-2-(phenylaminomethyl)pyrrolidine10 to form the desired cube (3) driven by the thermodynamic displacement of gaseous dimethylamine. The intermediate compounds (2) were not thoroughly purified as only an alkaline aqueous work-up was performed to destroy any traces of remaining n-butyllithium. The excess of 3-bis(dimethylamino)phosphinobromobenzene employed in the transformation of (1) to ensure the complete substitution of all chlorine atoms, as well as the excess of the chiral (S)-2-(phenylaminomethyl)pyrrolidine were successfully washed away during the precipitation process of (3) in hexane. This procedure was repeated until no phosphorus signal attributed to the small molecules could be detected by 31P NMR spectroscopy . The final spectrum contained only one signal from each of the desired SemiEsphos terminated POSS cored macromolecules.
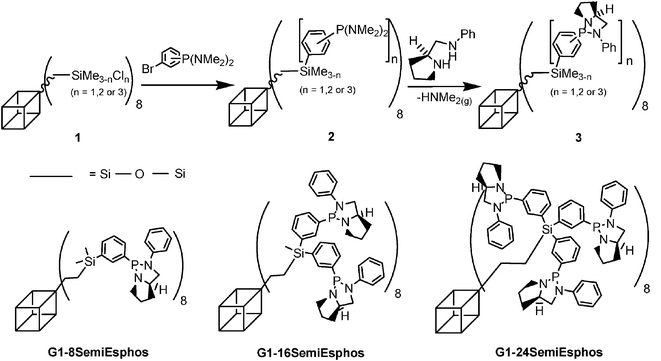 | ||
| Scheme 2 Preparation of generation 1 SemiEsphos terminated POSS cored macromolecules. | ||
29Si NMR was also very useful in monitoring this synthesis as three different signals, Si(T) (silicon at the vertex of the cube), Si–Cl and Si–Phenyl, are present in the final product. The purity of the final product was shown by the presence of a single 29Si NMR signal at –67 ppm. This resonance shows that the cube remains intact and that each corner has been substituted by the same group showing the presence of only one cube. Moreover, the nucleophilic substitution of the chlorine atoms on (1) by the lithium aryl entity was confirmed by the observation in the 29Si NMR spectrum of downfield signals characteristic of the Si–Phenyl bond at −1.3 ppm, −6.3 ppm and −10.1 ppm (respectively for G1-8SemiEsphos, G1-16SemiEsphos and G1-24SemiEsphos). In addition to the characterization of the three new G1 products by 31P and 29Si NMR spectroscopy , microanalysis also testifies to the purity of the macromolecules.
The POSS bound SemiEsphos ligands were tested in the rhodium catalysed asymmetric hydroformylation of vinyl acetate, to see whether crowding at the periphery might encourage bidentate binding and induce activity. To the best of our knowledge, the only other published use of dendrimer based catalysts in vinyl acetate hydroformylation is that of Alper et al. employing the achiral silica-supported polyamidoamine dendrimer complex (Rh-PPh2-PAMAM–SiO2).11
Catalytic solutions were prepared by mixing the G1 ligands with [Rh(acac)(CO)2] in toluene at the desired rhodium to phosphine ratio, before being transferred to a batch Hastelloy autoclave via canula. The conditions and results for the catalytic reactions are summarized in Table 1. Entries 1 and 2 show, consistent with the literature,7 that poor conversion (around 11.5%), low b : l ratio (0.5) and no ee are obtained when using monomeric SemiEsphos at different phosphine to metal ratios whilst entry 37 emphasizes the very good behaviour of ESPHOS in this reaction as total conversion is reached after 3 h. These three results will serve as standards in the evaluation of the activity of our macromolecular ligands.
| Entry | Ligand | P : Rh | Concentration/mol L−1 | Conversion (%) | Aldehyde yield (%)b | Aldehyde b : lc | Aldehyde ee (%) | Alcohol yield (%)d | Alcohol ee (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Catalyst prepared in situ from [Rh(acac)(CO)2] and the phosphine in toluene (4 mL) containing vinyl acetate (1 mL). PCO–H2 (1 : 1) = 40 bar. Temp. = 80 °C, t = 20 h. b Yield refers to 2-acetoxypropanal (1-acetoxypropanal decomposes under the reaction conditions to acetic acid and propenal). c Refers to aldehyde formation before aldehyde decomposition/hydrogenation. Determined from the ratio of branched chain product (aldehyde + alcohol) to acetic acid. d Refers to 2-acetoxy-1-propanol plus 1-acetoxy-2-propanol. e Acetoxyacetone (0.3–1.3%) is also a product. f t = 3 h. | |||||||||
| 1 | SemiEsphos | 6.0 | 0.01 | 12.9 | 2.3 | 0.4 | — | — | — |
| 2 | SemiEsphos | 3.0 | 0.01 | 10.2 | 2.5 | 0.3 | — | — | — |
| 3 | ESPHOS 7 ef | 1.5 | 0.01 | 100.0 | 34.9 | 15.9 | 76 (S) | 58.8 | 84 (S) |
| 4 | G1-8SemiEsphos | 3.0 | 0.01 | — ppt | — | — | — | — | — |
| 5 | G1-8SemiEsphos | 6.0 | 0.01 | — ppt | — | — | — | — | — |
| 6 | G1-8SemiEsphos | 6.0 | 0.001 | — ppt | — | — | — | — | — |
| 7 | G1-16SemiEsphos | 3.0 | 0.01 | — ppt | — | — | — | — | — |
| 8 | G1-16SemiEsphos | 6.0 | 0.01 | — ppt | — | — | — | — | — |
| 9 | G1-16SemiEsphos | 6.0 | 0.005 | 11.0 | 6.7 | 1.5 | — | — | — |
| 10 | G1-24SemiEsphose | 6.0 | 0.01 | 60.2 | 42.0 | 11.6 | — | 5.9 | — |
| 11 | G1-24SemiEsphos | 3.0 | 0.01 | 51.5 | 48.4 | 15.5 | — | — | — |
| 12 | G1-24SemiEsphos | 6.0 | 0.005 | 13.6 | 9.2 | 2.1 | — | — | — |
Upon mixing G1-8SemiEsphos and the rhodium precursor at a 3 to 1 phosphine to metal ratio (entry 4), a white precipitate instantaneously appeared. This precipitate could not be redissolved in common organic solvents even after addition of excess ligand. It is believed to be the result of rhodium cross-linking between two dendrimers leading to insoluble oligomeric species. This phenomenon, which suggests unidentate coordination to Rh, has already been observed previously in the case of diphenylphosphine terminated dendrimers bound to [Rh(acac)(CO)2] but at lower phosphine/rhodium ratios (below 3 : 1).12 For G1-8SemiEsphos increasing this ratio to 6 : 1 (entry 5) or decreasing the precursor concentration (entry 6) does not prevent the formation of such insoluble material. The same conclusions can be drawn about G1-16SemiEsphos (entries 7 and 8) except that no precipitate is present when the rhodium concentration is reduced to 0.005 mol dm−3 (entry 9). Activity in vinyl acetate hydroformylation is observed, but the conversion and branched selectivity are quite poor (11% and b : l = 1.5). When changing from G1-16SemiEsphos to G1-24SemiEsphos at 0.01 mol dm−3 of [Rh(acac)(CO)2], no precipitate is visible after mixing and significant activity in the hydroformylation of vinyl acetate is observed (60% of vinyl acetate is converted with a branched to linear ratio of 11.6, entry 10). This positive behaviour in both conversion and especially branched selectivity compared with SemiEsphos, G1-8SemiEsphos and G1-16SemiEsphos suggests that in G1-24SemiEsphos two SemiEsphos molecules may be constrained by the macromolecular architecture to bind the rhodium in a bidentate manner and hence behave more like ESPHOS. However, we cannot be completely certain that the whole POSS molecule is needed for this effect. It would have been better to compare G1-24SemiEsphos with the small molecule analogue where the Si of the tris(SemiEsphos) substituent is bound to a methyl group rather than via the ethylidene group to the POSS core. Unfortunately, despite many attempts, we were unable to prepare this compound.
Decreasing the ligand to metal ratio to 3 : 1 (entry 11) slightly diminished the yield (51.5%) but enhanced the branched to linear ratio to 15.5, comparable to that obtained with ESPHOS. Since no ee was obtained in the reactions using G1-24SemiEsphos, this ligand does not behave exactly like ESPHOS, although we note that asymmetric catalysis using dendritic ligands is sometimes unsuccessful.13,14 It is possible that the excess of ligand in our case may be responsible for the low enantioselectivity observed, since Abboud et al. have noted that in hydroformylation reactions using Rh-TANGPHOS, excess ligand leads to a sharp decrease in enantioselectivity.15 Alternatively, the bridge between the two SemiEsphos portions is quite different in G1-24SemiEsphos from that in ESPHOS, so, although bidentate coordination may occur and allow reactivity, the chiral environment may be very different.
We finally compared the performance of G1-16SemiEsphos and G1-24SemiEsphos under the only catalytic conditions where G1-16SemiEsphos is active (entries 9 and 12). The behaviour is very similar, suggesting that at low rhodium concentration and high SemiEsphos : Rh ratio, bidentate binding might also occur for G1-16SemiEsphos.
In conclusion, we have shown that SemiEsphos on the periphery of POSS based compounds generally gives unidentate binding to rhodium so that polymeric complexes precipitate. If crowding at the periphery of the molecule is increased, these ligands can become effective in rhodium catalysed vinyl acetate hydroformylation, suggesting that two SemiEsphos molecules which give low activity may be constrained by the molecular architecture to become bidentate and at least partially emulate the active ESPHOS.
We would like to thank the EC through the Network of Excellence (IDECAT, No. NMP3-CT-2005-011730) for support (N. R. V.)
Notes and references
- D. A. Tomalia and J. M. J. Frechet, Dendrimers and Dendritic Polymers, Elsevier B. V., Amsterdam, The Netherlands, 2005 Search PubMed.
- N. J. Ronde and D. Vogt, in Catalyst Separation, Recovery and Recycling; Chemistry and Process Design, ed. D. J. Cole-Hamilton and R. P. Tooze, Springer, Dordrecht, The Netherlands, 2005 Search PubMed.
- X. Helms and J. M. J. Frechet, Adv. Synth. Catal., 2006, 348, 1125 CrossRef.
- L. Ropartz, R. E. Morris, D. F. Foster and D. J. Cole-Hamilton, Chem. Commun., 2001, 361 RSC.
- K. J. Haxton, D. J. Cole-Hamilton and R. E. Morris, Dalton Trans., 2004, 1665 RSC.
- K. J. Haxton, D. J. Cole-Hamilton and R. E. Morris, Dalton Trans., 2007, 3415 RSC.
- S. Breeden, D. J. Cole-Hamilton, D. F. Foster, G. J. Schwarz and M. Wills, Angew. Chem., Int. Ed., 2000, 39, 4106 CrossRef CAS.
- K. Drewelies and H. P. Latscha, Angew. Chem., 1982, 94, 642 CAS.
- P. A. Jaffres and R. E. Morris, J. Chem. Soc., Dalton Trans., 1998, 2767 RSC.
- C. W. Edwards, M. R. Shipton, N. W. Alcock, H. Clase and M. Wills, Tetrahedron, 2003, 59, 6473 CrossRef CAS.
- S. C. Bourque and H. Alper, J. Am. Chem. Soc., 2000, 122, 956 CrossRef CAS.
- L. Ropartz, D. F. Foster, R. E. Morris, A. M. Z. Slawin and D. J. Cole-Hamilton, J. Chem. Soc., Dalton Trans., 2002, 1997 RSC.
- J. Brunner, J. Organomet. Chem., 1995, 500, 39 CrossRef CAS.
- L. I. Rodriguez, O. Rossell, M. Seco and G. Muller, J. Organomet. Chem., 2007, 692, 851 CrossRef CAS.
- A. T. Axtell, J. Klosin and K. A. Abboud, Organometallics, 2006, 25, 5003 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation and characterization of G1-8SemiEsphos, G1-16SemiEsphos and G1-24SemiEsphos. See DOI: 10.1039/b814582b |
| This journal is © The Royal Society of Chemistry 2009 |
