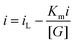Next generation screen printed electrochemical platforms: Non-enzymatic sensing of carbohydrates using copper(II) oxide screen printed electrodes
Nadeem A.
Choudhry
,
Dimitrios K.
Kampouris
,
Rashid O.
Kadara
,
Norman
Jenkinson
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Biology, Chemistry and Health Science, Division of Chemistry and Materials, Manchester Metropolitan University, Chester Street, Manchester, Lancs, UK M1 5GD. E-mail: c.banks@mmu.ac.uk
First published on 23rd October 2009
Abstract
The first example of a copper(II) oxide screen printed electrode is reported which is characterised with microscopy and explored towards the electrochemical sensing of glucose, maltose, sucrose and fructose. It is shown that the non-enzymatic electrochemical sensing of glucose with cyclic voltammetry and amperometry is possible with low micro-molar up to milli-molar glucose readily detectable which compares competitively with nano-catalyst modified electrodes. The sensing of glucose shows a modest selectivity over maltose and sucrose while fructose is not detectable. An additional benefit of this approach is that metal oxides with known oxidation states can be incorporated into the screen printed electrodes allowing one to identify exactly the origin of the observed electro-catalytic response which is difficult when utilising metal oxide modified electrodes formed via electro-deposition techniques which result in a mixture of metal oxides/oxidation states. These next generation screen printed electrochemical sensing platforms provide a simplification over previous copper oxide systems offering a novel fabrication route for the mass production of electro-catalytic sensors for analytical and forensic applications.
1. Introduction
The pursuit of non-enzymatic glucose sensing is a vigorous and competitive area of research. The development and fabrication of cost effective, simple, accurate, portable and rapid sensors for glucose are socially important aiding diabetics which represent ∼5% of the world population.1 Additionally glucose sensing has applications in medical and food industries, biochemistry and glucose-oxygen fuel cells.2,3 Related to this is the sensing of carbohydrates which finds widespread exploitation in analytical and forensic applications.4 For example, carbohydrate content of tobaccos can distinguish between varieties thus allowing, for example, to match an unknown tobacco found at a crime scene.4,5Chromatography coupled with electrochemical detection is widely used and reported for carbohydrate sensing,4 particularly in foodstuffs and determining food adulteration. The electrochemical oxidation of glucose is highly dependant on the chosen electrode and copper finds wide interest for the analysis of glucose, carbohydrates and amino acids.6,7 A range of electrode materials have been reported such as platinum, nickel, gold and silver for the sensing of carbohydrates but using these can be problematic due to poisoning/fouling of the electrode surface, especially at gold and platinum electrodes.8 Additionally the cost of these precious metals needs to be considered. In comparison, cobalt and copper electrodes are reported to provide enhanced stability, with low detection limits and wide analytical ranges achievable.9A range of advantageous approaches have been reported such as flower-shaped copper oxide nanostructures10 and nanospheres11 which are immobilised onto suitable electrode surfaces. In these cases and others where catalytic materials are simply immobilised onto an electrode surface, consideration needs to be given to surface stability. Other approaches have reported amperometric glucose biosensors based on the dispersion of glucose oxidase (GOx) and copper oxide within a graphite paste composite12 A variant on paste electrodes are screen printed electrodes. Screen printed electrodes are produced by spreading a thixotropic fluid evenly across a mesh screen which defines the geometry of the desired electrode. The thixotropic fluid or ink contains a variety of substances such as graphite, carbon black, solvents and polymeric binder. The mesh screen is a negative of the desired shape or electrode and various screens are used to build up the desired designs. Copper-plated screen printed electrodes have been reported for various analytes,13–15 and in particular Jumar and Zen have reported copper-plated screen-printed carbon electrodes for the amperometric detection of hydrogen peroxide where glucose oxidase was immobilized on to the copper layer.16
To the authors knowledge we report the first example of a copper oxide screen printed electrode where micron-sized copper(II) oxide is incorporated within the surface of the screen printed electrode. Such an electrode precludes the need for copper plating, greatly simplifying the electrode fabrication and provides a strategy for fabricating electrodes in large quantities. Copper(II) oxide screen printed electrochemical sensing platforms are explored for the non-enzymatic detection of carbohydrates, in particular, glucose sensing.
2. Experimental section
All chemicals used were of analytical grade and were used as received without any further purification from Sigma Aldrich. These were: copper(II) oxide powder (<5 μm, 98%), glucose, sucrose, maltose, fructose (all ACS reagents), potassium chloride (>99.0%), potassium phosphate monobasic (>99%) and sodium hydroxide (> 99%). All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm−1. A fresh solution of the chosen carbohydrate was prepared daily.Voltammetric measurements were carried out using a μ-Autolab III (Eco Chemie, The Netherlands) potentiostat/galvanostat and controlled by Autolab GPES software version 4.9 for Windows XP. All measurements were conducted using a three electrode configuration with a large surface area platinum wire as a counter and a saturated calomel electrode as the reference electrode. Connectors for the efficient coupling of the screen printed electrochemical sensors were purchased from Kanichi Research Services Ltd (http://kanichi-research.com/). In amperometric experiments, convection was applied via the use of a stirrer plate and a magnetic stirring bar rotating at 6000 rpm. Screen-printed carbon electrodes were fabricated in-house with appropriate stencil designs using a microDEK 1760RS screen printing machine (DEK, Weymouth, UK).
The surface topography was studied by surface profilometry (Dektak). The surface topography of each screen-printed electrode was measured by a Dektak ST stylus surface profilometer which has the capability of measuring step height down to a few nm. The Dektak is controlled by a PC running Windows with software offering several data processing functions as well as image capturing and storage.
Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-5600LV model.
3. Results and discussion
3.1 Characterisation of the copper oxide screen printed electrodes
We have reported previously the fabrication of screen printed electrodes17,18 and in this case, commercially purchased copper(II) oxide was mixed into the ink formulation prior to screen printing. This adversely affects the rheology of the ink which needs to be modified with organic solvents and the standard printing parameters that were previously used,17,18 need to be tailored to ensure an efficient printing process. Parameters that need to be monitored/tailored from adding in the copper(II) oxide are printing pressure, viscosity of the ink, dispersion of the metal oxide, time of drying and so on. These parameters depend largely on the ink formulation being utilised and need to be carefully considered. Increasing amounts of copper(II) oxide were incorporated into the screen printed electrodes over the range of 0–10% (MP/MI), where MP is the mass of particulate and MI is the mass of ink formulation used in the printing process.Fig. 1 depicts SEM images of the bespoke copper(II) oxide screen printed electrochemical sensing platform where a ‘webbed’ appearance is evident. Analysis of the electrode surfaces was explored with profilometric analysis where Ra values, which are the arithmetical mean surface roughness (in microns), were measured for the 2, 5 and 10% (MP/MI) copper(II) oxide screen printed electrode and found to correspond to 1.2, 1.8 and 2.5 respectively. In the absence of copper(II) oxide, the screen printed electrodes have a Ra value between 1–1.317 and clearly the introduction of the copper(II) oxide results in the electrode surface becoming more ‘coarse’ as the amount of copper(II) oxide is increased in the ink. Note that this surface roughness is critical since it defines the inherent reproducibility and electrochemical performance of the sensors. An additional benefit of using screen printing technology is that the oxidation state of the copper can be readily controlled and changed allowing users to easily identify exactly which oxidation states are responsible for their observed electrochemical response; this can sometimes be time-consuming and not straight forward when metals and their inherent oxides are produced in situvia electro-deposition.
 | ||
| Fig. 1 SEM images of a 5% copper oxide screen printed electrochemical sensing platform. | ||
It has been reported that copper (or copper oxide) electrodes provide superior and enhanced sensitivity for carbohydrates.19 In particular, Kanoet al. have shown for the electrochemical oxidation of glucose in sodium hydroxide, that copper(II) oxide modified electrodes are superior over copper(I) oxide modified electrodes; consequently we have focused on fabricating copper(II) oxide screen printed electrodes and exploring their use towards glucose and associated compounds.
3.2 Electro-catalytic sensing of carbohydrates
We turn to exploring the electrochemical sensing of carbohydrates using the copper(II) oxide screen printed electrodes, with Fig. 2A depicting the voltammetric profiles resulting from the addition of glucose where a quantifiable peak is observed at ∼ +0.6 V (vs.Ag/AgCl). Analysis of the peak height from glucose additions is shown in Fig. 2B where a linear response is observed over the range 100 μM to 500 μM, beyond which, the plot is observed to plateau. Based on this linear range (3 sigma) a limit of detection was found to correspond to 27 (±2) μM (IH/A = 0.08 A M−1 + 3.83 × 10−6 A; R2 = 0.992). The insert of Fig. 2A depicts the voltammetric response of the copper oxide screen printed electrode in sodium hydroxide only, which is likely due to the electrochemical oxidation of copper(II) to copper(III).20 The electrochemical performance of the copper oxide screen printed electrode was explored in pH 7 for glucose detection but no significant response was observed supporting the inference that the copper(III) oxide serves as a catalyst in the electrochemical oxidation.19 | ||
| Fig. 2 Cyclic voltammetric profiles (A) resulting from the addition of glucose into a 0.1 M NaOH solution using a 2% copper(II) oxide screen printed electrochemical sensing platform. The insert of (A) is the response of the copper(II) oxide screen printed electrode in 0.1 M NaOH. All scans recorded at 50 mVs−1vs.SCE. The analysis of the peak height versusglucose additions is shown in (B). | ||
Fig. 2B shows that at high glucose concentrations a Michaelis–Menten type response is observed. The catalytic reaction for the electrochemical oxidation of glucose may be described by the formation and decomposition of an intermediate charge transfer complex, similar to that of Michaelis–Menten kinetics:
| Cu(II)↔Cu(III) + e− |
where the apparent Michaelis–Menten constant,
 and the maximum current, iL = nFAK+2, the observed current, i, is expressed as21:
and the maximum current, iL = nFAK+2, the observed current, i, is expressed as21:The apparent Michaelis–Menten constant can be estimated using Lineweaver–Burke:
and Eadie–Hofstee transformations of the Michaelis–Menten equation:
where i is the observed current, iL is the maximum current observed at high glucose concentration, [G] is the concentration of glucose, and Km is the Michaelis–Menten rate constant. The average Km was estimated to be 0.33 mM. The low value indicates a good affinity of the copper oxide microdomains and the glucose substrate. This value is comparable to those previously reported, such as zinc oxide nanowires modified with glucose oxidase22 and with the reported value of 0.022 mM for a glassy carbon electrode modified with single walled carbon nanotubes, which in turn is modified with an ionic liquid, gold nanoparticles and glucose oxidase.23
The mechanism of the electrochemical oxidation of carbohydrates at copper oxide electrodes according to Xie and Huber24 involves the chemisorption of hydroxide ions on CuO surface lattices followed by oxidation of the hydroxide to a hydroxyl radical:
At adjacent lattice sites, the target analyte adsorbs onto the copper oxide surface:
The rate determining step involves the formation of a bridge cyclic intermediate and the abstraction of a hydrogen atom from the carbon in the alpha position to the functional group:
After abstraction, the analyte radical is rapidly oxidised to a carboxylate or other product24:
An alternative mechanism for the electrocatalytic oxidation of glucose at a copper oxide surface was later proposed by Kanoet al19 as:
3.3 Amperometric detection of carbohydrates
Next, attention was turned to exploring the amperometric determination of glucose using the copper(II) oxide screen printed electrodes. Fig. 3 depicts the response obtainable at a 2% copper(II) oxide screen printed electrode where a linear response was obtained over the range 0.5 mM to 6 mM (IH/A = 0.32 A M−1 + 3.3 × 10−5 A; R2 = 0.997). Clearly these approaches have potential for determining glucose levels in the range concerned with identifying hypoglycaemia when blood glucose levels fall below 3 mM. For lower levels of glucose, the 5% copper(II) oxide screen printed electrode was explored. Fig. 4 depicts the amperometric response obtained using a 5% copper(II) oxide screen printed electrode from glucose additions over the range 50 μM up to 1200 μM. Two linear portions are clearly evident. The first is from 50 to 600 μM (IH/A = 3.2 × 10−4 A M−1 + 3.3 × 10−8 A; R2 = 0.997) and the second from 650 to 1200 μM (IH/A = 2.4 × 10−4 A M−1 + 7.5 × 10−8 A; R2 = 0.998). Based on the first linear part, the limit of detection (based on three sigma) was found to correspond to 4 (±1) μM. This limit of detection is analytically competitive and compares to recent reports utilising dimethylglyoxime functionalized copper nanoparticles,25 flower-shaped copper oxide nanostructures,10CuO nanorod modified electrodes,26 and is superior over nickel hydroxide modified carbon ionic liquid electrodes.27 Clearly when microdomains of copper(II) oxide are utilised, they compare favourably to recent reports using nano-catalysts. | ||
| Fig. 3 Calibration plot resulting from amperometry using a 2% copper oxide screen printed electrochemical sensing platform resulting from additions of glucose into a 0.1 M NaOH solution. The potential was held at + 0.6 V (vs.SCE). | ||
 | ||
| Fig. 4 A typical amperometric response obtained at a 5% copper(II) oxide screen printed electrochemical sensing platform resulting from 50 μM additions of glucose into a 0.1 M NaOH solution. The potential was held at + 0.6 V (vs.SCE). Also shown is the analysis of the current from the glucose additions. | ||
The response of the 10% copper oxide screen printed electrode was observed to be highly un-reproducible with no stable response achievable. This is likely due to the reduction in the number of conductive pathways probably from ‘clumping’ of the copper(II) oxide in the ink forming large micron (and larger) sized particles. It is interesting to note that the 2% has only a slightly less analytical response in terms of sensitivity to low levels of glucose compared to the 5% screen printed electrode, but the 2% appears to be useful for extending the sensing range to higher glucose concentrations.
Next, we turn to exploring the copper(II) oxide screen printed electrode towards the sensing of other carbohydrates. Fig. 5A depicts the cyclic voltammetric profiles obtained at the copper oxide screen printed electrodes of the electrochemical oxidation of sucrose, maltose and fructose. No significant voltammetric response were observed for fructose while voltammetric profiles are observed at +0.99 V and +1.06 V for the maltose and sucrose (vs.SCE) respectively, as shown in Fig. 5A. Fig. 5B depicts the analysis from the amperometric measurements of glucose from holding the potential at +0.6 V. This was repeated for the case of sucrose and maltose which is found to have a reduced activity using the copper(II) oxide screen printed electrode compared to that observed for glucose. For maltose, a disaccharide of two α-1,4-limited glucose units, it has been shown that the number of electrons transferred during the oxidation is surface oxide dependant where ‘one unit’ is easy to oxidise but to increase the number of electrons, a significant amount of oxide required.20 While glucose is unaffected by the level of oxide, this suggests that disaccharides are sensitive to the micron sized copper(II) oxide domains. Based on this response the copper oxide domains allow a modest selective sensing of glucose over other carbohydrates.
 | ||
| Fig. 5 (A) Cyclic voltammetric profiles obtained using a 5% copper oxide screen printed electrochemical sensing platform recorded in 0.1 M NaOH solution containing 1 mM glucose, 1 mM fructose, 1 mM, maltose and 1 mM sucrose. The dotted line is the screen printed electrode in the absence of any carbohydrates. All scans recorded at 50 mVs−1. Part (B) compares the amperometric response obtained for glucose (circles), sucrose (triangles) and maltose (diamonds). The potential was held at + 0.6 V (vs.SCE). | ||
4. Conclusions
We have reported the first example of a copper(II) oxide screen printed electrode which has been characterised with microscopy and explored towards the non-enzymatic sensing of glucose. This next generation screen printed electrochemical sensing platform provides a simplification over previous copper oxide systems and provides a novel fabrication route for producing inexpensive, sensitive sensors which can be readily mass-produced. In comparison to other analytical systems, the screen printed electrodes which contain micron-sized copper(II) oxide particles, compares favourably with electrodes modified with nano-catalysts allowing low micro-molar up to milli-molar glucose to be readily detected. Additionally, we envisage that this electrode can be used in conjugation with chromatography precluding the need for electrode polishing/re-generation between measurements.This approach is beneficial over systems where the electro-deposition of copper is undertaken as this can result in differing copper oxidation states and the underlying catalytic mechanism may not be easily de-convoluted. In contrast, screen printed electrodes can be readily fabricated with copper(II) oxide and copper(I) oxide and explored with the analytical target to fully understand the underlying (electro)chemical processes.
We note that copper oxide finds use in other areas such as supercapacitors,28cyclohexanol oxidation,29lithium-ion batteries,30nitrite,31amikacin32 and sulfite detection33 and we expect that our copper oxide screen printed electrochemical sensing platforms can be beneficially utilised in such areas.
5. References
- A. Heller and B. Feldman, Chem. Rev., 2008, 108, 2482 CrossRef CAS.
- J. Lin, C. He, Y. Zhao and S. Zhang, Sens. Actuators, B, 2009, 137, 768 CrossRef.
- M. Tominaga, Y. Taema and I. Tangiuchi, J. Electroanal. Chem., 2008, 624, 1 CrossRef CAS.
- T. J. O'Shea, S. M. Lunte and W. R. LaCourse, Anal. Chem., 1993, 65, 948 CrossRef.
- C. M. Zook, P. M. Patel, W. R. LaCourse and S. Ralapati, J. Agric. Food Chem., 1996, 44, 1773 CrossRef CAS.
- P. Luo, F. Zhang and R. P. Baldwin, Anal. Chim. Acta, 1991, 244, 169 CrossRef CAS.
- L. Nagy, G. Nagy and P. Hajos, Sens. Actuators, B, 2001, 76, 494 CrossRef.
- W. R. LaCourse, in Advances in Chromatrography, 2009, vol. 47, pp. 247–281 Search PubMed.
- K. B. Male, S. Hrapovic, Y. Liu, D. Wang and J. H. T. Luong, Anal. Chim. Acta, 2004, 516, 35 CrossRef.
- A. Umar, M. M. Rahman, A. Al-Hajry and Y. B. Hahn, Electrochem. Commun., 2009, 11, 278 CrossRef CAS.
- E. Reitz, W. Z. Jia, M. Gentile, Y. Wang and Y. Lei, Electroanalysis, 2008, 20, 2482 CrossRef CAS.
- G. L. Luque, M. C. Rodríguez and G. A. Rivas, Talanta, 2005, 66, 467 CrossRef CAS.
- J. M. Zen, Y. S. Song, H. H. Chung, C. T. Hsu and A. S. Kumar, Anal. Chem., 2002, 74, 6126 CrossRef CAS.
- J. M. Zen, H. H. Chung and A. S. Kumar, Analyst, 2000, 125, 1633 RSC.
- D. M. Tsai, P. R. Shih, H. W. Tai, C. Y. Liu and J. M. Zen, J. Chinese Chem. Soc., 2005, 52 Search PubMed.
- A. S. Kumar and J. M. Zen, Electroanalysis, 2002, 14, 671 CrossRef CAS.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Sens. Actuators, B, 2009, 138, 556 CrossRef.
- R. O. Kadara, N. Jenkinson, B. Li, K. H. Church and C. E. Banks, Electrochem. Commun., 2008, 10, 1517 CrossRef CAS.
- K. Kano, M. Torimura, Y. Esaka, M. Goto and T. Ueda, J. Electroanal. Chem., 1994, 372, 137 CrossRef CAS.
- N. Torto, T. Ruzgas and L. Gorton, J. Electroanal. Chem., 1999, 464, 252 CrossRef CAS.
- H. Ju and D. Leech, Anal. Chim. Acta, 1997, 345, 51 CrossRef CAS.
- J. Zang, C. M. Li, X. Cui, J. Wang, X. Sun, H. Dong and C. Q. Sun, Electroanalysis, 2007, 19, 1008 CrossRef CAS.
- R. Gao and J. Zheng, Electrochem. Commun., 2009, 11, 608 CrossRef CAS.
- Y. Xie and C. O. Huber, Anal. Chem., 1991, 63, 1714 CrossRef CAS.
- Q. Xu, Y. Zhao, J. Zhong and J.-J. Zhu, Sens. Actuators, B, 2006, 114, 379 CrossRef.
- C. Batchelor-McAuley, Y. Du, G. G. Wildgoose and R. G. Compton, Sens. Actuators, B, 2008, 135, 230 CrossRef.
- A. Safavi, N. Maleki and E. Farjami, Biosens. Bioelectron., 2008, 24, 1655.
- V. D. Patake, S. S. Joshi, C. D. Lokhande and O. S. Joo, Mater. Chem. Phys., 2009, 114, 6 CrossRef CAS.
- M. Hasanzadeh, G. Karim-Nezhad, M. G. Mahjani, M. Jafarian, N. Shadjou, B. Khalilzadeh and L. A. Saghatforoush, Catal. Commun., 2008, 10, 295 CrossRef CAS.
- S. Y. Gao, S. X. Yang, J. Shu, S. Z. Zhang, Z. D. Li and K. Jiang, J. Phys. Chem. C, 2008, 112, 19324 CrossRef CAS.
- B. Sljukic, C. E. Banks, A. Crossley and R. G. Compton, Electroanalysis, 2007, 19, 79 CrossRef CAS.
- J. Z. Xu, J. J. Zhu, H. Wang and H. Y. Chen, Anal. Lett., 2003, 36, 2723 CrossRef CAS.
- M. Kiattipoomchai, M. Somasundrum, M. Tanticharoen and K. Kirtikara, Analyst, 1998, 123, 2017 RSC.
| This journal is © The Royal Society of Chemistry 2009 |