A microfluidic technique for monitoring bloodstream analytes indicative of C-peptide resistance in type 2 diabetes
Teresa
D'Amico Oblak
,
Jennifer A.
Meyer
and
Dana M.
Spence
*
Michigan State University, Department of Chemistry, East Lansing, Michigan, USA. E-mail: dspence@chemistry.msu.edu
First published on 27th November 2008
Abstract
A simple poly(dimethylsiloxane) (PDMS) microchip was employed to establish a relationship between red blood cell (RBC) antioxidant status and the ability of RBCs to interact with metal-activated C-peptide, a bio-active peptide reported to reduce some complications often associated with diabetes. It is known that the reduced form of glutathione (GSH) levels in the RBCs obtained from people with type 2 diabetes are lower in comparison to those RBCs obtained from healthy controls and accordingly, this correlation has the potential to implicate type 2 diabetes in high-risk individuals. A parallel channel microfluidic device for the quantification of GSH in age-based fractions, along with control and diabetic RBCs is described. Important to the fluorescence-based measurement is the simultaneous determination of the antioxidant without prior separation in either a six- or twelve-channel microchip. Here, we separated the RBCs using a density-based Percoll solution and quantitatively determined the concentration of GSH in younger, less dense RBCs to be increased more than 2-fold (336.7 ± 29.6 amol/RBC) than older, more dense RBCs (137.0 ± 25.3 amol/RBC). The ability of C-peptide to interact with the RBC membrane of the separated fractions was determined by immunoassay and it was found that the recovery of the C-peptide added to the younger RBCs increased by more than 40.6 ± 12.7% above basal levels while with the older cells C-peptide increased by only 9.18 ± 4.60%. These results suggest that GSH concentrations in the RBC may be useful in screening for resistance to C-peptidein vivo.
Introduction
The linear tripeptideL-γ-glutamyl-L-cysteinyl-glycine or glutathione (GSH) is the most abundant non-protein thiol found in the body.1GSH, a strong reducing agent, is often recognized as the major intracellular antioxidant defense within cells. Accordingly, GSH has the ability to scavenge reactive oxygen species (ROS) and reactive nitrogen species (RNS). Upon oxidation, GSH forms the thiolate anion (GS−) and two GS− species can further react to form oxidized glutathione (GSSG).There are established correlations between the concentration of the antioxidant, GSH, and a variety of disease states including HIV,2 cancer,3–5 and diabetes.6–8 Physiological concentrations of GSH are maintained by the activity of glutathione reductase (GR), an enzyme that catalyzes the conversion of the oxidized dimer, GSSG, to its reduced form, GSH. This process requires the co-factor, nicotinamide adenine dinucleotide phosphate (NADPH), a substrate produced during the pentose phosphate pathway. In red blood cells (RBCs), the availability of NADPH is solely dependent on the enzyme glucose-6-phosphate dehydrogenase (G6PD).
It is known that the RBCs obtained from people with type 2 diabetes have lower concentrations of GSH. This may be due, in part, to a decrease in the activity of GR found in the RBCs of people with diabetes.9,10 Accordingly, the decreased concentration of GSH in diabetic RBCs may not be the result of lesser amounts of the peptide, but rather the impairment of GR to successfully recycle GSSG to its reduced state. GSH concentrations have also been correlated to another relevant RBC substrate, adenosine triphosphate (ATP). Released by the RBC upon mechanical deformation, ATP is known to stimulate nitric oxide (NO) production in endothelial cells.11 We have previously reported a relationship between RBC-mediated ATP release and GSH concentrations in erythrocytes subjected to oxidative stress.11 Importantly, RBCs obtained from people with type 2 diabetes are known to be less deformable than those RBCs obtained from non-diabetic controls.6,8,11 This decrease in deformability is thought to be due to oxidative damage to the RBC membrane protein, spectrin.
Antioxidants, such as reduced GSH, protect spectrin from excessive oxidative stress. GSH concentrations are ultimately dependent on NADPH and the activity of G6PD, an activity that has been correlated to cell aging.12 Recently, a decrease in NADPH levels has been reported in older RBC fractions as compared to those of younger RBCs isolated from whole blood obtained from healthy rabbits.13
In addition to its role of protecting important RBC proteins, we suspect that GSH may also have a relationship to other RBC properties. Specifically, work recently published by Meyer et al. reported a 4-fold increase in ATP release from diabetic RBCs when incubated in a buffered solution that contained metal-activated C-peptide.14C-peptide is created in the pancreas during insulin production as proinsulin C-peptide. Initially, this 31 amino acid peptide was thought to have minimal biological activity,15 although C-peptide has been reported to reduce complications associated with diabetes in rat models.16–18C-peptide has also been shown to improve the deformability of RBCs19 and increase endothelial nitric oxide synthase (eNOS) activity.20
Inspired by these previous results involving C-peptide, we hypothesize a relationship between the antioxidant status of the RBC and its ability to interact with C-peptide. To investigate this hypothesis, we report on the development of a simple, parallel microfluidic device capable of quantitatively determining the concentration of GSH in RBC samples. The device was used to determine the concentration of GSH in density-separated RBCs and RBCs obtained from rat models of type 2 diabetes and controls. In order to establish a relationship between antioxidant status and C-peptide in RBCs, an immunoassay for C-peptide was performed (off-chip) to determine the ability of younger and older cells, as well as RBCs obtained from the rat subjects, to interact with metal-activated C-peptide. These results were then compared to determine if a correlation between the oxidative status of the cell and the ability of the RBCs to interact with metal-activated C-peptide exists. Such a relationship potentially provides evidence that RBC GSH levels may be a bloodstream-based biomarker indicative of C-peptide resistance in type 2 diabetes.
Experimental
Generation of washed red blood cells
To obtain rabbit RBCs, male New Zealand White rabbits (2.0–2.5 kg) were anesthetized with ketamine (8.0 mg/kg) and xylazine (1.0 mg/kg) followed by pentobarbital sodium (15 mg/kg iv). After tracheotomy, the rabbits were mechanically ventilated (tidal volume 20 mL/kg, rate 20 breaths/min; Harvard ventilator). A catheter was placed into a carotid artery, heparin (500 units, iv) was administered, and after 10 min, animals were exsanguinated. Rat RBCs were collected by cardiac puncture after the animals had been anesthetized with isoflurane. Blood was collected into vials (heparinized in the case of the rat blood), and the RBCs were separated from other formed elements in plasma by centrifugation at 500 × g at 4 °C for 10 min. The supernatant and buffy coat were removed by aspiration. Packed RBCs were resuspended and washed three times in PSS {in mM; 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 21.0 tris(hydroxymethyl)aminomethane, and 11.1 dextrose with 5% bovine serum albumin, pH adjusted to 7.4}. Cells were prepared on the day of use and experiments were finished within 8 h of removal from the animals. All procedures were approved by the Animal Investigation Committee.Type 2 diabetic rat models were obtained from Wayne State University and washed in PSS as described above. Each rat had an average weight of 700 g and sacrificed approximately 130 days after diabetic onset with a blood glucose average of 300 mg/dL.
Preparation of the microfluidic device
The device shown in Fig. 1 was used for all GSH studies. PDMS channel structures were produced following previously published methods.21 Masters for the production of PDMS microchannels were made by coating a 4″ silicon wafer (Silicon, Inc., Boise, ID) with SU-8 10 negative photoresist (MicroChem Corp., Newton, MA) using a spin coater (Brewer Science, Rolla, MO) operating with a spin program of 2000 rpm for 20 s. The photoresist was prebaked at 95 °C for 5 min prior to UV exposure with a near-UV flood source (Autoflood 1000, Optical Associates, San Jose, CA) through a negative film (2400 dpi, PageWorks, Cambridge, MA), which contained the desired channel structures. All channel structures were drawn in Freehand (PC version 10.0, Macromedia, Inc. San Francisco, CA). Following this exposure, the wafer was postbaked at 95 °C for 5 min and developed in Nano SU-8 developer (Microchem Corp.). The photoresist thickness was measured with a profilometer (Alpha Step-200, Tencor Instruments, Mountain View, CA), corresponding to the channel depth of the PDMS structures.![(a) Cross-section of the microfluidic device employed for RBC GSH determination, where each channel has dimensions of 200 µm width by 100 µm depth; (b) example of a microchip setup for generation of a GSH standard curve: B = blank, S1–S5 = RBC samples spiked with increasing concentrations of GSH stock; and (c) a sample standard curve to determine GSH concentration in a RBC sample. The concentration is calculated using the equation [GSH] = (bCS)/(mVX), where b is the y-intercept, CS represents the concentration of the stock GSH solution spiked into the sample, m is the slope, and VX is the volume of RBCs added to the sample. A blank sample was subtracted from each standard to account for background present in each measurement.](/image/article/2009/AN/b816740k/b816740k-f1.gif) | ||
| Fig. 1 (a) Cross-section of the microfluidic device employed for RBC GSH determination, where each channel has dimensions of 200 µm width by 100 µm depth; (b) example of a microchip setup for generation of a GSH standard curve: B = blank, S1–S5 = RBC samples spiked with increasing concentrations of GSH stock; and (c) a sample standard curve to determine GSH concentration in a RBC sample. The concentration is calculated using the equation [GSH] = (bCS)/(mVX), where b is the y-intercept, CS represents the concentration of the stock GSH solution spiked into the sample, m is the slope, and VX is the volume of RBCs added to the sample. A blank sample was subtracted from each standard to account for background present in each measurement. | ||
A 10 : 1 mixture of Sylgard 184 elastomer and curing agent (Ellsworth Adhesives, Germantown, WI) was used. This degassed mixture was poured onto the silicon wafer and cured at 75 °C for approximately 10 minutes. Another mixture, 5 : 1, was prepared and poured onto the 10 : 1 mixture and baked for an additional 7 min. After this time, the thermally sealed PDMS layers were removed from the master and inlet holes were punctured (using a 20 gauge luer stub adapter) through the chip as well as 1/8″ exit holes. The chip was reversibly sealed to a glass plate to alleviate the PDMS from adhering to the microscopy stage. A chip containing channels of 100 µm depth × 200 µm width × 2 cm length was used for all studies reported here. The channel depth corresponds to the height of the master, which was measured with the aforementioned profilometer.
Fluorescence determination of GSH using standard addition
The standard addition method was employed to perform quantitative determinations for GSH. The standards were prepared by combining 100 µL of a 1.0% hematocrit of lysed RBCs and varying amounts (0, 50, 100, 150, and 200 µL) of a 0.1 mM stock solution of GSH in separate vials. The GSH stock was prepared by dissolving 0.0307 g of GSH (Sigma Aldrich, St. Louis, MO) in phosphate buffer solution (PBS without Ca or Mg). The reaction mixtures were prepared to a final volume of 1.0 mL using varying volumes of distilled/deionized water. Also added to the mixture to aid in the labeling of GSH was 50 µL of glutathione transferase (GST). A GST solution was prepared by dissolving 1 mg of GST (Sigma Aldrich, St. Louis, MO) in 1 mL of PBS (approximately 50 units mL−1). A volume of 20 µL of 2.5 mM monochlorobimane (MCB) was added simultaneously with GST. MCB stock (10 mM) was prepared by dissolving 0.0227 g of MCB (Molecular Probes, Carlsbad, CA) into 1 mL of DMSO, followed by the addition of 9 mL PBS. After addition of the MCB/GST to the mixture, the solution was added to a gas-tight syringe and hydrodynamically pumped through the microchannel at 1.00 µL min−1. For the 12 channel analysis, 6 dual displacement syringe pumps (Harvard Apparatus, Holliston, MA) were loaded with syringes (2 syringes/pump) and started simultaneously to deliver the appropriate solutions to each channel (1 solution/channel). At t = 27 min, each syringe pump was stopped and a measurement was taken at t = 30 min on an Olympus MVX1 microscope (Olympus America, Melville, NY) with an electrothermally cooled CCD camera (Orca, Hamamatsu) and Microsuite Software (Olympus America). The microscope incorporated a blue-green fluorescent protein (BGFP) filter cube encompassing the excitation (370 nm) and emission (478 nm) wavelengths.Separation of red blood cells into age-based fractions
RBCs were separated into fractions based on cell density centrifugation using Percoll (Sigma Chemical Co, St. Louis, MO). Percoll consists of colloidal silica particles of 15–30 nm diameter (23% (w/w) in water) that are coated with poly(vinylpyrrolidone) (PVP). The PVP coating results in a completely non-toxic product ideal for use with biological materials. In a 15 mL tube, 2 mL of a high-density Percoll solution were added to the bottom of the tube. Next, 2 mL of a low-density Percoll solution were added slowly on top of the first 2 mL layer. Finally, 1 mL of RBCs (70% hematocrit) was added on top of the Percoll layers. The tube containing the Percoll and RBCs was centrifuged at 3000 × g at 25 °C for 15 min. After centrifugation, two layers of RBCs are present; those having a density of less than the top Percoll solution will appear at the top of the tube and represent those RBCs having the lowest density; these RBCs are considered the younger of the RBCs in the original RBC sample.22,23 Those below the higher density Percoll solution are the more dense, or aged, RBCs. These separated RBC fractions were removed by pipette and the hematocrit of each fraction was measured using a hematocrit centrifuge. All RBC samples were then diluted to the same hematocrit prior to analysis.Reagent preparation
Human C-peptide (American Peptide, Sunnyvale, CA), 0.25 mg (molecular mass 3020), was dissolved into 100 mL purified water (18.2 MΩ) to yield a concentration of 83 µmol/L. A solution of zinc chloride was prepared in purified water. The metal solution was then added in equimolar amounts to the C-peptide solution through a series of dilutions. Before adding to the RBCs, the metal–C-peptide mixture was diluted in PSS to avoid any cell lysis that may occur upon direct contact of RBCs with a non-buffered aqueous solution.C-peptide immunoassay
C-peptide and Zn2+ were added in equimolar amounts (20 nM) to PSS, followed by the addition of old or young RBCs. The resulting RBC suspension was at a 7% hematocrit. The suspensions incubated at room temperature for 2 hours. After incubation, the suspensions were centrifuged and washed two times at 500 × g for 10 minutes to remove any excess physiological salt solution and C-peptide/Zn2+. The packed RBCs were added to an appropriate volume of deionized water to lyse the RBCs. From this lysate, 150 µL were added to the wells of a C-peptide ELISA kit (Millipore, Billerica, MA, USA). The protocol provided in the kit was followed with modifications. The plate was read at 450 nm using a multi-detection microplate reader (Spectramax M5, Molecular Devices, Sunnyvale, CA, USA).Results
It has been established that ATP release is correlated to the antioxidant status of RBCs, often indicated by concentrations of GSH and NADPH.11 It is also known that aged erythroctyes are more subjected to oxidative stress due to the lack of G6PD12 and decreased NADPH concentrations within the cell, similar to that observed in people diagnosed with type 2 diabetes.13 To date, there has been no attempt to correlate GSH concentrations in RBCs with these cells' ability to interact with C-peptide. Establishing a GSH : C-peptide ratio may be a useful biomarker, as it could be indicative of a prediabetic stage.The method of simultaneous standard additions was employed on a parallel, six channel microfluidic device to determine the concentration of GSH in a RBC sample and a sample standard curve is shown in Fig. 1. The use of standard addition for a complex sample matrix such as RBCs removes the need for a separation step or sample pre-treatment that is required with other GSH determinations that do not utilize standard addition. Increasing volumes of a known GSH concentration were spiked into 1% lysed RBC samples to generate standards for the fluorescence analysis. A blank sample was used to subtract the background of the fluoroprobe, MCB, from each sample. The samples were incubated with MCB/GST and hydrodynamically pumped through the microfluidic channels simultaneously for 27 min before acquiring the fluorescence images (λex 370 nm, λem 478 nm) at t = 30 min. The GSH concentration in RBCs can be determined from the y-intercept (b) multiplied by the concentration of the GSH stock (CS) divided by the product of the slope (m) and the volume of RBCs added to each standard (VRBC). The GSH concentration in RBCs was determined to be 377.0 ± 23.1 amol/RBC (n = 7) and this value is in statistical agreement with previous reports.24
In an extension of the device, the GSH concentration of density-separated RBCs was also determined simultaneously on a twelve channel microdevice. Percoll solutions were used to separate the RBCs into two fractions, the ‘younger’, less dense layer and the ‘older’, more dense layer (Fig. 2a). Again, increasing volumes of a known GSH concentration were spiked into 1% young and 1% old RBC solutions followed by a 27 min incubation and pumping through the microchannel. As illustrated in Fig. 2b there is an approximate 2.5-fold increase in the GSH concentration of the young fraction (337 ± 29.6 amol/RBC) as compared to the old fraction (137.0 ± 25.3 amol/RBC) (n = 7). This decrease in the older cells is anticipated since it is known that NADPH concentrations are lower in chemically aged and old fractions of density-separated RBCs.13
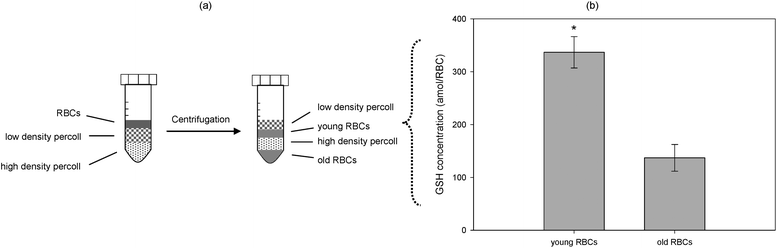 | ||
| Fig. 2 (a) Preparation of young and old RBC fractions using Percoll density solutions. A 2 mL solution of high-density Percoll is pipetted into the bottom of centrifuge tube followed by the dropwise application of a 2 mL low-density Percoll solution. A (70% hematocrit) RBC solution of 1.0 mL is carefully added on top of the density solutions and centrifuged at 3000 × g for 15 min. This process separates the solution into two distinct RBC bands representing young (top) and old (bottom) erythrocytes, and (b) the simultaneous quantification of GSH in Percoll density-separated RBC fractions, young and old, in a twelve channel microfluidic device (n = 7) employing standard addition. Error bars represent the standard error of the mean (SEM), while the asterisk indicates statistical significance of <0.001. | ||
Recently, we reported that C-peptide has the ability to restore decreased levels of ATP release from the RBCs of diabetic individuals to levels of healthy controls.14 The level of ATP release is dependent on the oxidative status of the RBC; therefore, an attempt was made to establish a relationship between GSH concentrations and the ability of younger and older erythrocytes to bind to exogenously-added C-peptide. Collectively, these previous results involving RBC antioxidant levels and C-peptide suggest a possible relationship between cellular GSH concentrations and C-peptide interaction with the cell membrane. To investigate this possible relationship, C-peptide was added exogenously to younger and older fractions of RBCs (70% hematocrit). A second aliquot of younger and older cells (70% hematocrit) was incubated with Zn2+-activated C-peptide. After a 2 h incubation, the RBCs were subjected to immunoassay to determine the increase in C-peptide bound to the RBCs relative to baseline levels in the cells (Fig. 3a). As shown in Fig. 3b, the younger RBCs had an increase of Zn2+-activated C-peptide of 40.6 ± 12.7%, a statistically significant difference in comparison to the older RBCs, which had an increase of only 9.2 ± 4.6% (n = 4, p < 0.001). In addition, similar to previous results, there was no increase in C-peptide concentration to either RBC fraction when the peptide was incubated in the absence of Zn2+.
 | ||
| Fig. 3 (a) The ELISA assay begins by pipetting 150 mL of young or old RBC fractions onto the ELISA plate and incubating for 1 h during which time the available C-peptide within the sample will bind to the anti-C-peptide antibody on the ELISA plate. The RBC solution is removed and a secondary antibody is added to the plate, incubated for an additional 1 h to conjugate, and the corresponding absorbance is measured and (b) the ability of density-separated fractions, young and old, to interact with C-peptide, a bio-active peptide known to relieve diabetic complications (n = 4). Young and old RBC solutions (70% hematocrit, respectively) were analyzed by an ELISA to monitor the concentration of C-peptide within the erythrocyte. Also analyzed by ELISA were solutions of young and old RBCs (70% hematocrit) incubated with physiologically relevant metal-activated C-peptide for 2 h. Error bars represent the SEM, while the asterisk indicates statistical significance of <0.001. | ||
Finally, the parallel, twelve channel microchip described above was employed to quantitatively determine the GSH concentrations in control and diabetic samples, simultaneously. The data in Fig. 4 illustrate the concentrations quantified from control (n = 3) and diabetic (n = 7) samples. The control RBC samples (319.7 ± 9.5 amol/RBC) have approximately 81% more GSH available than those of the RBC samples obtained from the diabetic rats (176.5 ± 27.4 amol/RBC). These results support previous work indicating that oxidant stress in the RBC coincides with lower concentrations of available GSH and subsequent ATP release.11 Interestingly, the concentration of GSH quantified from diabetic samples is not statistically different than the concentration derived from the older, more dense fraction of RBCs (p < 0.001) and the same is true for the control as compared to the young fraction (p < 0.001). This might suggest that density-separated RBCs, albeit with more analysis, could potentially be utilized as models for representing control and diabetic erythrocyte samples.
 | ||
| Fig. 4 The quantitative determination of GSH concentrations in diabetic (n = 7) and control (n = 3) RBC samples (1% hematocrit) utilizing a twelve channel microchip and standard addition. Error bars represent the SEM, while the asterisk indicates statistical significance of <0.001. | ||
Conclusions
Diabetes is often signified by a lack of insulin production or a resistance to available insulin. The role of insulin is to facilitate glucose transport across cell membranes where it is consumed for energy or stored by the cell. An excess of sugar in the bloodstream as a result of improper glucose transport can result in hyperglycaemia and other complications including retinopathy, renal failure, and cardiovascular disease. C-peptide is created in the pancreas during insulin production as proinsulin C-peptide and has been shown to alleviate complications associated with diabetes.16–18The work presented here establishes a relationship between GSH, a known antioxidant and the most abundant non-thiol found in RBCs, and the ability of erythrocytes to interact with metal-activated C-peptide. By employing standard addition, the need to separate the complex RBC matrix is removed as reported elsewhere.24 However, the application of this method to a microfluidic device enables a complete standard addition curve to be constructed simultaneously under a fluorescence microscope. The age-based fractions were also subjected to an ELISA examination to test their capacity to interact with C-peptide in a 2 h period. Importantly, the young RBCs, with high levels of available GSH, appear to interact nearly 3-fold more C-peptide than those of the older fraction. Importantly, this suggests that the antioxidant status of the RBC can affect the interaction of C-peptide. However, it also could mean that the older RBCs cleave C-peptide upon interaction, especially considering recent work showing that C-peptide can be internalized into cell membranes.25 Though further studies will need to be performed in this area, control experiments performed in our group also demonstrated that the levels of C-peptide in the supernatant of the older RBC fractions were 36% higher than those levels in the supernatant of the younger fractions.
Another significant finding is the correlation of GSH concentration between the age-based RBCs compared to that of control and diabetic individuals. While it would not be scientifically appropriate to claim that an ‘older’ RBC is physiologically identical to a type 2 diabetic RBC, further studies will be performed in the near future to determine if certain aspects of the aged RBC can be employed as a model of the diabetic RBC.
Such a device, like the one described above, is important when considering the current methods approved for diagnosing diabetes. The standard technique accepted is the fasting plasma glucose (FPG) analysis, which requires 8 h of fasting followed by a blood test. However, the test is not always reliable and can give a different result for the same individual depending on the time of day the test is administered. Often, hemoglobin A1c (HbA1c) is used in conjunction with the FPG test; however, it is not an approved method for initial diagnosis. Owing to the differences in GSH levels and C-peptide binding to the RBC, the data shown here may be of use in future high-throughput screening for diabetic-onset. Moreover, both of the analytes determined in this work (GSH and C-peptide) are in the bloodstream. The ability to measure both without extensive sample preparation would represent a simple diagnostic tool for the clinical setting. The use of the device will also enable flow-based phenomena (such as shear-induced release of ATP from the RBC) to be incorporated in a ‘microtitre-plate’ design.
Acknowledgements
This work was supported by the National Institutes of Health (DK 001788).References
- A. Meister and M. E. Anderson, Annu. Rev. Biochem., 1984, 711–760
.
- M. Repetto, C. Reides, M. L. Gomez Carretero, M. Costa, G. Griemberg and S. Llesuy, Clin. Chim. Acta, 1996, 255, 107–117 CrossRef CAS
.
- J. Gromadzinska, W. Wasowicz, M. Andrijewski, M. Sklodowska, O. Z. Quispe, P. Wolkanin, B. Olborski and A. Pluzanska, Neoplasma, 1997, 44, 45–51 Search PubMed
.
- S. Upadhya, S. K. Mohan, K. Vanajakshamma, M. Kunder and S. Mathias, Indian Clin. Biochem., 2004, 19, 80–83 Search PubMed
.
- R. Subapriya, R. Kumaraguruparan, C. R. Ramachandran and S. Nagini, Clin. Biochem., 2002, 35, 489–493 CrossRef CAS
.
- C. Costagliola, Clin. Physiol. Biochem., 1991, 8, 204–210 Search PubMed
.
- J. Aaseth and G. Stoa-Birketvedt, J. Trace Elem. Exp. Med., 2000, 13, 105–111 CrossRef CAS
.
- K. M. Beard, N. Shangari, B. Wu and P. J. O'Brien, Mol. Cell. Biochem., 2003, 252, 331–338 CrossRef CAS
.
- H. Freeman, K. Shimomura, R. D. Cox and F. M. Ashcroft, Biochem. Soc. T., 2006, 34, 806–810 Search PubMed
.
- H. Freeman, K. Shimomura, E. Horner, R. D. Cox and F. M. Ashcroft, Cell Metab., 2006, 3, 35–45 CrossRef CAS
.
- J. Carroll, M. Raththagala, W. Subasinghe, S. Baguzis, T. D. A. Oblak, P. Root and D. Spence, Mol. BioSyst., 2006, 2, 305–311 RSC
.
- G. G. De Schepper, C. J. F. Van Noorden and J. M. Houtkooper, Histochem. J., 1987, 19, 467 CrossRef CAS
.
- W. Subasinghe and D. M. Spence, Anal. Chim. Acta, 2008, 618, 227–233 CrossRef CAS
.
- J. A. Meyer, J. M. Froelich, G. E. Reid, W. K. A. Karunarathne and D. M. Spence, Diabetologia, 2008, 51, 175–182 CAS
.
- T. Kunt, T. Forst, A. Pfutzner, J. Beyer and J. Wahren, Pathophysiology, 1999, 5, 257–262 CrossRef CAS
.
- B. L. Johansson, S. Sjoberg and J. Wahren, Diabetologia, 1992, 35, 121–128 CrossRef CAS
.
- B. L. Johansson, A. Kernell, S. Sjoberg and J. Wahren, J. Clin. Endocrincol. Metab., 1993, 77, 976–981 Search PubMed
.
- Y. Ido, A. Vindigni and K. Chang, Science, 1997, 277, 563–566 CrossRef CAS
.
- T. Kunt, S. Schneider and A. Pfutzner, Diabetologia, 1999, 42, 465–471 CrossRef CAS
.
- T. Wallerath, T. Kunt and T. Forst, Nitric Oxide, 2003, 9, 95–102 CrossRef CAS
.
- D. Duffy, J. C. McDonald, O. J. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 4974–4984 CrossRef CAS
.
- A. Risso, M. Turello, F. Biffoni and G. Antonutto, Blood Cell. Mol. Dis., 2007, 38, 83 CrossRef CAS
.
- F. Omodeo-Sale, A. Motti, N. Basilico, S. Parapini, P. Olliaro and D. Taramelli, Blood, 2003, 102, 705 CrossRef CAS
.
- M. Raththagala, P. D. Root and D. M. Spence, Anal. Chem., 2006, 78, 8556–8560 CrossRef CAS
.
- E. Lindhal, U. Nyman, E. Melles, K. Sigmundsson, M. Stahlberg, J. Wahren, B. Obrink, J. Shafqat, B. Joseph and H. Jornvall, Cell. Mol. Life Sci., 2007, 64, 479–486 CrossRef
.
| This journal is © The Royal Society of Chemistry 2009 |
