DOI:
10.1039/B816230A
(Paper)
Analyst, 2009,
134, 176-181
Received
16th September 2008
, Accepted 4th November 2008
First published on
25th November 2008
Abstract
In this paper, we have constructed a low temperature plasma (LTP) probe using dielectric barrier discharge (DBD) and employed it for the detection of explosives on a variety of substrates under ambient conditions. Upon discharge, a transient, low-temperature non-equilibrium plasma comprising ions, electrons and metastable atoms are generated between the electrodes. Three common explosives, 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-trinitro-1,3,5-triazine (RDX), and pentaerythritol tetranitrate (PETN), were directly desorbed and ionized from solid surfaces, followed by subsequent analysis using the mass spectrometer in the negative ion mode. Limits of detection (LODs) were 500 fg for TNT, 1 pg for RDX, and 500 fg for PETN. The reliability of the method was characterized by a successful analysis of a mixture of the three explosives. The ion source also allowed direct detection of trace explosives on both conductive and non-conductive substrates, thus expanding the applicability of low temperature plasma desorption mass spectrometry.
1 Introduction
Detection of explosives is of great analytical importance because of its application in forensic analysis,1–3 public safety monitoring,4,5 and environmental analysis.6,7 As powerful instrumentation for structure elucidation, mass spectrometry (MS) plays an important role in the detection of explosives3,8–10 as it offers better sensitivity and specificity than any other common analytical technique. Also, rapid and direct detection without sample preparation is critical to security checks and criminal detection. Therefore, effective in-situ analysis of explosives in complicated contexts where potential contaminants exist is highly desirable.
Aiming at applying MS to the detection of explosives on different substrates and eliminating sample preparation to facilitate in-situ and real-time analysis, great efforts have been taken to develop various ionization and sampling techniques, including dielectric barrier discharge ionization (DBDI),11–13 desorption electrospray ionization (DESI)14–19 and direct analysis in real time (DART)20,21 mass spectrometry. In DESI, a pneumatically assisted electrospray is directed onto a surface with the generated secondary ions carried into the mass spectrometer. In DART, the ion source operates with the sample exposed to a dry gas stream (typically helium or nitrogen) consisting of excited neutral atoms or molecules. In a previous report by our group,11 a DBDI source with needle-plate configuration was constructed for the detection of TNT, RDX, and PETN under ambient conditions. In that design, a copper plate as a counter electrode needs to be attached to the bottom of the glass slide, on which explosives were present. Because only within a certain distance can the plasma be formed and high ionization efficiency be achieved, the shape, size, and thickness of the substrates greatly affect the performance of the ion source. However, our previous source was unable to ionize explosives on conductive substrates. Recently, a low temperature plasma (LTP) probe has been developed for ambient desorption and ionization, in which an AC electric field was employed to induce a dielectric barrier discharge (DBD) by using a specially designed electrode configuration.13 The LTP probe proved to be effective in explosive detection in that limits of detection (LODs) as low as 5 pg were achieved for RDX and TNT deposited on polytetrafluoroethylene (PTFE) surfaces. In comparison with DESI and DART, the LTP probe requires no spray solvents and the structure is simple, which enables it to be a potential ion source for portable mass spectrometers. In this work, we have constructed an LTP probe with a smaller opening of the discharge tube and employed it for the detection of explosives on a variety of substrates without sample preparation under ambient conditions. The small size of the sampling ion source makes it suitable to analyze explosives on various substrates and easy to operate. In the present design, the exit opening of only 2∼3 mm was used to allow the plasma to be blown out, resulting in increased temperature of the plasma and enhanced desorption of explosives from substrates. Results suggested that explosives on both non-conductive and conductive substrates were successfully detected, and the sensitivity was greatly improved in comparison with previous results.
2 Experimental
2.1 Chemicals and reagents
All chemicals were of analytical reagent grade and used without further purification. Methanol and hydrochloric acid were purchased from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). Water was deionized and further purified with a Milli-Q water purification system (Millipore, Milford, MA). Discharge gases of helium (99.998%), argon (99.99%) and nitrogen (99.99%) were purchased from Huayuan Gas Co. Ltd (Beijing, China). All the explosives were purchased from AccuStandard (New Haven, CT, USA), and explosive standards were prepared by diluting them to appropriate concentrations using a prepared 100/100/0.01 (v/v/v) CH3OH/H2O/HCl solution.
2.2 The low temperature plasma probe
The structure of the LTP probe in the present work is illustrated in Fig. 1. A quartz tube (30 mm o.d.) was employed in the fabrication of the LTP probe, inside which a 15 cm copper rod electrode (1.5 mm o.d.) was used as an internal electrode. A piece of copper sheet (70 mm × 40 mm) attached to the outside surface of the quartz tube was used as the counter electrode. An alternating voltage of 3.5–4.5 kV (2.5–30 W) was applied to both electrodes to generate an atmospheric-pressure plasma. In comparison with the LTP reported previously,13 a bigger overlap of the electrodes was implemented and a smaller opening of the probe was used. The discharge gas flowed through the tube via a side port. Samples deposited on various substrates were mounted on a 3D translation stage. The distance between the end of LTP probe and the glass slide was within 10 mm. Upon analysis, analytes were desorbed and ionized by the plasma and analyzed by MS.
 |
| | Fig. 1 Schematic diagram of the experimental setup (not to scale). | |
3 Results and discussion
3.1 Optimization of experimental parameters
Three explosives, 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-trinitro-1,3,5-triazine (RDX), and pentaerythritol tetranitrate (PETN), dissolved in the CH3OH/H2O/HCl solution were deposited on glass slides, then allowed to dry and analyzed in the negative ion mode. As illustrated in Fig. 2, characteristic ions of [TNT − H]− (m/z 226), [RDX + NO3]− (m/z 284), and [PETN + NO3]− (m/z 378) were detected. Ions derived from [RDX + Cl]− (m/z 257, 259) and [PETN + Cl]− (m/z 351, 353) are characterized by the presence of isotopes 35Cl and 37Cl, with the ratio of their relative abundances being 3 : 1.
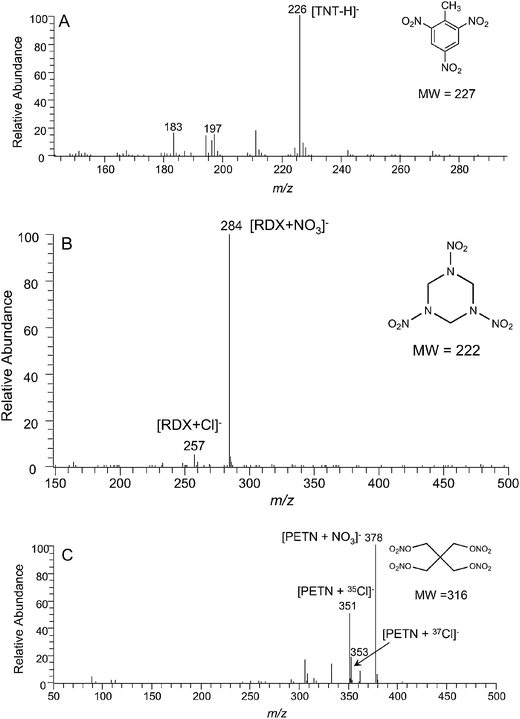 |
| | Fig. 2 Negative ion DBDI mass spectra of (A) TNT (50 ng), (B) RDX (50 ng), and (C) PETN (50 ng) deposited on a glass slide. | |
We further explored the relationship between fragmentation patterns and structural features of the explosives by collision induced dissociation (CID). The CID mass spectra of TNT, RDX, and PETN are shown in Fig. 3. The peak of [TNT − NO − H]− (m/z 196) in Fig. 3(A) corresponds to the quasi-molecular anions of [TNT − H]− (m/z 226) after loss of NO. The peak of [RDX + NO3 − NO2]− (m/z 238) in Fig. 3(B) corresponds to [RDX + NO3]− (m/z 284) after loss of NO2. In Fig. 3(C), the peak at m/z 315 represents quasi-molecular anions of [PETN − H]− and the peak of [PETN + NO3 − NO2]− (m/z 332) corresponds to [PETN + NO3]− (m/z 378) after loss of NO2.
![Negative ion DBDI tandem mass spectra of (A) [TNT − H]− (m/z 226), (B) [RDX + NO3]− (m/z 284), and (C) [PETN + NO3]− (m/z 378). The discharge power was 2.5 W and the flow rate of argon was 250 mL/min.](/image/article/2009/AN/b816230a/b816230a-f3.gif) |
| | Fig. 3 Negative ion DBDI tandem mass spectra of (A) [TNT − H]− (m/z 226), (B) [RDX + NO3]− (m/z 284), and (C) [PETN + NO3]− (m/z 378). The discharge power was 2.5 W and the flow rate of argon was 250 mL/min. | |
3.1.1 Effect of gas flow rate.
The gas flow rate is important to generate the DBD plasma. In the present study, mass spectra of RDX were obtained at different gas flow rates (50–400 mL/min) for optimization, as shown in Fig. 4. When the flow rate was set to zero, the plasma was not generated. A clean mass spectrum was obtained using gas flow rates from 50 to 250 mL/min. At even higher flow rates, ions irrelevant to RDX become more abundant, leading to increased background and a decreased signal-to-noise (S/N) ratio. Therefore, a gas flow rate of 250 mL/min (5.3 m/s) was selected for subsequent experiments. In comparison with the normal gas flow rates in DESI (>350 m/s)22 and DART (1500–3000 mL/min),20 the flow rate selected in the ion source is much lower, which is important for the application of the ionization technique in portable mass spectrometers.
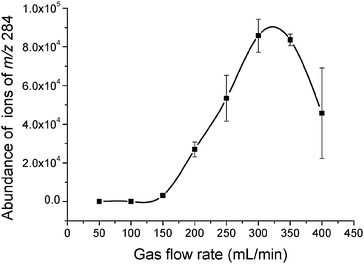 |
| | Fig. 4 Effect of gas flow rate (argon) on the abundance of ions of m/z 284. | |
3.1.2 Effect of power.
Powers ranging from 2.5 to 30 W were investigated for the generation of the non-equilibrium plasmas. It was observed that similar mass spectra were obtained utilizing different powers, using RDX as a representative case (Fig. 5). However, increased discharge power resulted in high plasma temperature, which would cause damage to the substrates on which the explosives were deposited and further lead to sample pollution and complicated mass spectra. Therefore, a 2.5 W power was selected and used throughout our experiments. It has a small size (L × W × H, 6.0 cm × 4.0 cm × 3.0 cm) and can be inexpensively obtained.
 |
| | Fig. 5 Negative ion mass spectra of 50 ng RDX deposited on a glass slide using different powers: (A) 2.5 W, (B) 5 W, (C) 7 W, and (D) 30 W. All mass spectra were obtained with background subtracted. The gas flow rate was 250 mL/min. | |
3.1.3 Effect of discharge gases.
Helium,23,24 nitrogen,25,26 and argon27 are commonly used to generate plasmas for the LTP probe. To test their applicability, 50 ng RDX was deposited on a glass slide followed by subsequent LTP-MS analysis. As illustrated in Fig. 6, similar spectra for RDX were obtained. In comparison with helium, little fragmentation and low background intensity were observed in mass spectra obtained using argon and nitrogen. Therefore, argon was selected as the discharge gas.
 |
| | Fig. 6 Negative ion mass spectra of 50 ng RDX deposited on a glass slide using different discharge gases: (A) argon, (B) nitrogen, and (C) helium. The discharge power was 2.5 W and the gas flow rate was 250 mL/min. | |
3.1.4 Effects of substrates.
The effects of a variety of substrates on the quality of the mass spectra were investigated. Fig. 7 shows the mass spectra of 50 ng PETN deposited on four different substrates of glass, PTFE, polyethylene, and cloth. Results indicated that PETN could be readily detected. In addition, to test whether the explosives can be detected from conductive substrates, 50 ng TNT and 50 ng RDX were deposited on copper foils to carry out LTP-MS analysis. Corresponding mass spectra obtained are shown in Fig. 8. It is concluded that both explosives were successfully detected. Therefore, it is confirmed that the method is capable to analyze explosives on both non-conductive and conductive substrates, making LTP-MS a more versatile ionization technique.
 |
| | Fig. 7 Negative ion mass spectra of 50 ng PETN deposited on different substrates: (A) glass, (B) PTFE, (C) polyethylene, and (D) cloth. | |
 |
| | Fig. 8 Negative ion mass spectra of 50 ng (A) TNT and (B) RDX deposited on a copper foil. | |
3.2 Analytical performance
To evaluate the sensitivity of the present system, aliquots (1 µL) of 500 fg/µL to 160 ng/µL PETN solutions were deposited on glass slides and allowed to dry for detection. LODs of the three explosives using the present LTP-MS system in the negative ion mode were listed in Table 1. The monitored ions were m/z 197, 284, and 378 for TNT, RDX, and PETN, respectively. Signal-to-noise ratios were automatically calculated using the instrument software of Xcalibur Home Page Version 1.4 SR1 from the extracted ion chromatograms. Compared with the LODs of DESI and the previous needle-plate DBDI-MS system, the present method has a much higher sensitivity.
Table 1 Comparison of LODs obtained using three different techniques for explosive analysis8,11
| Explosives |
DESIa |
Previous DBDIb |
Present LTP probeb |
| LOD |
S/N |
LOD |
S/N |
LOD |
S/N |
|
During DESI-MS analysis, TNT was deposited on plastics, and RDX and PETN were deposited on papers.
During previous DBDI and present LTP probe experiments, all explosives were deposited on glass slides.
|
| TNT (m/z 197) |
<1 pg |
12 : 1 |
10 pg |
8 : 1 |
500 fg |
8 : 1 |
| RDX (m/z 284) |
<1 pg |
9 : 1 |
100 pg |
10 : 1 |
1 pg |
9 : 1 |
| PETN (m/z 378) |
<100 pg |
5 : 1 |
1000 pg |
12 : 1 |
500 fg |
6 : 1 |
3.3 Sample analysis
In practical situations, explosives may be present accompanied by other contaminants on various substrates. This does not allow direct detection via the needle-plate DBDI source.11 However, this problem was solved using the ion source constructed in this work, which distinguishes itself as an effective approach to desorb and ionize explosives on different substrates. The mass spectra of PETN deposited on different objects made of different materials, including a beaker, plastic boxes (PTFE and polyethylene), and a pencil box (cloth), are provided in Fig. 9.
 |
| | Fig. 9 Negative ion mass spectra of 50 ng PETN deposited on different objects: (A) beaker made of glass, (B) plastic box made of PTFE, (C) plastic box made of polyethylene, and (D) pencil box made of cloth. | |
The present LTP-MS system can also be used for fast discrimination of explosive mixtures on a glass slide and the mass spectrum is shown in Fig. 10, which suggests the feasibility in real sample analysis. The observed characteristic peaks indicate a successful characterization.
 |
| | Fig. 10 Negative ion mass spectrum of a mixture of 50 ng PETN, 34 ng RDX, and 50 ng TNT on a glass slide. | |
In general situations, the explosives are present in complicated contexts and may suffer from various kinds of interferences. Therefore, to test the applicability of the ion source for real samples, we investigated the feasibility for the detection of TNT in a typical matrix. 10 µL of 500 ng/µL TNT solution mixed with a hand cream was deposited on a glass slide to form a thin layer less than 0.5 mm thick. Fig. 11 shows the obtained mass spectrum of TNT and the dominating peak (m/z 197) is indicative of TNT.
 |
| | Fig. 11 Mass spectrum of TNT mixed with a hand cream in the negative ion mode. | |
4 Conclusions
In comparison with other ion sources, the present design has the following advantages: (1) explosives on both non-conductive and conductive substrates can be directly desorbed and ionized under ambient conditions without restriction to substrates; (2) the ion source requires no spray solution, which is a requisite in DESI, and has a simple structure in contrast with the complex structure of DART; and (3) efficient desorption and ionization of the explosives on a variety of surfaces lead to a greatly improved sensitivity in comparison with the previous DBDI and a comparable sensitivity to that of DESI. All these characteristics, together with its small size and low power consumption, make this ion source a potential candidate to be integrated into a portable mass spectrometer, as well as suggesting its suitability for the in-situ detection of explosives in various complicated contexts.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (20535020 and 20635002).
References
- D. J. Klapec and D. Ng, J. Forensic Sci., 2001, 46, 1168–1173 CAS.
- X. M. Xu, A. M. van de Craats, E. M. Kok and P. de Bruyn, J. Forensic Sci., 2004, 49, 1230–1236 CAS.
- X. M. Zhao and J. Yinon, Rapid Commun. Mass Spectrom., 2002, 16, 1137–1146 CrossRef CAS.
- K. G. Furton, J. R. Almirall, M. Bi, J. Wang and L. Wu, J. Chromatogr., A, 2000, 885, 419–432 CrossRef CAS.
- G. W. Dicinoski, R. A. Shellie and P. R. Haddad, Anal. Lett., 2006, 39, 639–657 CrossRef CAS.
- I. Cotte-Rodriguez and R. G. Cooks, Chem. Commun., 2006, 2968–2970 RSC.
- A. N. Martin, G. R. Farquar, E. E. Gard, M. Frank and D. P. Fergenson, Anal. Chem., 2007, 79, 1918–1925 CrossRef CAS.
- Z. Takats, I. Cotte-Rodriguez, N. Talaty, H. W. Chen and R. G. Cooks, Chem. Commun., 2005, 1950–1952 RSC.
- C. S. Evans, R. Sleeman, J. Luke and B. J. Keely, Rapid Commun. Mass Spectrom., 2002, 16, 1883–1891 CrossRef CAS.
- Z. G. Wu, C. L. Hendrickson, R. P. Rodgers and A. G. Marshall, Anal. Chem., 2002, 74, 1879–1883 CrossRef CAS.
- N. Na, C. Zhang, M. X. Zhao, S. C. Zhang, C. D. Yang, X. Fang and X. R. Zhang, J. Mass Spectrom., 2007, 42, 1079–1085 CrossRef CAS.
- N. Na, M. X. Zhao, S. C. Zhang, C. D. Yang and X. R. Zhang, J. Am. Soc. Mass Spectrom., 2007, 18, 1859–1862 CrossRef CAS.
- J. D. Harper, N. A. Charipar, C. C. Mulligan, X. R. Zhang, R. G. Cooks and Z. Ouyang, Anal. Chem., 2008 DOI:10.1021/ac801641a.
- I. Cotte-Rodriguez, H. Hernandez-Soto, H. Chen and R. G. Cooks, Anal. Chem., 2008, 80, 1512–1519 CrossRef CAS.
- I. Cotte-Rodriguez, Z. Takats, N. Talaty, H. W. Chen and R. G. Cooks, Anal. Chem., 2005, 77, 6755–6764 CrossRef CAS.
- D. R. Justes, N. Talaty, I. Cotte-Rodriguez and R. G. Cooks, Chem. Commun., 2007, 2142–2144 RSC.
- N. T. Tiina, J. Kauppila, Tiia Kuuranne, Tapio Kotiaho, Risto Kostiainen and R. G. Cooks, Analyst, 2007, 132, 868–875 RSC.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Science, 2006, 311, 1566–1570 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471–473 CrossRef CAS.
- R. B. Cody, J. A. Laramee and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS.
- R. W. Jones, R. B. Cody and J. F. McClelland, J. Forensic Sci., 2006, 51, 915–918 CrossRef.
- Z. Takats, J. M. Wiseman and R. G. Cooks, J. Mass Spectrom., 2005, 40, 1261–1275 CrossRef CAS.
- B. M. Obradovic, S. S. Ivkovic and M. M. Kuraica, Appl. Phys. Lett., 2008, 92 Search PubMed.
- Y. T. Zhang, D. Z. Wang and M. G. Kong, J. Appl. Phys., 2005, 98 Search PubMed.
- F. Clement, E. Panousis, B. Held, J. F. Loiseau, A. Ricard and J. P. Sarrette, J. Phys. D: Appl. Phys., 2008, 41 Search PubMed.
- E. Panousis, L. Papageorghiou, N. Spyrou, J. F. Loiseau, B. Held and F. Clement, J. Phys. D: Appl. Phys., 2007, 40, 4168–4180 CrossRef CAS.
- H. Y. Luo, Z. Liang, B. Lv, X. X. Wang, Z. C. Guan and L. M. Wang, Appl. Phys. Lett., 2007, 91 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![Negative ion DBDI tandem mass spectra of (A) [TNT − H]− (m/z 226), (B) [RDX + NO3]− (m/z 284), and (C) [PETN + NO3]− (m/z 378). The discharge power was 2.5 W and the flow rate of argon was 250 mL/min.](/image/article/2009/AN/b816230a/b816230a-f3.gif)








