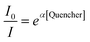Multiplexed energy transfer mechanisms in a dual-function quantum dot for zinc and manganese†
Maria Jose
Ruedas-Rama
and
Elizabeth A. H.
Hall
*
Institute of Biotechnology, Department of Chemical Engineering and Biotechnology, University of Cambridge, Tennis Court Road, Cambridge, UK CB2 1QT. E-mail: lisa.hall@biotech.cam.ac.uk; Fax: +44 (0) 1223 334161; Tel: +44 (0) 1223 334149
First published on 24th November 2008
Abstract
Photoexcited quantum dots (QDs) offer a wealth of mechanisms for interactions with the valence band holes or conduction band electrons, influencing electron–hole recombination. The potential to use combinations of these mechanistic pathways to achieve detection of different metal ions with one modified QD system has been tested. Dual-function water-soluble core/shell-modified CdSe/ZnS quantum dot nanoparticles have been created, that exploit two different fluorescence emission pathways for the detection of two heavy metal ions: Zn2+ and Mn2+. A QD-zincon system is proposed, which shows a static Perrin-type quenching mechanism, with sphere of action radii 1.1, 1.3 and 1.6 nm respectively, for 500, 540 and 620 nm QD emission. The QD-zincon system was produced using a layer-by-layer approach: mercaptopropionic acid-capped QDs were modified with a positive polyelectrolyte by electrostatic interaction and then a negatively charged chromogenic reagent, zincon, classically used for the determination of metals. QD-zincon is able to coordinate both Zn2+ and Mn2+ and, by exploiting two different mechanisms, QD-zincon conjugates can be tailored to respond to Zn2+ or Mn2+. Upon coordination of zincon with Mn2+, a dramatic enhancement of the fluorescence intensity results as the quenching interaction between zincon and QDs is deactivated, thereby ‘switching on the fluorescence emission’. The versatility of this system is demonstrated in terms of fluorescent emission wavelength, which could be selected across a wide range, through choice of QDs (examples are shown for λmax = 500, 540 and 620 nm). In contrast, in the case of Zn2+ detection, the mechanism is based on the radiationless resonance energy transfer (RET) from QDs acting as donor, to the acceptor zincon-Zn2+, since its absorption spectra offer adequate overlap with the emission spectra of QD540 and QD620, producing a useful analytical signal by the RET process. Using these different operating principles, CdSe/ZnS core/shell QD-zincon conjugates showed very good linearity in the range 10–1000 µM and 5–500 µM for Zn2+ and Mn2+ nanosensors, respectively, and RSDs about 3% (n = 10). In a study of interferences, the QD–zincon conjugate showed higher selectivity than the corresponding method with zincon in solution. The results from synthetic ionic mixtures suggested very good applicability in the determination of Zn2+ and Mn2+ in samples containing other metal ions, with just a small reduction of sensitivity at very high ionic concentration.
Introduction
The determination of trace amounts of heavy metal ions is of interest in fields including environmental analysis, process control, biology, and medicine. Traditional optical techniques for metal ion assay include inductively coupled mass spectroscopy1 and atomic/molecular absorption spectroscopy.2 They have been widely used due to their rapidity, simplicity, and high sensitivity. Also, simultaneous determinations of metals can be carried out by ion chromatography.3 However, previous pre-concentration and/or separation steps of the analytes from the matrix components are usually needed. On the other hand, cheaper techniques such as spectrophotometry4 and spectrofluorimetry5 have also been extensively used for the determination of these metal ions. The spectroscopic determination is normally preceded by a reaction with different reagents, but it is difficult to differentiate analytes in mixtures because of the strong spectral overlap when they react with organic ligands.Currently, Quantum Dot (QD) nanoparticles are being researched as selective ion probes in aqueous samples. Semiconductor nanomaterials have generated great research interest in the past two decades and the properties and applications of fluorescent QDs stand amongst the most exciting research fields bridging chemistry, physics and biology. Since the photoluminescence of QDs arises from the recombination of the exciton, it is expected that the changes of surface charge or ligand components of QDs would affect the efficiency of core electron–hole recombination and consequently the luminescence efficiency. Therefore, chemical sensing systems based on QDs can be developed using fluorescence changes induced by direct physical adsorption or chelating of ions on the surface of QDs activated by the exchanged ligand. For instance, based on these principles, the determination of Zn2+ was possible looking at the reversible photoluminescence activation process caused by adsorption on the surface of CdS QDs.6 In another work on Zn2+ determination, the formation of a Zn–cysteine complex on the surface of the cysteine-capped QDs, achieving a three-dimensional CdS QD network, was responsible for the activation of the surface states, producing an emission enhancement.7 Following this principle, various QD-based optical sensors have been developed for the detection of metal ions, where not only luminescence enhancement, but also quenching was observed, dependent on the nature and environment of the ions.8–12
Most of these systems were based on direct interaction of the metals with the surface of the QD nanoparticles, whereas the combination of luminescent QDs with the classical organic ligand or chromogenic reagents has been less characterised. In an earlier work we reported new QDs with surface covalently bonded azamacrocycles,13 acting as receptor for Zn2+ binding. In this instance it is the azamacrocycle (the ligand) interacting directly with QDs and accepting photoinduced hole transfer that separates the charge and means that recombination cannot occur, quenching the QD emission. When Zn2+ enters the azamacrocycle, this hole transfer pathway is stopped, switching on a Zn-dependent QD emission.
There is a potential wealth of different mechanisms for metal–ligand modulation of the QD fluorescence, so that the idea of combining different energy transfer response mechanisms to determine different ions in one system is exciting. This idea is explored in the work reported herein. One of the reagents more used in the past for the determination of metal ions has been 2-carboxyl-2-hydroxy-5-sulfoformazylbenzene (Zincon). Analytical reactions involving zincon as a chromogenic reagent have been used for spectrophotometric determinations of many metals in solution,14–16 such as Zn2+, Cu2+, Ni2+ and Co2+, and also immobilised on polymer membranes.17,18 The broad range of metals indicates its low selectivity. However, when a distinction can be established in the chemical reactivity or the mechanism of two or more species with a common reagent, this can be very useful in developing methods for the sequential or simultaneous determination of analytes in mixtures.
Indeed, the resolution of mixtures of zinc and copper, based on the differential kinetics or the variation of the stability of the corresponding complexes, with the pH of the medium have been developed.19–22 Thus, in this context, we have modified the surface of CdSe/ZnS core/shell QD nanoparticles with the non-fluorogenic reagent zincon to develop new metal ion nanosensors with fluorescent detection. Different mechanisms of response of QD-zincon conjugates towards zinc and manganese have served as the basis for the development of nanosensors for these metals.
Experimental
Materials
All experiments were performed with reagents of analytical-reagent grade, pure solvents and milli-Q water (used for the dilution of samples and reagents). Quantum Dots CdSe/ZnS core/shell with maximum emission at 500, 540 and 620 nm were purchased from Evident Technologies. The surfactant capping the QDs was a long chain (16 C) amine and some tri-n-octylphosphine/tri-n-octylphosphine oxide (TOP/TOPO). 2-Carboxyl-2-hydroxy-5-sulfoformazylbenzene (Zincon) and poly(allylamine hydrochloride) (average Mw ≈ 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (PAH+), were obtained from Sigma-Aldrich. All inorganic salts including zinc, manganese, nickel, cobalt, calcium, magnesium, iron, and copper chloride salts, and TRIS buffers were of analytical grade and used as obtained from Aldrich. 3-Mercaptopropionic acid (MPA) was purchased from Fluka. All standard chemical solutions were protected from sunlight and kept at about 5 °C in a refrigerator. Standard salt solutions and HCl and NaOH solutions were prepared in milli-Q water.
000) (PAH+), were obtained from Sigma-Aldrich. All inorganic salts including zinc, manganese, nickel, cobalt, calcium, magnesium, iron, and copper chloride salts, and TRIS buffers were of analytical grade and used as obtained from Aldrich. 3-Mercaptopropionic acid (MPA) was purchased from Fluka. All standard chemical solutions were protected from sunlight and kept at about 5 °C in a refrigerator. Standard salt solutions and HCl and NaOH solutions were prepared in milli-Q water.
Synthesis of water-soluble MPA-capped CdSe/ZnS nanoparticles
Water-soluble quantum dots (QD-MPA) were prepared by ligand exchange of lipophilic hexadecylamine-capped QDs by 3-mercaptopropionic acid, preserving the high luminescence quantum yield of QDs. The procedure was adapted from the surface–ligand exchange in previous reports.23–25 0.5 ml of commercial QDs (Evident Technologies: hexadecylamine as ligand) dissolved in toluene were left to react overnight with 0.5 ml of MPA, protected from light.25 After the ligand exchange, the particles were transferred to an aqueous phase by adding 1 M NaOH solution and shaking. The two-phase mixture was separated, and the water-soluble CdSe/ZnS QD nanoparticles were separated from the excess of MPA by precipitation of the particles with acetone and centrifugation, followed by the re-dissolution of the MPA-capped QDs in milli-Q water diluting 6 times the original concentration of QDs.Optimisation of QD/MPA/zincon ratios
Zincon has a negatively charged sulfonic group, therefore QDs with positive charge on their surface are expected to form stable QD-zincon conjugates by means of electrostatic interactions. Thus, following a layer-by-layer modification26 on QD-MPA, poly(allylamine hydrochloride) (PAH+) was chosen as positive electrolyte for achieving a positively charged surface distribution on the QDs, able to self-assemble with negative charged zincon. The assembly was carried out at pH 7.5 (10 mM TRIS buffer) to achieve stable conjugates. At this pH the MPA on the QDs is deprotonated (pKa ≈ 6.5)27 and PAH+ is fully protonated (pKa ≈ 9).28Since high density MPA capping on the QDs leads to a high negative charge on the surface, this also produces a concomitant increase in the attachment of subsequent layers. There are thus 3 levels of ‘tuning’ that will influence the final loading of the QD by zincon: the MPA/PAH+ surface ligand-density and the zincon concentration. At low MPA concentration on the QDs the quantity of PAH+ attached on the surface was also low, and therefore the subsequent quantity of zincon. Increasing the amount of MPA (and therefore PAH+) gave successful immobilisation of zincon, indicated by a higher quenching of QD luminescence observed. Thus the zincon-QD conjugate preparations were standardised as follows:
QD-MPA employed for QD-zincon conjugates were prepared with 500 µl of lipophilic QDs (82, 36, and 16 nmol of QD500, QD540, and QD620, respectively) and 500 µl of MPA (corresponding to 5800 µmol) for the ligand exchange step. 1 ml QD-MPA prepared as above was mixed with 1 ml of a solution of 1% PAH+ in TRIS buffer, 10 mM, pH 7.5, shaking during 30 minutes. To remove the excess of PAH+ (non-attached), QD-MPA-PAH+ nanoparticles were centrifuged and the residue was re-dissolved in milli-Q water. Straight afterwards, 1 ml of a solution of 250 µM zincon in milli-Q water was added to QD-MPA-PAH+ and shaking was used during 1 hour. In the last step, attachment of higher zincon concentrations caused higher quenching of the emission of QDs. As will be shown in the results, for Zn ion detection, a compromise is required between a high zincon concentration on the surface of the QDs, to introduce a Zn-binding ligand and sufficient sensitivity and range (i.e. requiring not high quenching). A solution of 250 µM zincon in milli-Q water was used here for the attachment of zincon on QDs. After self-assembly through electrostatic interaction, QD-MPA-PAH-zincon was purified twice by centrifugation and re-suspension in milli-Q water. The separation of zincon (non-immobilised) in the supernatant was required to ensure that the Zn2+ and Mn2+ response occurred just with immobilised zincon on the surface of QD nanoparticles. The resultant QD-MPA-PAH-zincon (labelled as QD-zincon) conjugates showed a purple colour corresponding to the immobilised zincon on the surface.
Spectrofluorimetric measurements of QD-zincon conjugates
Emission spectra were measured using a Cary-Eclipse Fluorescence Spectrofluorimeter (Varian). The spectrofluorimeter was equipped with a xenon discharge lamp (75 kV), Czerny-Turner monochromators, two detectors (sample and internal reference), an R-928 photomultiplier tube with manual or automatic voltage controlled using the Cary-Eclipse software. Instrument excitation and emission slits were set at 5 and 10 nm (for Mn2+ detection) and 10 and 10 nm (for Zn2+ detection), respectively, and the scan rate of the monochromators was 600 nm min−1. All samples were illuminated under an excitation wavelength of 350 nm and the emission was scanned from 475 to 670 nm, while the detector voltage was maintained between 550 and 600 V.In all experiments, sets of samples were prepared by adding 70 µl of Zn2+ or Mn2+ ion stock solutions in TRIS buffer 10 mM pH 7.5 into 70 µl of QD-zincon conjugate solution. These samples were placed in a quartz microcell (Starna) with a light path length of 10 mm (160 µm inner volume). To study response time, emission intensity was measured as a function of time, and CdSe/ZnS nanoparticle suspensions were scanned every 20 seconds during 30 minutes.
Effect of pH and ionic strength in QD-zincon conjugates
70 µl QD-zincon conjugates were exposed to 70 µl Zn2+ and Mn2+ solutions buffered with TRIS at different pH and concentrations. The responses towards Zn2+ and Mn2+ were evaluated in all those media and the linear response plots were calculated and compared. The direct effect of different pHs and ionic strengths on the intrinsic luminescence of the QD-PAH+ and QD-zincon conjugate was also studied.Calibration of the QD-zincon conjugate
Calibrations were constructed under similar conditions for both Zn2+ and Mn2+ nanosenors. 70 µl of Zn2+ or Mn2+ stock solutions (ranging from 1 µM to 1 mM) in 10 mM TRIS buffer at pH 7.5 were added to 70 µl of QD-zincon conjugates, respectively. The mixtures were placed in a quartz microcell for fluorescence scanning. Because of the broad metal ion response range in both cases, logarithmic representations were made. The data, corresponding to the average of three determinations, were fitted by standard least-squares treatment and equations for the calibration lines were calculated.Results and discussion
QD-zincon conjugate formation: preparative overview
The aim of this work was to demonstrate that a modified QD, with a single metal-ion sensitive ligand, could selectively detect different ions by using different energy transfer interactions with QDs and to propose and investigate the feasibility of a scheme for the detection of both Zn2+ and Mn2+. A critical component of this system is an ion-complexing chromogenic reagent forming a QD-chromogenic pair. In this instance, we have investigated zincon (2-carboxyl-2-hydroxy-5-sulfoformazylbenzene), which has been extensively used for the detection of many metals, because of the formation of water-soluble metal ion complexes. It has also been chosen here, as suitable for proof of concept, due to the presence of a negatively charged sulfonic group in its structure, which allows easy assembly on the positively charged surface of the QD-MPA-PAH+ nanoparticles to form a stable QD-zincon conjugate.As we reported previously,27 the concentration of MPA on the surface of the QD nanoparticles will influence the properties of the resultant water-soluble QDs, the negative charge distribution and their ability to interact with PAH+, and therefore with zincon in a subsequent step. QD-zincon conjugates could be formed with different QD : MPA ratios (with a stoichiometric excess of MPA between 50–500 × 103 for the lipophilic-capped QDs as supplied),27 followed by the electrostatic assembly of a layer of the positive electrolyte PAH+ (a solution of 1% PAH+ was enough to reach a high positive charge on the surface of the QDs). The incorporation of the layer of polyelectrolyte PAH+ produced neither a shift in the maximum emission nor a change in the luminescence intensity (Fig. 1a), which suggests that no change has occurred in the valence or conduction band energy barriers as a result of the surface interaction.26
 | ||
| Fig. 1 (a) Emission spectra of QD540-MPA (black), QD540-MPA-PAH+ (red), and QD540-MPA-PAH-zincon (250 µM zincon) (blue). (b) Emission spectra of QD540-MPA (black), QD540-MPA + 250 µM zincon in solution (red), and QD540-MPA + 250 µM zincon after centrifugation (blue). | ||
After immobilisation of zincon (concentration range 25–500 µM) on the positively charged QD-MPA-PAH+, quenching of the CdSe/ZnS QD nanoparticle luminescence was observed.
Emission spectra of QD540-MPA, QD540-MPA-PAH and the QD540-MPA-PAH-zincon conjugate at the same concentrations were compared under the same working conditions (Fig. 1a) and showed that the QD-MPA-PAH-zincon conjugates were strongly quenched (circa 75%). These conjugates also showed a purple colour (after purification by centrifugation to remove the zincon not attached), corresponding to the immobilised zincon on the surface. This suggests that the assembly of zincon on the surface was quite efficient. In contrast, only 7% quenching was observed for the QD540-MPA-zincon blank, and after centrifugation no change of colour was seen on the surface of the QDs, confirming that the attachment of the zincon anion is not possible on the anionic MPA surface (Fig. 1b).
Even though the emission intensity was reduced, there was no shift of the maximum emission of the QDs after zincon attachment, presumably since the electrostatic attachment on the surface did not modify the shell barrier energy of the QD nanoparticles. Similar QD-zincon conjugates were achieved with three different CdSe/ZnS core/shell QD populations (500, 540 and 620 nm). All these conjugates showed fluorescence quenching after the attachment of zincon, so the quenching mechanism is also not dependent on the QD band gap in this range.
Moreover, the stability of the QD-zincon conjugates at different pHs and ionic strengths was also tested. The emission intensity of QD-zincon conjugates was quite stable over a large range of ionic strengths. No changes in the luminescence signal of QD-zincon conjugates were observed by changing the concentration of TRIS buffer (pH 7.5) from 5 mM up to 100 mM, suggesting that the electrostatic interactions between the sulfonic group of zincon and the positive polyelectrolyte PAH+ were quite strong, and even the presence of quite high concentrations of background electrolyte did not interfere with these interactions.
Increasing the pH from 6 to 8 also caused negligible changes in the emission of the QD conjugates. However, at pHs higher than 8.5, enhancement of the emission of the QD conjugates could be observed. This is consistent with desorption of zincon from the surface of the QDs, since at this pH PAH+ is not fully protonated and its interaction with negatively charged zincon would be compromised. However, below this pH, the same emission intensity of the conjugate was retained for at least 20 days, with only a small decrease of emission thereafter. In summary therefore, this profile suggests that the layer-by-layer electrostatic interactions were quite stable and desorption of zincon did not happen easily. This was also substantiated visually by the purple colour that was retained on the QDs.
Mechanism of the quenching of QD-PAH by zincon
The mechanism whereby the QD-zincon complex showed quenching of the QD luminescence was first examined according to the Stern–Volmer mechanism. The quenching mechanism of the QD excited state may range from a collision with a quencher (dynamic mechanism) to a static mechanism arising from charge transfer (or electron tunnelling) or the overlap of molecular orbitals. As expected for these systems, in which the quencher is immobilised on the surface of the QDs, a dynamic mechanism is unlikely and the Stern–Volmer plots for all three different wavelength populations of QD-zincon conjugates (Fig. 2a) are clearly non-linear. However, this can result from several different models, including static quenching mechanisms and where distance-dependent quenching is involved. The non-linear quenching, associated with other semiconductor nanoparticles,29 including modified QDs,27 also has the same characteristic shape in the Stern–Volmer plot as seen here. In this instance a simple Perrin model, where quenching of the excited state occurs only within a sphere of action for the quencher, has been proposed, according to:where α = NAV, where NA is Avogadro's number and V the quenching or ‘action’ volume.
 | ||
| Fig. 2 Plots showing fits to (a) Stern–Volmer quenching and (b) Perrin model sphere of action quenching of QD500-PAH (■), QD540-PAH (●), and QD620-PAH (▲) upon addition of zincon. | ||
From this relationship a plot of ln(I0/I) vs. [zincon] will reveal V, and hence the radius (r) of the sphere of action for the zincon can be calculated for each QD system. In this instance (Fig. 2b), the sphere of action radii calculated from the gradient of the plot of ln(I0/I) vs. [zincon] for each QD population were 1.1, 1.3 and 1.6 nm respectively for QDs of λ = 500, 540 and 620 nm. In its simplest form, this relationship would account for such short range processes involving molecular orbital overlap and described by Dexter energy transfer mechanisms. The value of this action radius defines the distance for 50% quenching, so it gives some indication of the type of quenching mechanism. However, as shown by Murphy et al.30 similar curvature of the Stern–Volmer plot results from resonant energy transfer and at short distances the paradox of the Perrin model formula is that it cannot easily distinguish this mechanism mathematically from experimental data.31 However, noting that the higher quenching is obtained for QDs with lower spectral overlap with the zincon (see also Fig. 3), the dipole–dipole interaction seems to be less likely.
 | ||
| Fig. 3 Absorption spectra of zincon (orange), zincon-Zn2+ complex (blue) and zincon-Mn2+ complex (yellow); and normalized emission spectra of QD500 (black), QD540 (green) and QD620 (red). | ||
This partially quenched QD-zincon system provides the basis for a dual action metal-ion multiplexed detection mechanism. Thus, to achieve the dual action we have targeted two mechanisms (Scheme 1):
• metal-ion disruption of the QD-zincon quenching as a result of formation of a zincon-metal-A complex, causing recovery of fluorescence at the QD wavelength;
• RET or FRET from the QD as a donor to zincon-metal-B complex, as acceptor, producing further quenching or a second fluorescence emission at longer wavelength respectively.
 | ||
| Scheme 1 Mechanisms of detection of Mn2+ and Zn2+ with the QD-zincon system. | ||
Zinc and manganese response mechanisms
Since zincon is a chromogenic reagent widely used for metal ion determination by forming water-soluble complexes, we can anticipate the reaction of zincon attached on the surface of QDs with these metals. Thus, even through zincon in solution is not a fluorescent reagent for the determination of metals, the combination of zincon and QDs can achieve a sensitive and selective metal ion sensing system with fluorescent detection.The addition of Zn2+ or Mn2+ ions to QD-zincon conjugates produced changes of colour corresponding to the formation of the respective zincon-metal complexes on the surface of the QDs. The typical blue colour of the complex zincon-Zn2+ and the yellow colour of zincon-Mn2+ were observed (Fig. 3) a few seconds after addition of these metal ions. In addition, after centrifugation of the QD-zincon-metal complexes the colour of the corresponding complex could still be seen on the surface of the QDs.
Changes in the emission intensity of QD-zincon conjugates corresponding to the formation of these complexes with zincon were also observed after Zn2+ and Mn2+ complexation. Controls adding Zn2+ and Mn2+ to QD-PAH+ showed no change in the luminescence signal of the QDs (metal concentrations tested up to 10 mM).
However, the changes in the luminescence of QDs modified with zincon were different for Zn2+ and for Mn2+. After the formation of the zincon-Zn2+ complex, quenching of the emission of the QD-zincon conjugate was observed (Fig. 4b). In contrast, the addition of manganese to form the zincon-Mn2+ complex initiated an enhancement of the emission of the QDs (Fig. 4a). These differences can be anticipated by examining the emission spectra of the QDs and the absorption spectra of the complexes in Fig. 3. Zincon in solution shows a strong broad absorption maximum at 470 nm, which is shifted to 620 nm when the zincon-Zn2+ complex is formed. The zincon-Mn2+ complex shows a very weak absorption with a maximum at a wavelength of 423 nm.
 | ||
| Fig. 4 Emission spectra of three populations of QD-zincon responding to changes in Mn2+ concentration: (a) QD500, (c) QD540, (e) QD620; and to changes in Zn2+ concentration: (b) QD500, (d) QD540, (f) QD620. (10 mM TRIS pH 7.5.) | ||
As suggested above from the small Perrin radius, the QD-zincon quenching mechanism is probably caused by overlap of molecular orbitals on the ligand and one of the QD charge carriers (i.e. valance band holes or conduction band electrons), thereby disrupting the radiative recombination process. For example, if the valence band of the QD is at a lower energy than a molecular orbital on the zincon, the photogenerated hole on the QD can transfer to the ligand and become trapped, preventing recombination13 after photoexcitation. Thus, the complexation of Mn2+ or Zn2+ with the zincon must impact this mechanism, as a result of the change in energy levels from the zincon to the metal complex. When Zn2+ is complexed, the blue complex absorbs across the same wavelengths as the QD540 and QD620 emission. As proposed previously in other systems for metals,32,33 the mechanistic principle involved here is probably based on radiationless resonance energy transfer (RET) from a fluorophore, in this case the QDs acting as donor, to the acceptor zincon-Zn2+. Thus the QD-zincon fluorescence is quenched by complexing Zn2+. Fig. 3 also shows that the absorption spectrum of the zincon-Zn2+ complex presents adequate overlap with the emission spectra of QD540 and QD620, producing a useful analytical signal by the RET process. However, also consistent with this mechanism, the overlap is lower with the QD500 population. Thus, when Zn2+ was added to QD-zincon conjugates of the three different wavelengths, the quenching of QDs in response to Zn2+ (TRIS pH 7.5) was only observed with QD540 and QD620, but no changes of QD500 emission were detected (Fig. 4b). Nevertheless, the zincon-Zn2+ complex was formed in all cases, since the blue colour was always seen.
In order to characterise the RET process, the Förster distance,34R0, the distance between the donor and acceptor that yields 50% energy transfer efficiency, was calculated from the spectral properties of the donor and the acceptor and the donor quantum yield (see ESI†). The quantum yields of the QD500, QD540 and QD620 were calculated by using fluorescein34 and rhodamine B35 as reference in aqueous solution. The experimentally calculated quantum yields were 0.02. 0.22 and 0.25 for QD500, QD540 and QD620, respectively (see ESI†). The R0 values obtained for QD540/zincon-Zn2+ and QD620/zincon-Zn2+ pairs were 41.5 and 47.6 Å, respectively. In contrast, the value of R0 obtained for QD500/zincon-Zn was 11.10 Å, due to the poor overlap and the low quantum yield. The Förster distances obtained here for QD540 and QD620 are consistent with typical R0 values (20–60 Å) and indicate that resonance energy transfer can be a dominant mechanism for the interaction of zincon-Zn2+ and QDs.
In contrast, the zincon-Mn2+ absorption spectrum is coincident with the zinconλmax, but very weak, so that a similar RET mechanism is not anticipated. Instead, the enhancement of QD luminescence in response to changes in Mn2+ concentration was consistent with small shift in the absorption wavelength (Fig. 3), indicating an energy change and the significant decrease in the molar extinction coefficient. We can deduce that the overlap of molecular orbitals with one of the QD charge carriers is less efficient for the Mn2+-zincon complex and charge trapping is no longer favoured. This luminescence enhancement was observed, for all three QD-zincon systems (500, 540 and 620 nm), with no dependence of either emission wavelength or QD size on the mechanism (Fig. 4a).
Zinc and manganese calibration and analytical parameters of QD-zincon nanosensors
To assess whether QD-zincon systems were suitable for both Zn2+ and Mn2+ sensing, calibration curves were first obtained using separate buffered Zn2+ and Mn2+ solutions with 10 mM TRIS pH 7.5. As discussed above, an enhancement of the QD luminescence for the QD-zincon conjugate occurred in response to changes of the concentration of Mn2+ and a quenching of luminescence of the QDs was seen in response to Zn2+ (Fig. 4). In both cases, QD-zincon conjugates showed a very good linear response in fluorescence per decade change in concentration for both Zn2+ or Mn2+, in the range 5–500 µM (R2 = 0.9893 and 0.9985, for Zn2+ and Mn2+, respectively). The sensitivity and linear response range of the three QD-zincon conjugates (500, 540 and 620 nm) for Mn2+ detection were all quite similar, whereas for Zn2+, only 540 and 620 nm were appropriate for detection. For these QDs the sensitivity and linear response range were also similar, with no effect of the QD size.The Zn2+ response of QD-zincon conjugates (linear response range and slope) was not affected by changes in the ionic strength of the medium (at different TRIS buffer concentrations (pH 7.5) ranging from 5 to 100 mM, see ESI†, Fig. SI-1). However, the Mn2+ response was slightly dependent on the buffer concentration (pH 7.5), showing a decrease of the response range only at high ionic strengths (100 mM TRIS).
Reproducibility of the Zn2+ and Mn2+ sensitive QD540-zincon conjugates was also checked and established for ten independent analyses. 70 µl samples of QD540-zincon conjugate were exposed to ten aliquots of 70 µl of 250 µM metal ion solution buffered with 10 mM TRIS at pH 7.5. The relative standard deviation (RSD) of the response of these QD-based nanosensors was 3.1 and 3.3% for Zn2+ and Mn2+, respectively, showing very good reproducibility. The detection limit was estimated as the concentration of analyte that produced an analytical signal equal to three times the standard deviation of the background fluorescence.36 The calculated values were 0.57 and 0.37 µM for Zn2+ and Mn2+, respectively, with the corresponding quantification limit37 (number of repetitions of the blank, K = 10) being 1.89 and 1.26 µM. Thus, although the detection of Mn2+ with zincon has not been widely used, the sensitivity of the proposed QD-zincon conjugates was higher than other methods employing zincon in solution,19,22,38 and similar to other systems that use pre-concentration of the complex in a solid phase21 for the determination of Zn2+.
The response time of QD-zincon conjugates towards Zn2+ or Mn2+ depends on the formation constant of the corresponding complexes. In both cases the ∼50% response is within 60 s with 95% change in less than 3–4 minutes (see ESI†, Fig. SI-2). Although the response time is not normally shown in the solution methods with zincon, the reported systems with immobilised zincon on polymer membranes showed more than 10 minutes response time18,33 toward both Zn2+ and Cu2+, so faster response appears to be achieved here with this nanoparticle system. Moreover, continuous excitation of either QD-zincon or QD-zincon-M2+ produced a very stable luminescence signal, even after 30 minutes.
Reversibility of the zinc QD-zincon-based nanosensors was achieved by using the chelator agent EDTA. Release of Zn2+ to form the complex with EDTA in solution caused an enhancement of the QD luminescence in parallel with a disappearance of the blue colour, since its formation constant39,40 is higher at the working pH (7.5). However, the EDTA-Mn2+ complex is not formed under these conditions, and thus no changes of the emission intensity were observed after addition of EDTA to the QD-zincon-Mn2+ complex. In this case, the zincon-Mn2+ complex could be decomposed under acid conditions.41
Selectivity of proposed Zn2+ and Mn2+ sensitive QD-zincon conjugates
The fluorescent response of the QD-zincon conjugate in the presence of various metal cations was first evaluated with the separate solution method in order to check the selectivity of the proposed Zn2+ or Mn2+ nanosensors. QD-zincon conjugates were exposed to several metal cations at different concentrations (TRIS pH 7.5) and the emission spectra scanned. Table 1 shows a summary of the highest metal cation concentration tested with no effect on the emission of the QDs (without pre-treatment of the sample). Physiologically important cations which exist at high concentration in living cells, such as Ca2+, Mg2+, Na+ and K+ did not produce any changes in the emission of the QD-zincon conjugates even at quite high concentrations, which points to the applicability of the proposed nanosensors also in physiological samples. This selectivity is presumably due to either the poor complexation of alkaline metals or alkaline earth metals with zincon or that they do not change the spectral characteristics41 (see ESI†, Fig. SI-3).| Metal | Concentration/mM |
|---|---|
| Na+ | 100 |
| K+ | 50 |
| Ca2+ | 20 |
| Mg2+ | 5 |
| Ni2+ | 5 |
| Co2+ | 2.5 |
| Fe2+ | 1 |
| Cu2+ | 0.01 |
However, the selectivity of the QD-zincon conjugates depends not only on the selectivity of the zincon itself but also on a direct ion interaction with the QD. Other transition metal cations such as Ni2+, Co2+ or Fe2+ did not produce big changes of the emission of the QD-zincon conjugates, even though complexes of zincon with those metals are easily formed in solution15,41 under these conditions (see ESI†, Fig. SI-3). Potential interference by Fe2+ is caused by inner filter effects,7 but the selectivity can be increased when necessary by the addition of F− as a masking agent to form a colourless complex. On the other hand, Cu2+ produced a very strong quenching of the QD-zincon conjugate luminescence. A similar quenching of QD-PAH+, even without the zincon conjugate, suggests that even though zincon-Cu2+ can form a blue complex very similar to the one with Zn2+, and therefore may show similar quenching of the QD emission, there is potential for direct QD quenching. Indeed, it has been reported previously that transition metals with half-filled d-orbitals, such as Cu2+, can produce the quenching of QDs by electron transfer between the metal and the QDs facilitating the non-radiative recombination.7 As a result, Cu2+ can be a potential interfering agent in the determination of Zn2+ or Mn2+ with QD-zincon conjugates.
In addition to the separate solution method, the response of the proposed QD-zincon conjugates towards 200 µM of Zn2+ and Mn2+, separately, in the presence of the same concentration of other foreign metals was evaluated (TRIS pH 7.5). The results are summarized in Fig. 5. In the case of Zn2+ determination, similar quenching of the QD emission was observed in the presence of almost all tested metals (Fig. 5a). Even in the presence of Mn2+ we can see quenching of the emission of the QDs in response to Zn2+, which suggests that the formation of the zincon-Zn2+ complex is favoured over zincon-Mn2+. Only in the presence of Cu2+ the quantitative detection of Zn2+ was not possible, for the reasons explained above.
 | ||
| Fig. 5 Response of QD540-zincon conjugates in 10 mM TRIS pH 7.5 towards (a) 200 µM Zn2+ and (b) 200 µM Mn2+ in the absence and in the presence of a solution of a specified interference metal ion of the same concentration. The emission intensity of the QD540-zincon conjugate without metal is also shown. | ||
When Mn2+ detection was evaluated the enhancement of the QD emission was also quite similar in the absence and in the presence of most of the tested metals (Fig. 5b). Again, Cu2+, but also Co2+ and Zn2+, produced a negative interference, and quenching instead of enhancement was seen. Therefore, for the determination of Mn2+ in the presence of these metals, alternative strategies must be designed to improve the selectivity, as shown in the next section. Nevertheless, taking into account the low selectivity of the chromogenic reagent used here, the combination of zincon with QDs significantly increased the selectivity for the determination of low concentrations of Zn2+ and Mn2+.
Deconvolution of the zinc and manganese signals
Since QD-zincon nanoparticles can respond to changes of both Zn2+ and Mn2+ concentrations, the challenge is to design an approach for sensing one metal in the presence of another. In the first instance, it is clear that QD500 shows no spectral overlap with the Zn2+-zincon complex and thus Zn-dependent RET is not activated for this system in contrast to QD540. The fluorescence emission/quenching mechanism can also be selected according to pH. The pH dependency of the response of the QD-zincon conjugates towards these metals mirrored that of the corresponding zincon complexes in solution (see ESI†, Fig. SI-4). For instance, it is well known that the zincon-Zn2+ complex in solution is only formed in basic conditions, since the deprotonation of the phenolic and carboxylic groups is needed for the coordination with Zn2+. Indeed, different pHs have been widely used in many approaches for the selective determination of Zn2+, in the presence of other metals that also form complexes with zincon.20,21 Below pH 6.6 the complex is not formed, and no change in the emission of the QDs could be seen. By increasing the pH up to 8.8 the formation of the complex is favoured and therefore a higher response of the QDs towards Zn2+ was detected. (Higher pHs were not tested, since the pKa of PAH+ is ∼9, so zincon would be desorbed at this pH, and also Zn2+ can form a white precipitate, Zn(OH)2, under more alkaline conditions.)Thus, the sensitivity (slope of calibration curve) could be increased by increasing the pH. The slopes of the fluorescence of QD540-zincon vs. log [metal ion] at different pHs (10 mM TRIS buffer) are summarized in Table 2. From these data the Zn2+ calibration slope can be adjusted between pH 7 and 8.2 by a factor of 0.33(pH)−1.
| pH | Zn2+ detection slope | Mn2+ detection slope |
|---|---|---|
| 6.3 | — | 1.1612 |
| 7.0 | 0.8302 | 1.2823 |
| 7.5 | 1.0208 | 1.5378 |
| 8.2 | 1.2015 | — |
| 8.8 | 1.8520 | — |
Behaviour mirroring the solution ion complex was also seen for Mn2+ determination. The zincon-Mn2+ complex was only formed from pH 6.2 (see ESI†, Fig. SI-4), but at pHs higher than 8.0 a brown precipitate was seen in the Mn2+ solutions, so that enhancement of the QD-zincon response to Mn2+ was only observed at pH ≈ 6.2–8.0. Again, the sensitivity to Mn2+ determination was increased at higher pHs (Table 2), but the calibration slope for Mn2+ was not linear with pH.
Thus, from Table 2 and the discussion above, it can be seen that the selective formation of the zincon-Mn2+ complex in the presence of Zn2+ is possible in a range of pH 6.2–6.6. To demonstrate this, Mn2+ determination was carried out in samples without and with three different concentrations of Zn2+, 15, 62 and 250 µM buffered at pH 6.5 (TRIS). No significant differences were found in the presence of Zn2+ and a reduced sensitivity was only observed when high Zn2+ concentration was used (250 µM).
An alternative strategy for the selective detection of Mn2+ is to use EDTA as a masking agent for Zn2+, which forms a stronger complex with EDTA than zincon. Solutions of Mn2+ at different concentrations were spiked with 100 µM of Zn2+ (TRIS pH 7.5) and the detection of Mn2+ in the presence and the absence of 100 µM of EDTA was performed and compared. Negligible response towards Mn2+ was obtained when EDTA was not in the medium, and formation of the blue zincon-Zn2+ complex was clearly observed. However, when EDTA was added, the blue colour disappeared and the sensitivity of the Mn2+ determination was similar to that obtained without Zn2+ (the slopes of the calibration lines were 1.851 and 1.794 in the absence and in presence of Zn2+, respectively). Thus, successful selective sensing of Mn2+ can be achieved in the presence of Zn2+ (Fig. 6a,b).
 | ||
| Fig. 6 Emission spectra of QD540-zincon conjugates after addition of (a) Mn2+ solutions and (b) Mn2+ + Zn2+ + EDTA solutions. Response of (c) QD540-zincon and (d) QD620-zincon toward Zn2+ in the presence of different concentrations of Mn2+ (TRIS pH 7.5): 0 µM (■), 50 µM (●), and 150 µM (▲). | ||
From these data we can also deduce that when the sensing of Zn2+ in the presence of Mn2+ is desired, the approach is rather simple, since the most favoured complex is zincon-Zn2+. In order to test this, the detection of Zn2+ in samples without and with different Mn2+ concentrations (50 and 150 µM) was performed at pH 7.5 with QD-zincon conjugates of two different QD populations. The linear plot of each set of samples was calculated and compared (Fig. 6c,d). No significant differences were seen when Mn2+ was in the medium, with only a small increase of the calibration slope by increasing Mn2+ concentration found. Moreover, similar results were obtained with QD540 and QD620. In the case of a higher Mn2+ concentration present in the samples, the interference might be reduced with sodium ascorbate42 or sodium citrate20 as masking agent.
Moreover, determination of these metal ions in synthetic samples containing Ni2+, Fe2+, Co2+, Ca2+, Mg2+, Na+ and K+ at concentrations of 50, 100 and 250 µM of each metal (TRIS pH 7.5) was also tested. The mixtures were spiked with different concentrations of Zn2+ and Mn2+, and the response of the QD-zincon conjugates was tested and compared with a control without the metal ion mixture. In the case of Zn2+ sensing, no differences were found in the absence and in the presence of concentrations of all these metal ions (Fig. 7a). This indicates again that the more favoured complex is zincon-Zn2+. On the other hand, reduced sensitivity was observed at high concentrations of the ionic mixture for Mn2+ (Fig. 7b).
 | ||
| Fig. 7 Response of QD540-zincon conjugates towards (a) Zn2+ and (b) Mn2+ in the presence of different concentrations of mixtures of metal ions (Ni2+, Fe2+, Co2+, Ca2+, Mg2+, Na+ and K+): 0 µM (■), 50 µM (●), 100 µM (▲) and 250 µM (▼) of each metal in the mixture (TRIS pH 7.5). | ||
To put these results into perspective, we have examined the detection limit (<1 µM) and range obtained here for the proposed Zn2+ and Mn2+ nanosensors in the context of the detection of these metal ions in a variety of real samples. For example, the amount of zinc can range throughout the body from nanomolar concentrations in the cytosol of certain cells to millimolar concentrations in some neuronal vesicles.43
The natural zinc content of soils44 is estimated to be 1–300 mg/kg (15 µM–4.5 mM), which is covered completely by the QD-zincon system, but in natural surface waters, the concentration of zinc is usually below 10 µg/l (0.15 µM), and in groundwaters, 10–40 µg/l.45 The latter is at the limit of detection. In tap-water, the zinc concentration can be much higher as a result of the leaching of zinc from piping and fittings,44 and for routine testing of drinking water, these higher concentrations are of interest, up to 1.1 mg/l (17 µM) and even higher zinc concentrations (up to 24 mg/l) measured in wells.
Manganese content in the soil can vary widely. It is only 50 ppm in some localities, but can reach 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm in unleached alkali soils. In groundwaters subject to reducing conditions manganese can be leached from the soil and occur in high solution concentrations. However, manganese is rarely found in natural surface waters in concentrations above 1000 µg/l, but the World Health Organization (WHO) recently lowered the guideline maximum value for manganese in drinking water to 400 µg/l (7 µM). This is in the region of highest sensitivity for the Zn2+ QD-zincon system.
000 ppm in unleached alkali soils. In groundwaters subject to reducing conditions manganese can be leached from the soil and occur in high solution concentrations. However, manganese is rarely found in natural surface waters in concentrations above 1000 µg/l, but the World Health Organization (WHO) recently lowered the guideline maximum value for manganese in drinking water to 400 µg/l (7 µM). This is in the region of highest sensitivity for the Zn2+ QD-zincon system.
Normal values for manganese levels in human blood (serum)46 are below the detection range here at about 1.14 µg/l (20 nM), but both Zn2+ and Mn2+ are also found in several pharmaceutical and/or cosmetic preparations and in foods and food supplements at levels within the useful range for the QD-zincon system. The only ion that strongly interfered with the detection of Zn2+ and Mn2+ was Cu2+, showing quenching of the fluorescence. This remains the limitation of our system despite its higher sensitivity and selectivity compared to other methods based on zincon.19,22,38 Again, as shown above, when it is needed the selectivity can be enhanced by the addition of proper masking agents. The results indicate the utility of the proposed QD-zincon-based nanosensors for the determination of Zn2+ and Mn2+ in a variety of samples containing mixtures of different metals.
Conclusions
We have demonstrated that CdSe/ZnS core/shell QD nanoparticles can be efficiently modified with zincon by layer-by-layer self-assembly, producing partial quenching of QD luminescence properties. The resulting QD-zincon conjugate forms the basis for a dual mechanism multiplexed assay system for Zn2+ and Mn2+. Since zincon is a chromogenic reagent that can complex metals, QD-zincon conjugates offer a good system for the sensing of those metals. Selective fluorescence quenching of QD-zincon in the presence of Zn2+ was detected as a result of RET for QDs with the right spectral overlap, whereas for the Mn2+-zincon complex there is no overlap of QD emission and Mn2+-zincon absorbance so that RET is not favoured. Furthermore, the probable overlap of molecular orbitals on the metal-free zincon with one of the charge carriers on the QD, which produced the initial quenching, is disrupted by complexation with Mn2+, so that the zincon quenching effect is reversed, thus ‘turning on’ the emission of the QD nanoparticles.By using the combination of a conventional broadband chromogenic reagent, such as zincon, with QDs, we succeeded in the development of very simple fluorescent detection sensors for Zn2+ and Mn2+, with high selectivity. Indeed, higher selectivity than classic methods, employing the same reagent, can be achieved. Moreover, a considerable asset of these QD-zincon conjugates is the ability to select the emission wavelength according to the choice of QD, thereby, avoiding overlap with other signals and resolution against background fluorescence to be tuned.
The suitability of these ion-sensitive QDs for the determination of Zn2+ and Mn2+ in samples containing a high concentration of other metal ions was also demonstrated. The samples tested were synthetic ionic mixtures, and QD-zincon conjugates were able to detect Zn2+ and Mn2+ with minimal reduction in sensitivity. To make this system more flexible and robust for analysis, work is now underway to produce 3-D structures containing modified QDs, which can be used as a solid phase system for analysis.
Acknowledgements
The authors acknowledge support from the BBSRC (BBD0013071)/EPSRC (GR/T17007/01) and the Newton Trust, Cambridge for the funding.References
- C. N. Ferrarello, M. Montes Bayón, J. I. García Alonso and A. Sanz-Medel, Anal. Chim. Acta, 2001, 429, 227–235 CrossRef CAS.
- J. A. Sweileh, Microchem. J., 2000, 65, 87–95 CrossRef CAS.
- L. E. Vanatta, J. C. Vanatta and J. Riviello, J. Chromatogr., A, 2000, 884, 143–150 CrossRef CAS.
- M. J. Ayora-Cañada, M. I. Pascual-Reguera and A. Molina-Díaz, Anal. Chim. Acta, 1998, 375, 71–80 CrossRef.
- I. Grabchev and J. M. Chovelon, Dyes Pigm., 2007, 77, 1–6.
- D. E. Moore and K. Patel, Langmuir, 2001, 17, 2541–2544 CrossRef CAS.
- Y. Chen and Z. Rosenzweig, Anal. Chem., 2002, 74, 5132–5138 CrossRef CAS.
- L.-G. Liang, X.-P. Ai, Z.-K. He and D.-W. Pang, Analyst, 2004, 129, 619–622 RSC.
- J.-L. Chen and C.-Q. Zhu, Anal. Chim. Acta, 2005, 546, 147–153 CrossRef CAS.
- M. T. Fernández-Arguëlles, W. J. Jin, J. M. Costa-Fernández, R. Pereiro and A. Sanz-Medel, Anal. Chim. Acta, 2005, 549, 20–25 CrossRef.
- K. M. Gattás-Asfura and R. M. Leblanc, Chem. Commun., 2003, 2684–2685 RSC.
- J. Li, D. Bao, X. Hong, D. Li, J. Li, Y. Bai and T. Li, Colloids Surf., A, 2005, 257–258, 257–271.
- M. J. Ruedas Rama and E. A. H. Hall, Anal. Chem., 2008, 80, 8260–8268 CrossRef CAS.
- J. Ghasemi, A. Niazi and R. Leardi, Talanta, 2003, 59, 311–317 CrossRef CAS.
- J. Ghasemi, S. Ahmadi and K. Torkestani, Anal. Chim. Acta, 2003, 487, 181–188 CrossRef CAS.
- N. Maniasso, E. A. G. Zagatto and R. E. Santelli, Anal. Chim. Acta, 1996, 331, 17–22 CrossRef CAS.
- I. Oehme, S. Prattes, O. S. Wolfbeis and G. J. Mohr, Talanta, 1998, 47, 595–604 CrossRef CAS.
- S. Rastegarzadeh and V. Rezaei, Sens. Actuators, B, 2008, 129, 327–331 CrossRef.
- R.-M. Liu, D.-J. Liu and A.-L. Sun, Talanta, 1993, 40, 511–514 CrossRef CAS.
- P. Richter, M. I. Toral, A. E. Tapia and E. Fuenzalida, Analyst, 1997, 122, 1045–1048 RSC.
- M. J. Ruedas-Rama, A. Ruiz-Medina and A. Molina-Díaz, Anal. Sci., 2005, 21, 1079–1084 CrossRef CAS.
- L. K. Shpigun, Y. V. Shushenachev and P. M. Kamilova, Anal. Chim. Acta, 2006, 573–574, 360–365 CrossRef CAS.
- W. C. W. Chan and S. Nie, Science, 1998, 281, 2016–2018 CrossRef CAS.
- G. P. Mitchell, C. A. Morkin and R. L. Letsinger, J. Am. Chem. Soc., 1999, 121, 8122–8123 CrossRef CAS.
- F. Patolsky, R. Gill, Y. Weizmann, T. Mokari, U. Banin and I. Willner, J. Am. Chem. Soc., 2003, 125, 13918–13919 CrossRef CAS.
- S. Jaffar, K. T. Nam, A. Khademhosseini, J. Xing, R. S. Langer and A. M. Belcher, Nano Lett., 2004, 4, 1421–1425 CrossRef CAS.
- M. J. Ruedas Rama and E. A. H. Hall, Analyst, 2008, 133, 1556–1566 RSC.
- S. Muller, G. Koenig, A. Charpiot, C. Debry, J.-C. Voegel, P. Lavalle and D. Vautier, Adv. Funct. Mater., 2008, 18, 1767–1775 CrossRef CAS.
- J. C. Scaiano, M. Laferrière, R. E. Galian, V. Maurel and P. Billone, Phys. Status Solidi, 2006, 203, 1337–1343 Search PubMed.
- C. B. Murphy, Y. Zhang, T. Troxler, V. Ferry, J. J. Martin and W. E. Jones, Jr, J. Phys. Chem. B, 2004, 108, 1537–1543 CrossRef CAS.
- J. S. Stroud, Appl. Opt., 1968, 7, 751–757 CrossRef CAS.
- A. Kashiwada, Y. Nakamura and K. Matsuda, Sens. Actuators, B, 2005, 108, 845–850 CrossRef.
- C. Cano-Raya, M. D. Fernández-Ramos and L. F. Capitan-Vallvey, Anal. Chim. Acta, 2006, 555, 299–307 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, New York, London, Moscow, Dordrecht, 2nd edn, 1999 Search PubMed.
- D. Magde, G. E. Rojas and P. Seybold, Photochem. Photobiol., 1999, 70, 737–744 CrossRef CAS.
- IUPAC, Spectrochim. Acta, Part B, 1978, 33, 242–245.
- ACS Committee on Environmental Improvement, Anal. Chem., 1980, 52, 2242–2249 CrossRef.
- R. M. Rush and J. H. Yoe, Anal. Chem., 1954, 26, 1345–1347 CrossRef CAS.
- J. K. Nyborg and O. B. Peersen, Biochem. J., 2004, 381, E3–E4 CrossRef CAS.
- J. Laib, C. F. Shaw, D. H. Petering, M. K. Eidsness, R. C. Elder and J. S. Garvey, Biochemistry, 1985, 24, 1977–1986 CrossRef CAS.
- E. Hilario, I. Romero and H. Celis, J. Biochem. Biophys. Methods, 1990, 21, 197–207 CrossRef CAS.
- D. A. Drum, G. J. Shugar, S. L. Bauman and J. Lauber, Environmental Field Testing and Analysis Ready Reference Handbook, McGrawHill Profesional, New York, 2000 Search PubMed.
- J. Silvia and R. Williams, The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, Claredon Press, Oxford, 1991 Search PubMed.
- J. O. Nriagu, Zinc in the environment. Part I, Ecological cycling, John Wiley, New York, NY, 1980 Search PubMed.
- C. G. Elinder, L. Friberg, G. F. Nordberg and V. B. Vouk, Handbook on the toxicology of metals, Elsevier Science Publishers, Amsterdam, 2nd edn, 1986 Search PubMed.
- A. Favier and D. Ruffieux, J. lnher. Metab. Dis., 1983, 6, 90 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: calculation of the quantum yield of QD540 and QD620, and Förster distance (R0) for the pairs QD540-Zincon-Zn2+ and QD540-Zincon-Zn2+. Response of QD540-zincon conjugates toward Zn2+ at different ionic strengths. Response time of QD540-zincon conjugates towards Zn2+ and Mn2+. Absorption spectra of zincon and complexes of zincon with different metals. Absorption spectra of zincon-Zn2+ and zincon-Mn2+ at different pHs. See DOI: 10.1039/b814879a |
| This journal is © The Royal Society of Chemistry 2009 |