Carbon-fiber microelectrodes for in vivo applications
Megan L.
Huffman
and
B. Jill
Venton
*
Department of Chemistry, University of Virginia, P.O. Box 400319, Charlottesville, VA 22904, USA. E-mail: bjv2n@virginia.edu; Fax: +1 (434) 924-3710; Tel: +1 (434) 243-2132
First published on 31st October 2008
Abstract
Carbon-fiber microelectrodes (CFMEs) have been a useful tool for measuring rapid changes in neurotransmitters because of their small size, sensitivity, and good electrochemical properties. In this article, we highlight recent advances using CFMEs for measuring neurotransmitters in vivo. Dopamine has been a primary neurotransmitter of interest but direct electrochemical detection of other neurochemicals including nitric oxide and adenosine has also been investigated. Surface treatments have been studied to enhance electrode sensitivity, such as covalent modification or the addition of a layer of carbon nanotubes. Enzyme-modified microelectrodes that detect non-electroactive compounds further extend the usefulness of CFMEs beyond the traditional monoamines. CFMEs continue to be used in vivo to understand basic neurobiological mechanisms and the actions of pharmacological agents, including drugs of abuse. Advances in sensitivity and instrumentation now allow CFMEs to be used for measurements of natural dopamine release that occur during behavioral experiments. A new technique combining electrochemistry with electrophysiology at a single microelectrode facilitates a better understanding of neurotransmitter concentrations and their effects on cell firing. Future research in this field will likely concentrate on fabricating smaller electrodes and electrode arrays, as well as expanding the use of CFMEs in neuroscience beyond dopamine.
 Megan Huffman | Megan L. Huffman received her B.S. in chemistry from Mary Baldwin College in Staunton, Virginia. She is currently a chemistry graduate student under Dr B. Jill Venton at the University of Virginia. Her research interests include examining the properties of different types of carbon-fiber microelectrodes and making neurotransmitter measurements in rat brain slices. |
 Jill Venton | B. Jill Venton is an Assistant Professor at the Department of Chemistry and Neuroscience Graduate Program at the University of Virginia. She received her Ph.D. from the University of North Carolina and was previously a postdoctoral fellow at the University of Michigan. Her research interests include the study of new carbon electrodes, in vivo measurements of neurotransmission, and high-speed capillary electrophoresis separations. |
Introduction
Carbon-fiber microelectrodes (CFMEs) were introduced nearly 30 years ago when Pujol and colleagues first used them with normal pulse polarography to measure the oxidation of several neurotransmitters, including dopamine, norepinephrine and serotonin.1 Today, the most popular applications for carbon-fiber electrodes are still as sensors for neurotransmitters. CFMEs have many advantages for biological applications. First, carbon fibers are biologically compatible and not toxic to cells. Second, their electrochemistry has been well characterized and they have good electrochemical properties.2 Third, because the carbon fibers used for electrodes are usually less than 10 μm in diameter, they are amenable for implantation and cause less tissue damage than larger conventional electrodes.3Another main advantage of CFMEs is their ability to make rapid measurements when used with fast electrochemical techniques such as amperometry, high-speed chronoamperometry and fast-scan cyclic voltammetry (FSCV). Constant-potential amperometry is commonly used to quantify neurotransmitter release and can be used with rapid sampling rates, often up to 500 Hz. However, it offers little selectivity and thus is commonly used to measure vesicular release of neurotransmitters from single cells where the identity of the neurotransmitter is known.4 High-speed chronoamperometry applies a high frequency square wave potential to the electrode. The waveform allows measurement of both oxidative and reductive currents, and the ratio of these currents offers improved selectivity over amperometry since major neurotransmitters such as dopamine and serotonin have different ratios.5,6 FSCV is a rapid scanning technique that provides an output of current versus voltage, the cyclic voltammogram, which aids in analyte identification. Fast scanning produces a large charging current but this current is relatively stable at carbon fibers and can be subtracted from the analyte signal to give a background-subtracted cyclic voltammogram. FSCV is most commonly used for in vivo studies because it provides some selectivity.
This review looks at the advances in carbon-fiber microelectrode research for in vivo studies over the past three years. Many of the electrochemical studies focus on new surface treatments to improve sensitivity or selectivity of electrodes. Detection of non-electroactive analytes may be achieved by modification of microelectrodes with enzymes. For in vivo studies, CFMEs continue to be important in understanding basic mechanisms of neurotransmission and the effects of pharmacological agents. While detection of neurotransmitter activity has traditionally relied on electrical stimulation of neurons, behaviorally-evoked changes in dopamine neurotransmission can now be measured. In addition, both electrochemical measurements of neurotransmitter dynamics and electrophysiological measurements of cell firing can be performed at a single electrode. These developments are described below.
Electrochemistry innovations
A continuing focus for carbon-fiber microelectrode studies is improving the sensitivity and selectivity of the electrode. With increasing interest in measuring complex mixtures, the ability to detect multiple compounds or one analyte in the presence of interferents is necessary. Because carbon surfaces are easily modified, the most common method of improving electrode performance is to coat the carbon-fiber surface. Molecules may be covalently attached to oxide groups on the electrode surface, tuning surface properties such as charge and active site density. Alternatively, polymers or redox hydrogels can be used to coat the surface, and materials such as carbon nanotubes are often embedded in them to improve sensitivity or selectivity. Enzymes can also be attached to electrodes, allowing amperometric detection of non-electroactive compounds.Electrode treatments
Dopamine is one of the best characterized neurotransmitters for electrochemical detection by CFMEs. Some of the earliest efforts to treat electrodes focused on increasing the selectivity of CFMEs for dopamine over anionic interferents such as ascorbic acid.7,8 The traditional method has been to apply a coating of Nafion, an anionic polymer that repels negatively-charged ascorbic acid and prevents it from reaching the electrode. However, Nafion coatings decrease the electrode response time for all compounds. In a recent study, the Wightman group covalently modified CFMEs with 4-sulfobenzene and found increased sensitivity and selectivity for catecholamines, but without the decrease in electrode response time.9 However, unlike Nafion, 4-sulfobenzene is not completely impermeable to anions, and some current from ascorbic acid could still be detected.Another method to change the surface properties of microelectrodes is etching of the surface. Our lab has recently shown that flame-etching of carbon-fiber electrodes increases the sensitivity for neurotransmitters while decreasing the sensitivity of the electrode to changes in pH.10 These small electrodes show increased current per unit surface area compared to both normal electrodes and electrodes that have been electrochemically etched. They are robust enough to be implanted in vivo, and the increased sensitivity allows detection of dopamine release after a single electrical pulse stimulation.
Carbon nanotube coatings of larger glassy carbon electrodes have been found to increase electrode sensitivity and decrease electrode fouling.11 Several labs have recently explored different methods for coating CFMEs with carbon nanotubes. For example, CFMEs have been modified with carbon nanotubes suspended in room temperature ionic liquids (RTILs).12 The nanotube coating promoted direct electron transfer to glucose oxidase, and distinct oxidation peaks for mixtures of dopamine and ascorbic acid were detected using square wave voltammetry. Multi-walled carbon nanotubes (MWCNTs), suspended in dimethylformamide, have also been used as a coating to measure ascorbic acid in vivo with differential pulse voltammetry.13 The Wang group tested coatings of Nafion-suspended MWCNTs on CFMEs.14 They found that dopamine could be detected in the presence of ascorbic acid using differential pulse voltammetry. Our lab used a similar coating strategy to test whether nanotube-modified microelectrodes could be used with fast-scan cyclic voltammetry (FSCV).15 We were able to detect serotonin and dopamine simultaneously in vivo with FSCV. Serotonin detection is problematic because its oxidation products adsorb to the electrode, forming an insulating layer that decreases electrode sensitivity. However, less electrode fouling occurred when the microelectrodes were coated in carbon nanotubes. This is shown in Fig. 1(a) where the current for normal, untreated electrodes decreases more rapidly with repeated exposures to serotonin than for nanotube-coated electrodes. Dopamine and serotonin could be detected simultaneously by distinguishing their reduction peaks in the cyclic voltammogram [Fig. 1(b)]. These studies have shown that the benefits of carbon nanotube modification can be applied to CFMEs and used for in vivo detection of neurotransmitters.
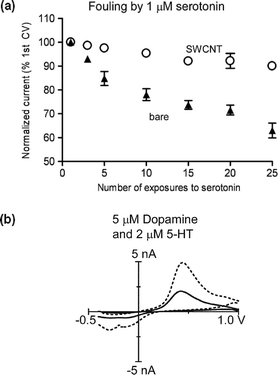 | ||
| Fig. 1 The Venton lab used carbon nanotube-modified CFMEs for measurement of serotonin and dopamine. (a) Effects of nanotubes on fouling of the electrode by serotonin. Bare electrodes (▲) have a larger decrease in current after repeated exposure to serotonin (5-HT) than single-walled carbon nanotube-modified electrodes (SWCNT, ○), showing that carbon nanotubes protect the electrode from fouling. (Reproduced with permission from Fig. 6 of ref. 15.) (b) Detection of 2 μM serotonin and 5 μM dopamine simultaneously in vitro. The carbon nanotube-modified electrode (dotted line) shows two reduction peaks, which allow the differentiation of dopamine and serotonin more easily than at a bare electrode (solid line). (Reproduced with permission from Fig. 2 of ref. 15.) | ||
New compounds
While carbon-fiber microelectrodes have traditionally been used to monitor monoamine neurotransmitters such as dopamine and serotonin, new research is aimed at expanding the number of compounds than can be detected. For example, CFMEs have been used to detect purines such as guanine or uric acid.16 Our group has used fast-scan cyclic voltammetry for the direct detection of the purine adenosine, an endogenous neuromodulator that influences both neurotransmission and cerebral blood flow. Adenosine changes can be measured on a sub-second timescale using CFMEs.17 Using this method, we detected adenosine and dopamine efflux in vivo in the caudate-putamen after electrical stimulation of dopamine neurons (Fig. 2).18 The false-color plot in Fig. 2 shows the fast-scan cyclic voltammetry data collected in the rat caudate-putamen. The x-axis plots time and the y-axis potential, with the switching potential of the triangular waveform in the center of the axis. Current changes are represented in false-color. Oxidative currents are shown in green, while reductive currents are blue. The green area around +0.6 V immediately after the five second mark is due to dopamine oxidation, while the green area at +1.5 V starting at 7.3 s is adenosine oxidation. The top plots show traces for changes at the oxidation potential for dopamine and adenosine, and adenosine release lasts longer and is delayed from dopamine release.![Measurement of electrically-stimulated dopamine and adenosine in the striatum of anesthetized rats using CFMEs. (a) Concentration profile for dopamine, taken at 0.6 V, the oxidation potential for dopamine [located at the red dashed line in the color plot (e)]. (b) Concentration profile for adenosine, taken at 1.5 V, the oxidation potential for adenosine (yellow dashed line in the color plot). Note that adenosine release is slower and lasts longer following stimulation than that of dopamine. (c) A cyclic voltammogram (CV) taken at 6 s (white dashed line on the color plot), showing primarily dopamine. (d) A CV taken at 7.3 s (blue dashed line on the color plot), showing a mixture of adenosine and dopamine. (e) Color plot represents all of the data, with scanned voltage on the y-axis, time on the x-axis and current in false-color. (Reproduced with permission from Fig. 2 of ref. 18. Copyright 2008, Blackwell Publishing.)](/image/article/2009/AN/b807563h/b807563h-f2.gif) | ||
| Fig. 2 Measurement of electrically-stimulated dopamine and adenosine in the striatum of anesthetized rats using CFMEs. (a) Concentration profile for dopamine, taken at 0.6 V, the oxidation potential for dopamine [located at the red dashed line in the color plot (e)]. (b) Concentration profile for adenosine, taken at 1.5 V, the oxidation potential for adenosine (yellow dashed line in the color plot). Note that adenosine release is slower and lasts longer following stimulation than that of dopamine. (c) A cyclic voltammogram (CV) taken at 6 s (white dashed line on the color plot), showing primarily dopamine. (d) A CV taken at 7.3 s (blue dashed line on the color plot), showing a mixture of adenosine and dopamine. (e) Color plot represents all of the data, with scanned voltage on the y-axis, time on the x-axis and current in false-color. (Reproduced with permission from Fig. 2 of ref. 18. Copyright 2008, Blackwell Publishing.) | ||
Direct detection of nitric oxide (NO) has also been achieved with CFMEs. Nitric oxide is an important gaseous signaling molecule that has roles in smooth muscle relaxation, neurotransmission and neurodegeneration. Owing to its high reactivity, measuring nitric oxide in biological systems can be difficult and the high oxidation potential needed for amperometric detection can lead to interference from other electroactive compounds. Carbon fibers modified with Nafion and o-phenylenediamine have been used to measure nitric oxide concentrations amperometrically.19 The polymer acts as a molecular filter, keeping molecules larger than nitric oxide away from the electrode. The Nafion also reduces side reactions such as nitrate oxidation, improving the sensitivity of the electrode. The limit of detection was in the nanomolar range, with a linear response to nitric oxide concentrations between 100 and 1000 nM. Selectivity for NO was better against anions such as ascorbic acid than cations, but the sensor was still 18 times more sensitive for NO than dopamine. In addition, the background current of the electrode was not affected by glutamate, arginine or N-methyl-D-aspartate (NMDA), compounds used to stimulate nitric oxide release in vivo. This electrode was able to detect nitric oxide release after glutamate stimulation of rat hippocampal brain slices.19
Amperometric enzyme sensors
Biosensors based on CFMEs modified with enzymes have been developed to allow selective monitoring of a specific compound. There are two possible methods for attaching the enzyme: direct covalent bonding of the enzyme to the electrode, or entrapping the enzyme in a polymer film on the electrode surface. Chemical attachment promotes direct electron transfer from the enzyme to the electrode. In polymer films, the mode of detection is either direct electron transfer to enzymes or the detection of electroactive products after an enzymatic reaction.An example of the direct attachment approach is the detection of superoxide at a modified carbon-fiber microelectrode. The superoxide anion can induce oxidative stress in living cells. Its potential role as a non-classical messenger molecule in response to bacterial infection creates interest for the measurement of the presence and flux of superoxide anion. Recently, the Ohsaka group developed a biosensor for this anion and the schematic is shown in Fig. 3(a).20 The carbon-fiber microelectrode was first coated with a layer of gold nanoparticles, which were then modified with cysteine. The cysteine was used to immobilize superoxide dismutase (SOD) onto the electrode. This is the first demonstration of using self-assembled monolayer chemistry based on gold–thiol bonds to modify CFMEs for direct detection of electron transfer to enzymes. The biosensor was found to be sensitive and to have a fast, stable response for long periods of time. In addition, the sensor response was resistant to interferents, making it useful for in vivo applications.
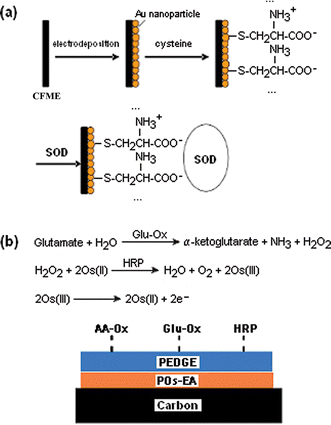 | ||
| Fig. 3 Enzyme-modified carbon-fiber microelectrodes. (a) The Ohsaka lab developed a superoxide anion biosensor. First, gold nanoparticles were electrodeposited onto the carbon fiber. Next, the gold nanoparticles were modified with cysteine, which was then used to immobilize superoxide dismutase (SOD) onto the electrode. Direct electron transfer between the SOD and the carbon fiber allowed for detection of superoxide anion. (Adopted and reproduced with permission from Scheme 1 of ref. 20. Copyright 2005, Elsevier B.V.) (b) The Westerink lab developed a glutamate sensor.22 Ascorbate oxidase (AA-Ox), glutamate oxidase (Glu-Ox) and horseradish peroxidase (HRP) were immobilized onto poly(ethylene glycol) diglycidyl ether (PEDGE). PEDGE acts as a cross-linker to bind the enzymes to an osmium-containing redox polymer (POs-EA). Glutamate is detected by a cascade of enzymatic reactions that produce osmium(III) which can be oxidized at the carbon surface. AA-Ox is included in the sensor to circumvent the action of ascorbate, which can interfere with the cascade by reducing Os(III), oxidized HRP or H2O2. | ||
For polymer-coated electrodes, immobilization of enzymes within coatings of redox hydrogels has been of particular interest. When hydrated, redox hydrogels mediate electron transfer between the electrode and the enzyme. However, when the hydrogel liquid is added to a mixture of enzymes it can precipitate, leading to a heterogeneous enzyme–polymer mixture that unevenly coats an electrode. The Michael lab showed that adding the surfactant sodium dodecyl sulfate (SDS) to the coating solution can be used to reduce precipitation and improve the overall homogeneity of the enzyme–polymer mixture.21 This results in more reproducible electrode fabrication.
The Westerink lab has used redox hydrogels to develop a carbon-fiber microelectrode-based glutamate sensor. By optimizing the composition of the redox hydrogel–enzyme coating and automating the coating system, they were able to improve the reproducibility of their coatings.22 This glutamate microsensor contains three enzymes: glutamate oxidase for glutamate detection, horseradish peroxidase to mediate electron transfer to osmium, and ascorbate oxidase to reduce ascorbic acid interference; as well as two polymeric components: poly(ethylene glycol) and an osmium redox polymer [Fig. 3(b)]. The electrode was also coated with Nafion. This microsensor was characterized for selectivity, sensitivity, and biofouling.23 They found that it is most sensitive for glutamate, but is also mildly susceptible to interference from ascorbic acid and uric acid. Though the microsensor has high sensitivity for hydrogen peroxide and oxygen, it may be used to analyze microdialysate samples because the hydrogen peroxide response can be subtracted out and the low oxygen levels present in the brain have little effect on the microsensor. This electrode is routinely used by this group for in vitro and in vivo studies.23
In vivo applications
The primary application for carbon-fiber microelectrodes has been for real-time monitoring of neurotransmitter changes. Dopamine continues to be a key target for analysis because of its good electrochemistry and importance to neurobiology. Studies of other monoamine neurotransmitters such as norepinephrine and anions such as ascorbic acid are growing in popularity. CFMEs are useful measurements in a variety of preparations, including studies of single cells, brain slices and experiments in living animals. In this section we overview recent research on in vivo applications. The main areas of investigation include using CFMEs to understand biological mechanisms of neurotransmission, effects of pharmacological agents, and the regulation of behaviorally-evoked release.Basic mechanisms of neurotransmission
The rapid nature of electrochemical measurements using CFMEs has allowed a better understanding of the dynamics of monoamine neurotransmission. For example, studies of electrically-stimulated dopamine release in anesthetized mice have been used to understand the reserve pool, a group of vesicles that is not released during normal activity. Mice lacking synapsin, a protein that tethers vesicles to the reserve pool, have greater dopamine release, indicating that a larger portion of vesicles can be released.24 Fast-scan cyclic voltammetry at CFMEs has also been used to understand how traumatic brain injuries affect dopaminergic transmission.25 Stimulated dopamine release was evoked in anesthetized rats and dopamine overflow levels were lower and dopamine clearance was slower after injury. These data indicate that dopamine agonists are of therapeutic value for treating brain injury by increasing dopamine levels that have been reduced by trauma.The mechanisms of other neurotransmitters have also been studied. Tanila and co-workers have used amperometry and FSCV at Nafion-coated CFMEs to characterize the basic mechanisms of norepinephrine release in the dentate gyrus of the hippocampus.26 They found that norepinephrine release patterns are similar to dopamine. In addition, the replenishment of a readily releasable pool of norepinephrine after stimulated release occurs at a rate similar to that of dopamine. Transient release of norepinephrine after natural stimuli could be observed after administration of a norepinephrine uptake inhibitor to raise concentrations. Chronoamperometry at CFMEs has been used to determine the clearance of exogenously-applied serotonin in various brain regions of the anesthetized rats.6 Both serotonin transporters and norepinephrine transporters were found to contribute to the clearance of serotonin.
Effects of pharmacological agents
CFMEs also continue to be used to study the effects of drugs and pharmacological agents. In particular, many researchers have focused on characterizing the effects of addictive drugs on dopamine release. Two studies have looked at the effect of ethanol on dopamine activity. Budygin and co-workers have found that a moderate dose of ethanol decreases dopamine signals but does not significantly alter either Km, the affinity of the dopamine transporter for dopamine, or the maximum rate of uptake (Vmax), which is related to the number of dopamine transporters.27 The Wightman lab observed similar results with larger does of ethanol, which caused a dose-dependent decrease in stimulated dopamine release.28 With the larger doses, the maximal rate of uptake, Vmax, appears to decrease.CFMEs have also been used to study the spontaneous release of dopamine after pharmacological agents without electrical stimulation. An example of this is the demonstration that cocaine causes an increase in fast spikes of dopamine release, called dopamine transients, as well as a slower increase in basal dopamine levels.29,30 Cheer et al. showed that cocaine, ethanol and nicotine all increased dopamine levels in the brain immediately after an acute dose was administered intravenously.31 Administration of a cannabinoid antagonist reduced dopamine release, indicating that these increases were mediated through cannabinoid receptors. Dopamine transients can also be measured in anesthetized rats after administration of a dopamine uptake inhibitor, nomifensine, and a D2 dopamine receptor antagonist, haloperidol.32 In anesthetized animals, few dopamine transients are observed without pharmacological manipulation.
Detection of behaviorally-evoked dopamine transients
One of the newer applications of CFMEs is the detection of behaviorally-evoked dopamine transients. Recent advances in low-noise instrumentation and sensitivity of CFMEs33 now make it possible to observe short-lived dopamine release events in the 50–100 nM range. Behavioral paradigms such as operant behaviors, where an animal presses a lever for a desired reward such as drug or food delivery, allow the study of synchronization of specific behaviors and dopamine release. For example, the first behavioral studies of this kind looked at the role of dopamine in cocaine self-administration and found dopamine transients both immediately before and after rats pressed a lever to deliver a dose of cocaine, indicating a role in anticipation and reward.34 Further studies to explore the mechanism of those transients showed that even if the animal was extinguished from self-administration (by turning off the cocaine pump so that pressing the lever did not result in reward), dopamine was still transiently released before the animal pressed the lever (Fig. 4).35 However, dopamine released after the lever press was severely diminished if the animal did not receive cocaine. This shows that there are different mechanisms for controlling spontaneous dopamine release. Voltammetry at microelectrodes has also been used to demonstrate that dopamine is released after a reward, but if that reward can be predicted, dopamine is then released due to the predictive stimulus and not when the reward is administered.36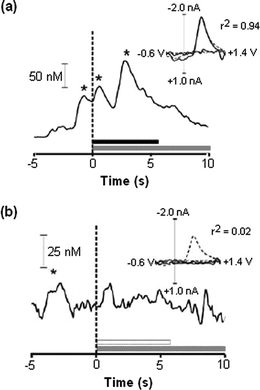 | ||
| Fig. 4 Carbon-fiber microelectrodes were used to study dopamine release during operant behavior. (a) Freely-moving rats were given an i.v. dose of cocaine (horizontal black bar) when they pressed a lever (vertical dashed line at time = 0). Dopamine levels rose when the rats were presented with the lever (first asterisk), and again when cocaine was administered (second and third asterisks). Researchers gave the rats a drug-related cue when the lever was pressed and cocaine provided (horizontal gray line). The voltammogram insert confirms that dopamine is detected by comparing behaviorally-evoked release (black line) to electrically-evoked release (dotted line). (b) When the rats were given a saline infusion in response to pressing the lever, there is no rise in dopamine levels. There is, however, a rise in dopamine when the rats are presented with the lever. This indicates that dopamine is involved in anticipatory behavior. (Adapted with permission from Fig. 2 and Fig. 3 of ref. 35. Copyright 2005, Elsevier B.V.) | ||
Wightman, Carelli and co-workers have also studied the effect of electrode placement in the rat brain on dopamine signals, either naturally-evoked or electrically-stimulated.30 Surprisingly, sites with the highest frequency of behaviorally-evoked transients were not the same as those that saw the greatest stimulated release. Though it has long been observed that electrode positioning affects signals, this is the first systematic study that has been published. Detection of behaviorally-evoked neurotransmitter changes will allow a better understanding of the concentrations of neurotransmitters available to signal in the brain and the time course of that signaling. These experiments will no doubt be expanded in the future to encompass a variety of behaviors as well as other neurotransmitters besides dopamine.
Electrochemistry combined with electrophysiology
In 1990, Williams and Millar used a combination of fast differential ramp voltammetry and steady-state electrophysiology to measure both dopamine release and neuronal activity in response to electrical stimulation.37 However, combining the two techniques is technically challenging and only recently has the Wightman group developed reliable instrumentation and software to combine electrochemical and electrophysiological measurements in freely behaving animals. The experimental paradigm is shown in Fig. 5(a). Fast-scan cyclic voltammetry is performed at the carbon-fiber working electrode and the cyclic voltammograms provide data on the identity and amount of neurotransmitter released. Between cycles, instead of being held at a holding potential, the electrode is switched from current measurement to voltage measurement. This allows the electrode to be used for electrophysiological measurements of neuronal firing patterns of neurons adjacent to the electrode. The advantage of this technique is that both the chemical signal, dopamine, and its potential effects on neuronal firing of target neurons can be measured simultaneously.![Electrochemistry combined with electrophysiology experiment. (a) Schematic of the timing of the experiment. The electrode voltage is ramped for cyclic voltammetry every 100 ms. Between ramps, the potential waveform is disconnected (represented by railroad tracks) and an electrophysiology experiment is performed by measuring voltage changes due to nearby cell firing. Action potentials are monitored and the rate of firing over a given time period is calculated. (b) Example of experimental data collected from an intercranial self-stimulation (ICS) experiment. The red line represents the average dopamine concentration elicited when an animal pressed a lever for a rewarding stimulation. The green dashed line shows when the lever press occurred. The histogram shows mean firing rates for neural activity determined from the electrophysiological data. The data above the histogram show a tick for every action potential detected from 24 different lever presses (each on a vertical line). When ICS takes place, there is a transient increase in dopamine levels and a transient decrease in neuronal firing frequency. [Part (b) was reproduced with permission from Fig. 1 of ref. 38, Copyright 2005, National Academy of Sciences, USA.]](/image/article/2009/AN/b807563h/b807563h-f5.gif) | ||
| Fig. 5 Electrochemistry combined with electrophysiology experiment. (a) Schematic of the timing of the experiment. The electrode voltage is ramped for cyclic voltammetry every 100 ms. Between ramps, the potential waveform is disconnected (represented by railroad tracks) and an electrophysiology experiment is performed by measuring voltage changes due to nearby cell firing. Action potentials are monitored and the rate of firing over a given time period is calculated. (b) Example of experimental data collected from an intercranial self-stimulation (ICS) experiment. The red line represents the average dopamine concentration elicited when an animal pressed a lever for a rewarding stimulation. The green dashed line shows when the lever press occurred. The histogram shows mean firing rates for neural activity determined from the electrophysiological data. The data above the histogram show a tick for every action potential detected from 24 different lever presses (each on a vertical line). When ICS takes place, there is a transient increase in dopamine levels and a transient decrease in neuronal firing frequency. [Part (b) was reproduced with permission from Fig. 1 of ref. 38, Copyright 2005, National Academy of Sciences, USA.] | ||
Two recent studies using this technique have been made in animals performing intracranial self-stimulation (ICS). The rats have a stimulating electrode implanted in the area where dopamine cell bodies are located. When the rats press a lever, they are given a stimulation pulse, similar to electrical stimulation experiments. This paradigm is highly rewarding and the rats willingly press the lever for this stimulation. Fig. 5(b) shows example data. The red line is the average dopamine concentration in the nucleus accumbens core and the histogram shows neuronal firing. Pressing the lever during ICS results in a transient increase in dopamine and a transient decrease in neuronal activity [Fig. 5(b)]. Further characterization of the signal was performed using pharmacological agents and they found that the inhibition of neuronal firing after ICS was predominantly regulated by GABAA receptors, not dopamine receptors.38,39 Follow-up studies have used iontophoresis to deliver drugs specifically to the area of interest and have found anticipatory dopamine released before ICS and mediation of ICS behavior by D1 receptors in the nucleus accumbens shell.39 The ability to measure both electrochemical and electrophysiological data simultaneously at a carbon-fiber microelectrode will allow for better understanding of both the time course of transient dopamine signaling and its effects on postsynaptic firing.
Conclusions and future directions
Carbon-fiber microelectrodes are ideal chemical sensors because they allow rapid measurements of chemical changes. This unprecedented temporal resolution has facilitated a better understanding of the basic signaling mechanisms of dopamine, in particular how it mediates neuronal firing and its pharmacology. The recent research in enhancing the sensitivity and selectivity of CFMEs will facilitate detection of even lower concentrations of analyte. In particular, future research will continue to expand the number of compounds that can be detected using CFMEs. A greater number of enzyme electrodes will likely be developed to selectively monitor specific compounds and the electrochemical detection of other electroactive neurotransmitters and neuromodulators explored. Application of surface chemistry, materials science and polymer chemistry knowledge will strengthen these endeavors. In addition, new carbon electrodes will continue to be developed that may ultimately replace carbon fibers. For example, carbon coatings on tungsten wires offer the ruggedness of metal electrodes with the electrochemical properties of carbon.40 Development of small, carbon nanotube-based electrodes is also a possible area of research.The application of CFMEs for studying in vivo neurotransmitter dynamics is coming into its prime. The technology and electrochemical techniques are sufficiently well-developed that they may now be more widely implemented by neuroscientists. Initially, many studies will continue to focus on dopamine neurotransmission, as this is the best characterized molecule for electrochemical detection. There are still a wide variety of behaviors and diseases in which dopaminergic neurotransmission is not well understood. CFMEs will also likely be used to better understand other monoaminergic systems in vivo, such as epinephrine, norepinephrine and serotonin. Also, arrays of microelectrodes for the simultaneous measurements of multiple analytes might be useful for study of neurotransmitter interactions. Ceramic-based microelectrode arrays on platinum have been developed with good sensitivity to such compounds as glutamate, choline and acetylcholine with temporal responses on the level of seconds.41,42 Continued development of these non-carbon electrodes, and perhaps an extension of these techniques using carbon with its unique electrochemistry, would facilitate detection of an even wider variety of neurotransmitters. Thus, as interest from neuroscientists grows, there will be demand for more advanced microelectrode technology and the field will continue to see advances.
Acknowledgements
Work from the Venton lab was funded by grants from the NSF (CHE 0645587) and NIH (R21-EB007830).References
- J. L. Ponchon, R. Cespuglio, F. Gonon, M. Jouvet and J. F. Pujol, Anal. Chem., 1979, 51, 1483–1486 CrossRef CAS.
- A. C. Michael and R. M. Wightman, in Laboratory Techniques in Electroanalytical Chemistry, ed. P. Kissinger and W. R. Heineman, Marcel Dekker, New York, 1996, ch. 12, pp. 367–402 Search PubMed.
- A. G. Ewing, M. A. Dayton and R. M. Wightman, Anal. Chem., 1981, 53, 1842–1847 CrossRef CAS.
- N. Sasakawa, N. Murayama and K. Kumakura, Cell. Mol. Neurobiol., 2005, 25, 777–787 CrossRef CAS.
- A. Gratton, B. J. Hoffer and G. A. Gerhardt, Neuroscience, 1989, 29, 57–64 CrossRef CAS.
- L. C. Daws, S. Montanez, W. A. Owens, G. G. Gould, A. Frazer, G. M. Toney and G. A. Gerhardt, J. Neurosci. Methods, 2005, 143, 49–62 CrossRef CAS.
- G. A. Gerhardt, A. F. Oke, G. Nagy, B. Moghaddam and R. N. Adams, Brain Res., 1984, 290, 390–395 CrossRef CAS.
- G. Nagy, G. A. Gerhardt, A. F. Oke, M. E. Rice, R. N. Adams, R. B. Moore, M. N. Szentirmay and C. R. Martin, J. Electroanal. Chem., 1985, 188, 85–94 CrossRef CAS.
- A. Hermans, A. T. Seipel, C. E. Miller and R. M. Wightman, Langmuir, 2006, 22, 1964–1969 CrossRef CAS.
- A. M. Strand and B. J. Venton, Anal. Chem., 2008, 80, 3708–3715 CrossRef CAS.
- M. Musameh, J. Wang, A. Merkoci and Y. Lin, Electrochem. Commun., 2002, 4, 743–746 CrossRef.
- Y. Liu, X. Q. Zou and S. J. Dong, Electrochem. Commun., 2006, 8, 1429–1434 CrossRef CAS.
- M. N. Zhang, K. Liu, L. Xiang, Y. Q. Lin, L. Su and L. Q. Mao, Anal. Chem., 2007, 79, 6559–6565 CrossRef CAS.
- S. R. Hocevar, J. Wang, R. P. Deo, M. Musameh and B. Ogorevc, Electroanalysis, 2005, 17, 417–422 CrossRef CAS.
- B. E. K. Swamy and B. J. Venton, Analyst, 2007, 132, 876–884 RSC.
- A. Brajter-Toth, K. A. El-Nour, E. T. Cavalheiro and R. Bravo, Anal. Chem., 2000, 72, 1576–1584 CrossRef CAS.
- B. E. K. Swamy and B. J. Venton, Anal. Chem., 2007, 79, 744–750 CrossRef.
- S. Cechova and B. J. Venton, J. Neurochem., 2008, 105, 1253–1263 CrossRef CAS.
- N. R. Ferreira, A. Ledo, J. G. Frade, G. A. Gerhardt, J. Laranjinha and R. M. Barbosa, Anal. Chim. Acta, 2005, 535, 1–7 CrossRef CAS.
- Y. Tian, L. Q. Mao, T. Okajima and T. Ohsaka, Biosens. Bioelectron., 2005, 21, 557–564 CrossRef CAS.
- J. J. Mitala and A. C. Michael, Anal. Chim. Acta, 2006, 556, 326–332 CrossRef CAS.
- W. H. Oldenziel and B. H. C. Westerink, Anal. Chem., 2005, 77, 5520–5528 CrossRef CAS.
- W. H. Oldenziel, G. Dijkstra, T. I. F. H. Cremers and B. H. C. Westerink, Anal. Chem., 2006, 78, 3366–3378 CrossRef CAS.
- B. J. Venton, A. T. Seipel, P. E. Phillips, W. C. Wetsel, D. Gitler, P. Greengard, G. J. Augustine and R. M. Wightman, J. Neurosci., 2006, 26, 3206–3209 CrossRef CAS.
- A. K. Wagner, J. E. Sokoloski, D. Ren, X. Chen, A. S. Khan, R. D. Zafonte, A. C. Michael and C. E. Dixon, J. Neurochem., 2005, 95, 457–465 CrossRef CAS.
- L. Yavich, P. Jakala and H. Tanila, J. Neurochem., 2005, 95, 641–650 CrossRef CAS.
- S. R. Jones, T. A. Mathews and E. A. Budygin, Synapse (N. Y.), 2006, 60, 251–255 CrossRef CAS.
- D. L. Robinson, T. J. Volz, J. O. Schenk and R. M. Wightman, Alcohol Clin. Exp. Res., 2005, 29, 746–755 CrossRef CAS.
- G. D. Stuber, M. F. Roitman, P. E. M. Phillips, R. M. Carelli and R. M. Wightman, Neuropsychopharmacology, 2004, 30, 853–863 CrossRef CAS.
- R. M. Wightman, M. L. A. V. Heien, K. M. Wassum, L. A. Sombers, B. J. Aragona, A. S. Khan, J. L. Ariansen, J. F. Cheer, P. E. M. Phillips and R. M. Carelli, Eur. J. Neurosci., 2007, 26, 2046–2054 CrossRef.
- J. F. Cheer, K. M. Wassum, M. L. Heien, P. E. Phillips and R. M. Wightman, J. Neurosci., 2004, 24, 4393–4400 CrossRef CAS.
- B. J. Venton and R. M. Wightman, Synapse (N. Y.), 2007, 61, 37–39 CrossRef CAS.
- M. L. A. V. Heien, P. E. M. Phillips, G. D. Stuber, A. T. Seipel and R. M. Wightman, Analyst, 2003, 128, 1413–1419 RSC.
- P. E. M. Phillips, G. D. Stuber, M. L. Heien, R. M. Wightman and R. M. Carelli, Nature, 2003, 422, 614–618 CrossRef CAS.
- G. D. Stuber, R. M. Wightman and R. M. Carelli, Neuron, 2005, 46, 661–669 CrossRef CAS.
- J. J. Day, M. F. Roitman, R. M. Wightman and R. M. Carelli, Nat. Neurosci., 2007, 10, 1020–1028 CrossRef CAS.
- G. V. Williams and J. Millar, Neuroscience, 1990, 39, 1–16 CrossRef CAS.
- J. F. Cheer, M. L. A. V. Heien, P. A. Garris, R. M. Carelli and R. M. Wightman, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 19150–19155 CrossRef CAS.
- J. F. Cheer, B. J. Aragona, M. L. A. V. Heien, A. T. Seipel, R. M. Carelli and R. M. Wightman, Neuron, 2007, 54, 237–244 CrossRef CAS.
- A. Hermans and R. M. Wightman, Langmuir, 2006, 22, 10348–10353 CrossRef CAS.
- J. J. Burmeister, F. Pomerleau, P. Huettl, C. R. Gash, C. E. Werner, J. P. Bruno and G. A. Gerhardt, Biosens. Bioelectron., 2008, 23, 1382–1389 CrossRef CAS.
- J. J. Burmeister and G. A. Gerhardt, TrAC, Trends Anal. Chem., 2003, 22, 498–502 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
