Water, electricity, and between… On electrowetting and its applications
Romi
Shamai
a,
David
Andelman
*b,
Bruno
Berge
c and
Rob
Hayes
d
aDepartment of Applied Physics, The Hebrew University of Jerusalem, Givat Ram 91904, Jerusalem, Israel
bRaymond and Beverly Sackler School of Physics and Astronomy, Tel Aviv University, Ramat Aviv, Tel Aviv 69978, Israel. E-mail: andelman@post.tau.ac.il
cVarioptic SA, Batiment Tony Garnier, 24B rue Jean Baldassini, 69007 Lyon, France
dLiquavista, de Witbogt 10, 5652 AG Eindhoven, The Netherlands
First published on 14th November 2007
Abstract
Imagine a drop of water lying on a surface, pulled into a ball by surface tension. With electricity it is possible to change the shape of the drop and cause it to flatten out. This is electrowetting, a physical phenomenon which has aroused great interest in recent years as it has found new applications. Here we will describe the phenomenon and two of its applications: variable-focus liquid lenses and paper-thin, video-rate, reflective color displays.
Romi Shamai received a BSc in Physics from Tel Aviv University in 2006 and is currently completing his MSc thesis in the field of optofluidics at the Nanophotonics and Optofluidics Laboratory, Department of Applied Physics at the Hebrew University of Jerusalem, under the supervision of Dr Uriel Levy. |
David Andelman received a BSc in Physics and Computer Sciences from Tel Aviv University in 1975 and a PhD in Physics from MIT in 1984. After a year as a Juliot–Curie Fellow at College de France in Paris and two years as a post-doctoral fellow at Exxon Research and Engineering Co. in Annandale, New Jersey, he joined the School of Physics of Tel Aviv University in 1987, where he is now a Professor of Physics. His interests lie within soft condensed matter and include physical properties of polymers, bio-membranes and soft charged matter. |
Bruno Berge received his PhD in Physics from the University of Grenoble, followed by two years of post-doctoral research at the University of Chicago. He has been appointed as a senior scientist at the French center for scientific research (CNRS) and Full Professor at Ecole Normale Superieure de Lyon, before founding Varioptic Co. for developing optical consumer solutions using electrowetting. |
Rob Hayes completed his Bachelor’s degree (honours) in Chemistry (U. Melbourne) and Master’s degree in Mineral Processing (U. South Australia) before receiving his PhD in Physical Chemistry from Brunel University (UK) in 1989. He subsequently worked for 10 years as a Research Fellow/Senior Research Fellow at the IWRI (Sth. Australia). In 1999 he moved to Philips Electronics in Eindhoven where he focussed primarily on materials and process development for novel electro-optic devices based on electrowetting. Since the formation of Liquavista he has held the position of Chief Technical Officer. |
The physics of wetting
Go into the kitchen and do a small experiment: place a drop of water on a smooth, clean glass surface (a plate, for example), and another drop on a Teflon frying pan or on greased baking paper. You will be able to see the difference in the behavior of the drops: on the glass the drop flattens out whereas on the Teflon or the greased paper it turns into a ball. We say the drop wets the glass, whereas on a hydrophobic (“water hating”) surface such as Teflon the wetting is only partial (Fig. 1). For a general review see Ref. 1. | ||
| Fig. 1 Left: A drop of water on a surface with low polarity (hydrophobic), such as Teflon or greased baking paper. The drop draws itself into a ball and there is little wetting. Photograph: Vincent Kwong, UBC. Right: A drop of water on a surface of higher polarity. The drop flattens out and high wetting occurs. Photograph: Betty Winter. | ||
The spherical shape of a drop is a result of intermolecular forces between the molecules of which it is made. A molecule located within the drop is equally attracted in all directions by the molecules surrounding it, and so the total force exerted on it is zero. However, a molecule located near the drop surface is attracted only by its inner neighbors, and so feels a resultant force in the direction of the neighboring molecules (Fig. 2). This effective attraction of a molecule on the surface creates surface tension. Surface tension is a physical quantity measured in units of force per unit length, or equivalently in units of energy per unit area, and it expresses the amount of energy necessary to enlarge the surface by one surface unit. Since the sphere has the lowest surface area per given volume, it is easy to understand that this is also the state with the lowest surface energy, and that is what causes the drop to take on a spherical shape.
 | ||
| Fig. 2 The molecules within the drop are equally attracted in all directions, whereas the molecules on the surface of the drop are attracted inward to their neighbors—and that is the source of surface tension. | ||
In different fluids intermolecular forces possess different character and intensity. In organic fluids, such as oil, the attractive forces are a result of momentary electric polarization of the electrons. This polarization creates a non uniform distribution of electrons in the molecules, and as a result a mutual attraction is created between every two molecules, similar to the attraction between two magnets with opposite polarity. The forces responsible for the attraction are called van der Waals forces, after the 19th century Dutch scientist. The surface tension between oil and air resulting from these forces is about 20–50 millijoule per square meter (mJ m−2). Water is a fluid with many special characteristics resulting from the large permanent dipole of water molecules and their intermolecular hydrogen bonds. Among other things this leads to the relatively high value of surface tension between water and air: 72 mJ m−2. In mercury, which is a metallic liquid at room temperature, the attractive forces are a result of the free conduction electrons as in solid metals, and the surface tension reaches 485 mJ m−2.
Surface tension at the interface of two materials depends on their mutual properties, and not just on one of them. For example, the surface tension of a water drop in air is different from the surface tension of that same drop in an oil medium. Therefore, when we place a liquid drop on a surface, the behavior of the drop depends not only on characteristics of the liquid, but also on characteristics of the material of which the surface is made. In general, we can say that if the polarization of the material making up the surface (that is, the ability of the electric charge in a molecule to distribute so that an electric dipole is created) is higher than the polarizability of the liquid, total wetting will take place, as in the case of a water drop on glass or metal. In other cases, as in the case of Teflon or a greasy surface, partial wetting will take place (see the different wetting states in Fig. 1 and 3).
 | ||
| Fig. 3 Different degrees of wetting. On the left, the wetting is low and the contact angle is large. On the right, the wetting is high and the contact angle is small. | ||
The noted British physicist Thomas Young, working at Cambridge University, found in 1805 that the contact angle θ (the angle created between the outer surface of the liquid and the surface on which it lies, see Fig. 3) depends on three surface tensions: the surface tension between the liquid and the solid surface γSL, between the surface and the air γSG, and between the liquid and the air γLG. At equilibrium the three lateral forces acting on the drop are balanced as the drop does not move (see Fig. 3).
| γLGcosθ + γSL−γSG = 0 | (1) |
The Young equation relates the cosine of the angle θ to the three surface tensions:
| cosθ = (γSG−γSL)/γLG | (2) |
The two extreme wetting limits are θ = 180°, which corresponds to a no wetting case (spherical drop), while θ = 0° is the complete wetting case where the liquid spreads uniformly over the surface and creates a thin liquid layer. The intermediate case of θ = 90° is achieved when the fraction of surface tensions in the Young equation (2) becomes very small.
Electrowetting
Some decades after Young's discoveries, in 1875, a French physicist named Gabriel Lippmann investigated effects of electrocapillarity which laid the basis of modern electrowetting.2 Lippmann worked at the Sorbonne at the end of the 19th and beginning of the 20th century, and was Marie Curie's doctorate supervisor. He later received the Nobel Prize for physics in 1908 for his work on color photography. For more details on Lippmann's work and electrowetting in general, see Ref. 3,4. Based on his findings, a term due to electric polarization was added to the Young equation. This generalized equation is called today the Young–Lippmann equation:| γLGcosθ = γSG−γSL + ½CV2 | (3) |
 | ||
| Fig. 4 Top: A water drop placed on a hydrophobic surface with a high contact angle. Bottom: Electrowetting of the surface. Operation of voltage between the drop and the electrode changes the distribution of charges due to the dielectric insulator and significantly decreases the contact angle. The two surface coatings are drawn not to scale. | ||
The dimensionless electrowetting number,
| W = ½CV2/γLG, | (4) |
Beyond the Young–Lippmann equation
The validity of the Young–Lippmann equation has been checked in experiments with many materials, and it gives a fairly good description for a large number of systems. Nonetheless, many aspects of electrowetting need further investigation.The most striking effect is the saturation of the contact angle under application of high voltages (Fig. 5).5 However, in applications, it is common to work with low enough voltages so that the Young–Lippmann regime always applies. Another issue is the contact angle hysteresis between repeated voltage on/voltage off states. Clearly, in applications the stability of the system should be maintained even after millions of changes between the different wetting states.
 | ||
| Fig. 5 Measurement of the contact angle as a function of electric voltage, compared to the theoretical prediction from the Young–Lippmann equation. At voltages lower than −110 volts (depending on the system) the contact angle is saturated, in contradiction to the theoretical prediction. The figure is adapted with permission from Ref. 5. | ||
The Young–Lippmann equation describes only the static state of a wetting system in equilibrium. Much of the current research is devoted to dynamic processes such as the flow of thin liquid films under electric field and other related issues.6–8
Another interesting limit occurs when the applied voltage is frequency dependent (AC voltage). When the frequency is in the 10–100 KHz range or higher (system dependent), the water droplet loses its ionic conductivity and behaves as a bulk dielectric material. However, for low enough frequencies, electrowetting can be shown to remain the dominant effect.9
These points manifest the ever-growing interest in understanding the phenomena of electrowetting on a fundamental level, and we expect this interest to persist in years to come.
In order to increase wetting and to control it, various salts are dissolved in water, providing a source of ions for electric charges moving freely in the liquid. In Lippmann's time the operation of a voltage between the metal and electrolyte gave rise to electrochemical reactions, similar to those within an electric battery. Over time these reactions lead to irreversible changes such as oxidation or pollution of the metal–electrolyte interface. They diminish the electrowetting effect to such a degree that it becomes useless in applications which require many cycles of changes in the contact angle over extended period of time.
A few years ago a solution to this problem was found.3 By adding a thin layer of insulating material between the metal surface and the water drop (Fig. 4), electrochemical reactions of the electrode are prevented. This method is called electrowetting on dielectric (EWOD) and is a technological breakthrough because electrowetting can be made stable for a long period of time. The insulating layer thickness and dielectric constant determine the capacitance (per unit area) of the region of contact between the surface and the drop. Special materials, with large dielectric constant and high endurance to strong voltages in thin layers are being developed and used in order to increase the surface capacitance. Furthermore, it is important that the insulating layer will be hydrophobic (or covered with a second hydrophobic layer) in order to increase the contact angle in the placid state (no applied voltage). The hydrophobic coated surface ensures a larger range of contact angle variation with applied electric field as can be seen in Fig. 5 and as is important in the applications discussed next.
Applications
Electrowetting has a number of interesting applications which have recently been developed. They are all based on the fact that it is possible using an external electric field, with no mechanical parts, to control movement or quick change (hundredths of a second) between a number of states of the system. It is important that systems can be miniaturized to scales of less than a millimetre and still be controlled with great precision using a minuscule amount of energy for a long period of time. Applications from recent years include transport of liquids for purposes of changing the characteristics of optical conductors and creating optical switches,10,11 cooling of electronic circuits by transport of cold drops across them,12transport and mixing of micro-drops for purposes of printing,13 suction of liquids into microtubes,14 and lab-on-a-chip applications for analysis of the chemical composition of liquids, particularly physiological liquids (blood, urine…) by means of transport of drops of the fluid to examination cells on the chip.15 There, they are mixed with other chemical materials and undergo various optical measurements, all at minuscule length scales of less than one millimetre.Variable lenses
An interesting and important application of electrowetting which has seen great development recently is the creation of an optical lens with a varying focal length.16,17 In this application a small drop of oil is placed in a sealed glass cell filled with water. The drop which is a few millimetres in size has a nearly perfect spherical shape, and so it can serve as a lens (Fig. 6 and 7). By applying only a few dozen volts, the shape of the drop may be changed within a hundredth of a second and, thus, its focal length may be adjusted as required. In this way it is possible to take pictures of objects in a wide range of distances varying from a few centimetres to infinity while preserving the focus. Varying the focal length has been realized up to now using a system of lenses moving in relation to one another. The new application enables increased miniaturization, lower sensitivity to mechanical faults, and is already in use in minuscule digital cameras found in mobile phones. Further applications of variable lenses are being developed at present for consumer products as well as for a wide range of industrial and medical implementations. | ||
| Fig. 6 A water drop in air can focus light like a lens. As early as the 17th century the English scientist Stephen Gray used a water drop as a lens for a microscope he built.18 Photograph: Gady Fishel. | ||
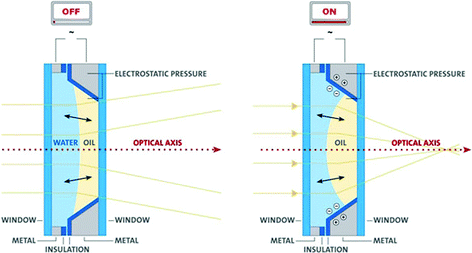 | ||
| Fig. 7 Left: A vertical cut through an optical lens consisting of an oil drop in a water medium. Here electric voltage is not turned on and the concave oil drop diffuses the light due to the refractive index of oil which is larger than that of water (Snell's law). Right: Here electric voltage is turned on, and under its influence the water pushes the oil drop to become convex and focus the light passing through it. | ||
Fig. 8 shows an example of a liquid lens embedded inside a camera module. This electrowetting-based liquid lens allows a very simple structure of miniature cameras, with no moving parts. It is a key advantage for the durability of the camera within mobile applications which are often subject to mechanical shock.
 | ||
| Fig. 8 Electrowetting variable focus liquid lens. Left: A miniature liquid lens inside its metal case. The case diameter is 10.5 mm. Right: The same lens with its electronic control as is used for digital cameras in mobile phone applications. Courtesy of Varioptic Co. | ||
Another advantage of liquid lenses is their high optical quality, even though the solid supporting parts are processed using conventional machining techniques. Because the lens itself is liquid, its surface has a self-healing property. Due to its surface tension, the liquid surface remains smooth on a nanometre scale (10−9 m) even if the supporting solid surface is polished on a micrometre scale (10−6 m). Another desired property of these lenses is that when they are perturbed (e.g., mechanical agitation), the perturbation does not propagate easily from the lens outside the perimeter toward the central region. As a result, liquid lenses have a highly precise optical surface, equivalent to that obtained in glass lenses after elaborated and delicate polishing work.
Electronic paper
Another promising use of electrowetting is in the development of electronic paper (e-paper). E-Paper is a display surface very similar to paper in terms of the reader's sensation, which can display varying content as does a computer or television screen, just as if it had been “printed anew” before our very eyes.These characteristics are expected to transform e-paper in the future to a substitute for regular paper in many areas. Instead of newspapers and books we can carry with us only a thin flexible surface, as large as we wish, on which we can read the entire daily paper or a book before bed. Among the paper's illustrations, video articles and film clips might also appear. Turning a page of the book, leafing through the newspaper or watching a film would be done on the e-paper in a way reminiscent of internet surfing today.
We would like to note here that there is a great deal of research effort invested in the subject of manufacture of e-paper, with preliminary successes based on a number of different technological directions. However, we will focus here only on an innovative development based on electrowetting technique, which has recently been introduced.19,20 Researchers succeeded in creating very thin display surfaces of a size suitable for use as electronic device screens such as mobile phones or Pocket PCs. The reaction time of the display surfaces they manufactured is sufficiently quick to display movies.
These display surfaces are unique in that they reflect light, as opposed to displays which emit light and which are familiar to us from television and computer screens. Reading and viewing a light-reflecting display is similar to looking at and reading regular printed paper, which we see by means of natural or artificial surrounding light that shines on the page and is reflected to our eyes. In this method the eye is not exposed to bright light emitted from the screen, but to light arriving from the natural light of the environment, which the reader can adjust at will. Therefore, a light-reflecting display is less tiring for the eye and healthier than a light-emitting display. An additional advantage is in looking at the screen in strong lighting conditions such as daylight, where a light-emitting display has difficulty competing with the sunlight intensity, whereas a light-reflecting display makes use of it.
Due to these advantages, e-paper has the potential to serve in the future a variety of display needs, and perhaps even to replace computer and television screens in use today. However, the achievements of the current development are intended for use as display screens for small electronic instruments only, and much additional research and technological effort is required before it will be possible to buy e-paper at the nearest store to replace a computer screen.
Pixels based on electrowetting
In every display screen (CRT, plasma, LCD…) the picture is made up of a large number of basic units called pixels (picture elements). The characteristics of a single pixel determine the characteristics of the entire screen—its size, reaction time, and the spectrum of hues it can create or reflect. First, we will explain how a monochromatic pixel unit based on the electrowetting technique works, as shown in Fig. 9. The bottom of the cell is made of white light-reflecting material which is also an electric insulator, and its upper cover is made of transparent material. The cell is filled with transparent water and a bit of insoluble oily liquid painted with opaque paint. An electric potential difference is applied between an electrode in contact with the bottom of the cell and a needle (another electrode) connected to the cell side. The cell has two wetting states. Without applying voltage, the layer of oil wets the entire bottom of the cell and its opaque color reflects light with the color of the oil. The liquid layer of oil is under the water since the surface tension between the water and the insulator is higher than the surface tension between the oil and the insulator. This is the state with the lowest surface energy.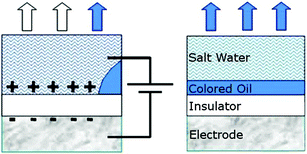 | ||
| Fig. 9 Side view of two monochromatic pixel states based on electrowetting. On the right the reflected light is blue (the color of the layer of oil) with no electric voltage, and on the left, with application of the voltage, the light is white (the color of the insulating layer). Adapted with permission from Ref. 19c. | ||
With the application of the electric voltage the free charges in the water will be attracted to the appropriate electrodes and the water will wet the bottom of the cell while pushing the oil to the corner of the cell. In this state the cell will become almost completely transparent, and light will be reflected from the white bottom. Namely, in both states of the cell there is full reflection of light but in two different colors (for example blue—the color with which the oil has been dyed, and white—as the color of the cell bottom), with the possibility of going from one state to another very quickly, and repeating the process hundreds of millions19 of times with no decrease in performance. This is how the monochromatic pixel works.
In recent years, as a result of intense research at Philips Corporate Labs, this cell has been miniaturized to a size of 3 microns,19 though mainly used at the size of 160 microns (0.16 millimetres). This size enables good picture resolution and is common in electronic displays. Similarly, the dimensions of the basic cell are small enough so that the force of gravity operating on the liquids is negligible in comparison to the forces of surface tension, and so inversion of the cell or a change in its spatial direction do not disturb its performance. The range of voltages in which the cell operates today is 15–20 volts; this has been decreased in recent years from values of over 200 volts. The technological aim is to decrease it to less than ten volts,21 an aim that is achievable by the choice of suitable materials or a decrease in thickness of the insulator layer between electrode and water.
Most of the contemporary and future uses of the cell as a pixel in a picture are based on building a large two-dimensional array of pixels, with the possibility of individual control of each of the pixels by means of electric voltage (similar to a plasma or LCD screen). The transition rate between two wetting states of a water drop in electrowetting is governed mainly by hydrodynamic forces (liquid viscosity) and friction of the drop boundary with the solid surface. A typical rate is measured to be less than 10 milliseconds. It enables quick change between static pictures as well as presentation of video, whose proper display requires a refresh time of about 25 pictures per second.
Colored pixel
From the principle of the monochromatic cell it is easy to explain the principle of its generalization to a colored pixel. This may be done in two ways: the first and more traditional method is to connect a trio of cells in red, green and blue colors (RGB), which are the optical primary colors (resulting from characteristics of the human eye), and by controlling the brightness of each cell to create a unit with the desired color (Fig. 10). The general picture looks good although the resolution using this method is three times lower than the corresponding resolution on a monochromatic screen, because a colored unit is three times as big as a monochromatic unit. | ||
| Fig. 10 A colored electrowetting pixel composed of three monochromatic pixels in the optical primary colors (RGB). Adapted with permission from Ref. 19c. | ||
A different demonstrated approach, is to manufacture a basic colored unit with three layers of colored oil, each in one of the three primary colors of ink (i.e., the three optical primaries for subtractive mixing): cyan, magenta and yellow (CMY). The unit is constructed of two cells placed one on top of the other (Fig. 11). The top cell has a layer of yellow oil at its top surface. The bottom cell contains two layers of oil: magenta at its top surface and cyan at its bottom. Again, gravity does not play a role on the “inverted” oil since the forces of surface tension for such tiny liquid layers are far stronger. With this method there is no loss of resolution since each pixel is capable of creating the entire spectrum of colors on its own. The final color of the pixel, as with color printing, is determined by control of the surface area taken up by the colored oil at each layer by means of electrowetting.
 | ||
| Fig. 11 A colored electrowetting pixel with layers of oil in different colors (CMY) placed above each other. Adapted with permission from Ref. 19c. | ||
Advantages of the electrowetting display
The electrowetting screen has particularly low energy consumption, since most of the energy is required for the transition between states. As long as the picture is static there is no movement of charges and no consumption of energy, because the color is created by external light which is reflected. Compared to the light-emitting LCD-based video screens, the electric consumption of an electrowetting screen is indeed about five times lower.An additional advantage of the electrowetting screen over the LCD display is the wide viewing angle, which results from the fact that light is reflected in all directions. Light is emitted from a regular LCD screen primarily in the direction of the center and the picture cannot be viewed from a large side angle, a well-known problem with every laptop screen. However, with an electrowetting screen it is possible to look at the screen from the sides and yet see a high-quality picture, with a high contrast ratio, an important feature as compared with paper.
We must note here that an additional technology for manufacturing of light-reflecting e-paper, which is not based on electrowetting, is called electrophoretic display.22 With this technology, the pixel is a microscopic capsule containing black and white particles moving in opposite directions under the influence of an electric field, so that two color states may be displayed. This technology is already in use for signs and thin flexible display surfaces, but it is incapable of displaying video because its reaction time is about a second.
A colored light-reflecting screen based on electrowetting has many advantages in comparison with accepted technologies. It is very thin, suitable in speed to video display, does not limit the viewing angle and is very economic with electricity. These advantages are summarized in Table 1, comparing displays based on electrowetting and principal other technologies.
| Display technology | Accepted use | Pixel size | Speed of reaction | Viewing angle | Thickness | Light emitting or reflecting | Electric consumption | |
|---|---|---|---|---|---|---|---|---|
| CRT | Regular television screens | 0.21 mm | Video rate | Wide | ≈50 cm | Emitting | High | |
| LCD | Flat computer screens | 0.28 mm | Video rate | Limited | A few mm | Emitting | Low to medium | |
| Electro-phoretic microcapsule | Black and white electronic paper | 0.15 mm | 1 s between pictures | Wide | <1 mm | Reflecting | Very low | |
| EWOD | Flat screens and colored electronic paper | 0.16 mm | Video rate | Wide | <1 mm | Reflecting | Very low | |
In summary, electrowetting is a fascinating physical phenomenon. Although it was first discovered in the 19th century, the phenomenon is still not fully understood, and is of great contemporary interest in light of its new and varied applications. In this article we have focused on its applications as variable lenses and electronic paper. The former are already used in mobile phones, while the latter may soon become an inseparable part of our reading and viewing experience of written text, pictures and graphic-visual information, and may take the place of the regular computer screen, the book or newspapers (see Fig. 12 and 13).
 | ||
| Fig. 12 Realization of the use of electronic paper for reading a newspaper. This is an archetype of Plastic Logic Co. based on microcapsule technology. | ||
 | ||
| Fig. 13 An example of a Liquavista Company electrowetting screen in a small electronic device. | ||
Most probably you are reading this article on paper which has been produced in an industry which consumes many millions of tons of wood and thousands of tons of ink each year, with grave ecological implications. It is interesting to consider when we will no longer require these resources and will be able to read the morning newspaper by means of pixels of oil, water and a little electricity…
Interesting websites on electrowetting and e-paper:
•http://www.liquavista.com– website of Liquavista, a company that develops display surfaces based on electrowetting.•http://www.varioptic.com– website of Varioptic, a French company which manufactures variable optical lenses focused by electrowetting.
•http://www.eink.com– a company which manufactures electronic paper based on electrophoretic technology.
•http://www.ece.uc.edu/devices/Research.html– website of an electrowetting research laboratory at the University of Cincinnati (Ohio, USA).
•http://www.ee.duke.edu/research/microfluidics– website from a lab at Duke University (North Carolina, USA) showing videos of transport of microdrops by means of electrowetting.
Acknowledgements
We thank Roy Beck, David Bergman, Yoram Burak, Michael Cogan, Moshe Deutsch, Haim Diamant, Daniel Harries, Judy Kupferman, Uri Nevo, Val and Adrian Parsegian, Elie Raphael, Shimon Reich, Adi Shafir, Michal and Moshe Siman-Tov, and Yoav Tsori for their helpful comments and suggestions.References
- For a general review on wetting see: P.-G. de Gennes, F. Brochard-Wyart, D. Quere, Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves, Springer, 2003 Search PubMed.
- G. Lippmann, Ann. Chim. Phys., 1875, 5, 494 Search PubMed . For an English translation, see Ref. 3.
- C. Quilliet and B. Berge, Curr. Opin. Colloid Interface Sci., 2001, 6, 34 CrossRef CAS.
- F. Mugele and J.-C. Baret, J. Phys.: Condens. Matter, 2005, 17, R705 CrossRef CAS.
- A. Quinn, R. Sedev and J. Ralston, J. Phys. Chem. B, 2005, 109, 6268 CrossRef CAS.
- T. B. Jones, M. Gunji, M. Washizu and M. J. Feldman, J. Appl. Phys., 2001, 89, 1441 CrossRef CAS.
- L. Y. Yeo and H. C. Chang, Mod. Phys. Lett. B, 2005, 19, 549 CrossRef CAS.
- L. Y. Yeo and H. C. Chang, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 011605 CrossRef.
- T. B. Jones, Langmuir, 2002, 18, 4437 CrossRef CAS.
- S. Yang, P. Mach, T. Krupenkin and J. A. Rogers, Appl. Phys. Lett., 2002, 81, 202 CrossRef.
- Z. Wan, H. Zeng and A. Feinerman, Appl. Phys. Lett., 2006, 89, 201107 CrossRef.
- V. K. Pamula and K. Chakrabarty, Proceedings of the 13th ACM Great Lakes symposium on VLSI, Washington, D.C., USA, 2003, ACM Press, New York, 2003, p. 84 Search PubMed.
- F. Mugele, J.-C. Baret and D. Steinhauser, Appl. Phys. Lett., 2006, 88, 204106 CrossRef.
- C. P. Collier, K. P. Giapis, J. Y. Chen and A. Kutana, Science, 2005, 310, 1480 CrossRef CAS.
- R. B. Fair, Microfluid. Nanofluid., 2007, 3, 245 CrossRef CAS.
- B. Berge and J. Peseux, Eur. Phys. J. E, 2000, 3, 159 CrossRef CAS.
- S. Kuiper and B. H. W. Hendriks, Appl. Phys. Lett., 2004, 85, 1128 CrossRef CAS.
- S. Gray, Philos. Trans., 1697, 19, 539 Search PubMed.
- (a) R. A. Hayes and B. J. Feenstra, Nature, 2003, 425, 383 CrossRef CAS; (b) T. Roques-Carmes, R. A. Hayes and L. J. M. Schlangen, J. Appl. Phys., 2004, 96, 6267 CrossRef CAS; (c) B. J. Feenstra and R. A. Hayes, Electrowetting Displays, white paper, http://liquavista.com/files/LQV060828XYR-15.pdf.
- J. Heikenfeld and A. J. Steckl, Appl. Phys. Lett., 2005, 86, 151121 and 011105 CrossRef.
- S. Berry, J. Kedzierski and B. Abedian, Curr. Opin. Colloid Interface Sci., 2006, 303, 517 CAS.
- B. Comiskey, J. D. Albert, H. Yoshizawa and J. Jacobson, Nature, 1998, 394, 253 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
