Composite multilayered biocompatible polyelectrolyte films with intact liposomes: stability and temperature triggered dye release†
Dmitry
Volodkin
*abd,
Youri
Arntz
ab,
Pierre
Schaaf
c,
Helmuth
Moehwald
d,
Jean-Claude
Voegel
ab and
Vincent
Ball
ab
aInstitut National de la Santé et de la Recherche Médicale (INSERM), Unité 595, 11 rue Humann, 67085 Strasbourg Cedex, France. E-mail: Dmitry.Volodkin@medecine.u-strasbg.fr
bUniversité Louis Pasteur (ULP), Faculté de Chirurgie Dentaire, 1 place de l'Hôpital, 67000 Strasbourg Cedex, France
cInstitut Charles Sadron (ICS), Centre National de la Recherche Scientifique, Unité Propre 22, 6 rue Boussingault, 67083 Strasbourg Cedex, France
dMax-Planck Institute for Colloids and Interfaces (MPI), D-14424 Potsdam, Germany
First published on 8th November 2007
Abstract
The design of new quasi-2D biocompatible films able to release a drug in a controlled manner through the application of physical stimuli is of outstanding interest in biomaterials science. Herein, construction of composite nanofilms with multiple strata of stabilized large unilamellar liposomes is developed. The film has a multilayered architecture formed by the layer-by-layer (LbL) technique utilising two biocompatible polyelectrolytes, hyaluronic acid and poly-L-lysine (HA and PLL), onto which phospholipid liposome “interlayers” are adsorbed and subsequently embedded by further polyelectrolyte adsorption. First of all the morphology of the film surface is characterised and compared to the morphology obtained for a film containing the same polyelectrolytes but stiff polystyrene (PS) latex particles instead of the soft phospholipid vesicles. Both morphologies appear to be similar suggesting that the embedded vesicles keep their spherical shape. As a second step, it has been shown that carboxyfluorescein (CF) encapsulated in the vesicles remains inside without sustained release at room temperature indicating that the liposomes stay intact upon LbL immobilization. A substantial fraction (about 70%) of the vesicles absorbed onto the polyelectrolyte film are desorbed upon further polyelectrolyte adsorption to reach the embedded state. Hence to achieve high loading of the film, multiple vesicle depositions have to be performed. It is shown that the total number of deposited vesicles on the film is proportional to the number m of vesicle deposition steps (up to m = 3). The encapsulated dye can be released in a controlled manner by a temperature increase above the main phase transition temperature of the lipid mixture used to prepare the vesicles. Release kinetics is faster for film-entrapped vesicles than for those in solution due to destabilization of the lipidic bilayer by the polyelectrolyte environment. The developed composite films represent a new surface biocoating able to preserve, in native state, or to release in a controlled way, surface immobilized materials under external stimuli.
1 Introduction
Advanced biomaterials research is one of the most important and dynamically developing areas in modern surface science and technology. Modification of surfaces with functional biomolecules can be achieved by various approaches including chemical grafting and physical adsorption.1 15 years ago, an alternative technique to the classical self assembled monolayer grafting or Langmuir and Langmuir–Blodgett deposition, the LbL adsorption technique was developed. It allows immobilization of a controlled amount of compounds, polyelectrolytes, inorganic particles or active biomolecules on the surface of the material of interest as soon as it carries a non zero density of permanent surface charges.2–6 The fundamental physical mechanisms behind the LbL technique were extensively studied7–12 but are yet not fully understood. This deposition method allows to construct thin surface films based on the consecutive adsorption of species having the ability to interact with each other, for instance oppositely charged species, but also polymers modified with host molecules and polymers modified with their complementary hosts13–15 or polymers interacting only by means of hydrogen bonds.16Surface bio-functionalization can be achieved by means of the LbL method either by utilizing active molecules (proteins and enzymes,17–20DNA,21–25 viruses26) as building blocks for LbL assembly, by adsorbing covalently modified polyelectrolytes,27,28 and by passive loading of active molecules inside the preformed films.29–31
It has to be noted that in modern biomaterials science one aims not only to produce biocompatible films on the surface of implant materials but essentially to confer these coatings with molecules able to reduce inflammatory response upon implantation or with growth or differentiation factors able to guide the subsequent cellular response.1 These molecules are released from the biocoating in order to interact with cells in solution (so-called soluble signalling system).1 Hence both the amount of loaded substance and its controlled release from the biocompatible coating is of fundamental importance. In this context it has to be noted that by single incorporation of active molecules or deposition of functionalized polyelectrolytes, a low amount of immobilized biomolecules can be deposited at each step, however with the possibility to control the immobilization depth and hence providing the accessibility to cells adhering at the top of the LbL architecture.32,33 Moreover, conformation of surface adsorbed active molecules can be changed which may lead to reduction in biological activity. So, one of the main challenges is to increase the load of the LbL films with high amounts of biomolecules which are shielded from the surrounding medium. Thus, engineering of thin films, which are able to retain/release a sufficient amount of protected functional molecules is an important task in modern nanotechnology and biomaterial development.
The low permeability of lipid vesicles allows encapsulation of molecules in a different range of sizes (ions, dyes, pharmaceuticals, enzymes and proteins, etc) above a lowest molecular mass for which molecules diffuse through the lipidic bilayers. In addition, lipid biocompatibility makes liposomes perfect reservoirs to shield various kinds of molecules. These features allow utilization of the liposomes in both medical and non-medical fields.34 Modification of solid surfaces with liposomes as reservoirs filled with encapsulated material seems to be attractive for surface bio-functionalization. However, adsorption of the fragile vesicles onto a solid surface is not an easy task. Liposomes are rather unstable and, in general, they undergo collapse or/and fusion when coming in contact with solid surfaces as well as polyelectrolyte films.35–38 This leads to spreading of vesicles on solid support and formation of so-called supported lipid bilayers. A recent review describing the mechanism of lipid bilayer formation from liposomes is presented by Richter et al.35
Up to the present time, only a few ways of surface coating with intact vesicles have been reported. The deposition of vesicles on solid surfaces has been carried out where the vesicles were preliminarily stabilized by the formation of a polymerized silica layer on the vesicle surface forming so-called cerasomes.39,40 Another way is vesicle surface grafting by host–guest interactions, for instance utilising avidin-biotin (streptavidin) chemistry41,42 or by oligonucleotide tethers.43 A recent review on this topic is given by Christensen and co-authors44 where the authors focused on the main contributions to date on the fabrication and applications of surface-immobilized vesicles.
Our previous studies showed that PLL-coated vesicles are not disrupted upon adsorption onto charged polyelectrolyte films made from poly-L-glutamic acid and a synthetic polycation poly(allylamine hydrochloride).45,46 Recently, we have studied in details a way for sterical stabilization of liposomes by means of PLL surface coating.47–49 The main findings are:
(i) The vesicle coating can be modulated by an electrostatic attractive interaction depending on the polymer size and the kinetics of the polymer adsorption.
(ii) The preservation of the coated vesicles is strongly affected by the mixing conditions and polymer/lipid ratio.
(iii) The layer of the biopolymer is located exclusively on the vesicle surface and does not induce any changes upon adsorption on the surface of “solid” liposomes, however it has a strong effect on the membrane integrity of “fluid” ones.
We have found the optimal conditions to prepare stable single polymer-coated liposomes which can retain a high amount of encapsulated dye (CF) for a long time as long as the temperature is kept lower than the main transition temperature of the used lipids.47
In this work we have constructed and characterized polyelectrolyte multilayer LbL-constructed films made from the biodegradable and biocompatible hyaluronic acid and poly-L-lysine and containing up to three strata of PLL-stabilized liposomes. These liposomes were also integrated into polyelectrolyte multilayer films by means of the LbL technique. The films were characterized and their properties were compared with properties of similarly prepared films containing non-deformable stiff latex particles. The retention and temperature triggered release of a small molecular mass model dye (CF) was also investigated. We thus developed an architecture able to immobilize onto a solid surface a large variety of molecules and to release them in a controlled manner.
2 Experimental
2.1 Materials
The used lipids, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt) (DPPG) were purchased from Avanti Polar Lipids (USA), whereas cholesterol (CL, ref. number C-8667) was purchased from Sigma (USA). PS latex particles, Polybead Sulfate 0.2 μm microspheres (Polysciences Inc., Warrington, PN, USA). Tris(hydroxymethyl)aminomethane (Tris, Sigma, T1503, USA), NaCl (Prolabo, France), Poly-L-lysine hydrobromide (PLL) with viscosimetric molecular masses of 280 kDa (Sigma, ref. P1399) and 28 kDa (Sigma, ref. P7890), hyaluronic acid (HA, Lifecore Biomedical, ref. 002570, USA), Triton X-100 (Aldrich, 23,472-9), 5(6)-carboxyfluorescein (Fluka, ref. 21877), Sephadex G-50 (Aldrich 27,114-4, USA) and Sephadex G-200 (Pharmacia, Sweden) were used without further purification. Throughout this study, 10 mM Tris containing 15 mM NaCl, pH 7.4 was used and will be mentioned in the text as Tris-buffer. The water used in all experiments was prepared in a three-stage Millipore Milli-Q Plus 185 purification system and had a resistivity higher than 18.2 MΩ cm.2.2 Liposome preparation and characterization
Unilamellar liposomes loaded with CF were prepared by hydration of a lipidic film with 0.2 mg mL−1 CF solution in Tris-buffer followed by mechanical extrusion. The vesicles were characterized as described elsewhere.31,47 The average hydrodynamic vesicle diameter was found to be 133 ± 2 nm as calculated from dynamic light scattering measurements carried out at 25 °C using an HPPS 500 apparatus (Malvern Instruments, UK). Light scattered by the sample was detected at an angle of 173° with a laser source operating at a wavelength of 632.8 nm. The analysis of DLS autocorrelation functions of the scattered light intensity has been carried out with the ALV-correlator v3.0 software.2.3 Stabilization of liposomes and PS-latexes by PLL coating
Vesicles or PS-latexes were coated with PLL following the procedure described in our previous study.31,47 Briefly, 0.5 mL of the PLL solution (only one fraction of chromatographed PLL was taken for better resolution of the liposome and PLL eluted peaks; see Fig. S1, ESI†) in a 2 mL-Eppendorf tube was placed in an Eppendorf Thermomixer Compact (Sigma, USA) at room temperature under an agitation speed of 950 rpm. The same volume of particle solution (concentration of the vesicles or PS particles was 2.2 × 1012 particles mL−1) was dropped at a constant rate during 1 min into the agitated PLL solution.31,47 The PLL-coated nanoparticles were separated from non-bound PLL by gel-permeation chromatography on Sephadex G-200 (see Fig. S2, ESI†).2.4 Preparation of PLL/HA films
The (PLL/HA)n polyelectrolyte multilayer films were prepared with the LbL technique using a dipping robot (Riegler&Kirstein GmbH, Berlin, Germany). The film was deposited on microscopy cover glasses (12 mm in diameter, Marlenfeld GmbH, Germany). Before deposition the glass slides were cleaned with consecutive incubation in hot solutions (60 °C) of 2% Hellmanex (Hellma GmbH, Germany), 0.01 M sodium dodecyl sulfate, and 0.1 M HCl during 15 min for each solution followed by multiple rinsing with pure water. The film build-up was pursued at 25 °C by alternating dipping of the glass slides into PLL and HA solutions (in Tris-buffer) with an intermediate washing step with Tris-buffer. Before use, polyelectrolyte solutions were filtrated through an 0.22 μm filter. Each dipping step lasted over 10 min. So, the abbreviation (PLL/HA)12 is given to the films prepared by 24 alternating polyelectrolyte adsorption steps (layers) or 12 pairs of layers.2.5 Vesicle adhesion to the polyelectrolyte film
Native vesicles and PLL-covered vesicles (either with or without separation of the non-bound PLL molecules) were put in contact with (PLL/HA)12/PLL and (PLL/HA)12 films, respectively, in order to check for their adhesion to the films. The used films have surface charge opposite to the charge of the vesicles to favour adhesion by electrostatic interactions.The glasses coated with the above mentioned films were placed into the well of 24-well tissue culture plates containing 200 μL of Tris-buffer. Then 0.52 mL of vesicle suspension in Tris-buffer (lipid content 0.029 mg mL−1) was injected into the well. After 2 h incubation, the glass was washed with Tris-buffer to remove unbound vesicles and stored in the same buffer or additionally covered with (PLL/HA)2 or HA/PLL/HA layers in the case of native and PLL-covered vesicles, respectively. Before characterization, both preparations were either stored in Tris-buffer or dried during 2 days in an air atmosphere and at room temperature.
2.6 Preparation of PLL/HA films with integrated vesicles or PS-latexes
PLL-covered vesicles were adsorbed on (PLL/HA)12 film as described above. The PS latexes were adsorbed in the same manner and at the same particle concentration as the vesicles (1.9 × 1011 particles mL−1). Liposomes and latex particles were used in excess of about two and five times compared to the amount needed to form a compact monolayer, respectively. The excess of adsorbed particles was removed by multiple washing with 1 mL of Tris-buffer. Then the film was covered with three additional layers, namely with HA/PLL/HA, under the same conditions as used to prepare the (PLL/HA)12 film. The procedure of liposome adsorption and additional coverage with HA/PLL/HA was repeated up to two or three times in order to prepare films containing two or three liposome “interlayers”.In order to analyse the amount of adsorbed vesicles, the film was quantitatively removed from the glass substrate by addition of 200 μL of 0.05 M NaOH followed by 5 min incubation with this solution. This treatment allows for a complete removal of the multilayer film by deprotonation of the PLL molecules. Then 1 mL of 0.5 M Tris-buffer (pH 8.3) containing 0.25 M NaCl and 1% of Triton X100 was added. The mixture was heated to 75 °C for 20 min and then cooled down to room temperature. This treatment allows for the decomposition of the lipidic bilayer. The fluorescence intensity was then measured with a VersaFluor fluorometer (BioRad, Germany). The excitation wavelength was set at 490 nm and the emission was measured at 520 nm. Each obtained value is the average of at least three independent experiments and is given with its standard deviation. A calibration curve for CF was constructed in the presence of the same medium used to remove the film from the glass substrate and to destroy the vesicles.
To study the interactions between CF in solution and the polyelectrolyte film, a (PLL/HA)12 film was put in contact with 500 μL of a 6.7 × 10−5 mg mL−1 CF solution. The amount of free CF used for this experiment was the same as that present in the vesicle suspension upon the vesicle adsorption procedure (see above). After 10 min or 2 h of incubation with the free CF solution, the film was washed with Tris-buffer to remove unbound CF, and the film was then subjected to the same procedure as described for a liposome-containing film in order to determine the amount of bound CF.
2.7 Atomic force microscopy (AFM)
To perform AFM measurements, the polyelectrolyte films (with or without integrated particles) were dried during 2 days in ambient air and at room temperature. Imaging in dry state was carried out due to difficulties to image a very soft gel-like (PLL/HA)12 film in the wet state. The AFM measurements were performed using a Nanoscope III (Digital Instruments, Santa Barbara, CA) equipped with silicon nitride cantilevers (model MLCT-AUHW, Park Scientific, Sunnyvale, CA) having a spring constant of 0.03 N m−1. The AFM was operated in constant force contact mode for dried samples or in tapping mode in order to image the samples immersed in Tris-buffer (after preparation the samples were stored in the buffer). Several scans were imaged over a given surface area in order to ascertain that there was no sample damage induced by the tip. Deflection and height mode images were acquired simultaneously at a fixed scan rate (between 2 and 4 Hz) with a resolution of 512 × 512 pixels.The thickness of the HA/PLL film was measured by the scratching technique using four film samples prepared on different glass supports. Average height and width of film-embedded liposomes or PS-particles were calculated by cross-section analysis of minimum 60 individual particles using software WSxM 4.0 Develop 7.0, Nanotec Electronica (Spain).
2.8 Confocal laser scanning microscopy (CLSM)
CLSM micrographs were taken with a Zeiss LSM 510 scanning system (Zeiss, Germany) equipped with a 40× oil immersion objective with a numerical aperture of 1.4. The excitation wavelength was set at 488 nm and the emission was collected between 505 and 530 nm. The glass slide with the polyelectrolyte films covered with Tris-buffer was fastened in cylindrical holes of the home-made sample holder in a direction perpendicular to the laser beam. The suspension of PLL-covered vesicles was added to the solution under the glass to obtain a total vesicle concentration of 1.3 × 1011 particles mL−1. After 40 min of contact between the liposome solution and the substrate, the glass was washed three times with Tris-buffer to remove unbound vesicles. Finally, the film was imaged by CLSM.2.9 Quartz crystal microbalance (QCM)
QCM measurements were performed using the dissipation enhanced QCM-D300 system from Q-Sense (Gothenburg, Sweden) fitted with an axial flow chamber QAFC 302. The QCM-D technique is based on a measure of the changes in the resonance frequency F of a quartz crystal when some material is adsorbed on the crystal surface from solution.50,51 The crystals used in this study were coated with a SiO2 layer of about 50 nm in thickness.Half a millilitre of PLL and HA (at 0.5 mg mL−1) solutions in Tris-buffer or a Tris-buffer solution without polyelectrolyte were injected into the measurement cell at intervals of 10 min. The buffer was injected in between the alternating injections of the polyelectrolytes. The temperature of the measuring cell was stabilized at 22.0 ± 0.1 °C. Thus, the (PLL/HA)n film was formed on the crystal surface in a similar way as those prepared using the dipping robot.
Changes in the resonance frequencies, ΔFν, were measured at the first, third, fifth, and seventh overtones of the excitation wave (ν = 1, 3, 5 and 7, respectively) hence at about 5, 15, 25 and 35 MHz, respectively. During the whole film build up process, the ΔFν value was continuously recorded.
2.10 Fourier transform infrared spectroscopy (FTIR)
The FTIR experiments were performed using an Equinox 55 spectrometer (Bruker, Wissembourg, France) fitted with a liquid nitrogen cooled detector. For the buildup of the multilayers, D2O was used as solvent instead of H2O. All the spectra during the multilayer buildup were collected by accumulating 512 interferograms at 2 cm−1 resolution. The (PLL/HA)8 films were deposited on a trapezoidal ZnSe crystal by allowing the PLL, buffer, HA and Tris-buffer solutions to circulate alternating atop the crystal surface. Solution circulation was allowed for 5 min by means of a peristaltic pump. The spectra were decomposed using the SPSERV software (SPSERV, version 3.20, Dr. Csaba Bagyinka, Institute of Biophysics, Biological Research Center of the Hungarian Academy of Sciences, Szeged, Hungary).After the (PLL-HA)8 film has been deposited at 25 °C, the thermostated bath, connected to the top of the circulation cell into which the ZnSe crystal was fitted, was heated to 45 °C and the infrared spectra were regularly recorded and compared to that of the film deposited at 25 °C in order to study if the multilayer film does not undergo decomposition upon heating.
3 Results and discussion
3.1 Matrix for vesicle integration
In order to embed phospholipid vesicles into polyelectrolyte multilayer architectures, the right choice of both vesicles and the film matrix has to be done. The film should be thick enough to include the vesicles. The size of the vesicles (133 ± 2 nm in diameter) was chosen because smaller vesicles could be less stable due to increased curvature energy but larger vesicles are too fragile and deformable. Thus, the appropriate thickness of the polyelectrolyte bed has to be in the micrometer range. At the same time, the film should be highly hydrated to contain enough water to keep the vesicles in their usual environment. The PLL/HA film constructed by the LbL technique should meet all these requirements and was hence chosen for this study.52–54 These films reach a thickness of a few microns after the deposition of about 10–20 pairs of layers. Their gel-like nature55 and biocompatibility make them an excellent candidate for liposome embedding.We have studied the PLL/HA film formation for HA of fixed molecular mass (400 kDa) and PLL of different molecular masses, 28 and 280 kDa. The evolution of the frequency shifts ΔFν/ν and of the dissipation changes measured by QCM-D during the film build up are given in Fig. S3, ESI.†
Exponential growth of the film formed with 28 kDa PLL is attributed to the diffusion of polyelectrolytes into the entire film during each build up cycle.52–54 The films prepared with higher molecular mass PLL (280 kDa) exhibit linear growth and are very thin as deduced from Fig. S3, ESI.† It is even not clear if the support is contentiously covered by the film. It seems that larger PLL sterically restricts polyelectrolyte diffusion inside the film as found in a previous study.54
Thus, in order to obtain polyelectrolyte multilayer films of sufficient thickness, we systematically built up the films with PLL having 28 kDa as a viscosity averaged molecular mass. The same PLL was used for adsorption on the vesicle surface before their deposition on the multilayer film.
Morphology analysis of the (PLL/HA)12 film showed that its surface is flat and geometrically suitable for adsorption of spherical vesicles and subsequent embedding under additional polyelectrolyte layers.
Drying of the film resulted in shrinkage by a factor of 2–3. The films made from the same polylelectrolytes but in the presence of 150 mM NaCl undergo a shrinkage by a factor of 4 upon drying.54 The dry film is characterized by relatively low roughness (mean roughness Ra is 1.7 nm over a 10 μm × 10 μm surface) and has a thickness of 92 ± 13 nm (the film in buffer is 243 ± 28 nm thick). Strong shrinkage due to removal of water points towards a highly hydrated state of the film. This gel-like behaviour is in agreement with microrheology experiments performed on HA/PLL multilayer films of about 10 μm in thickness.55
3.2 Vesicle adhesion to the PLL/HA film and embedding
Unilamellar and negatively charged phospholipid liposomes were prepared by mechanical extrusion and were passively loaded with CF as a fluorescent marker to detect them and to analyse their integrity upon adsorption and subsequent embedding under additional coverage with polyelectrolytes. Indeed even a transient opening of the lipidic bilayer would lead to CF leakage from the vesicle interior. Together with the main component DPPC, the vesicles contained 10% w/w of the lipid DPPG that carries a negative charge at pH 7.4 and 10% w/w of cholesterol CL in order to increase their rigidity.Native vesicles do not adsorb in an intact form on the surface of positively charged (PLL/HA)12/PLL film as studied by AFM and CLSM (Scheme 1, a → d). This could be due to disruption of liposomes interacting with charged polyelectrolyte surfaces.35–38
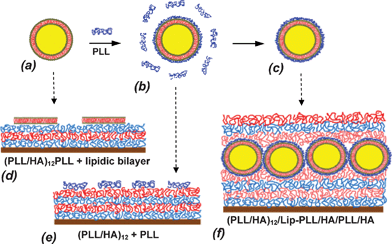 | ||
| Scheme 1 Scheme of vesicle stabilization by PLL covering (a → b) followed by separation of well-covered single vesicles from excess of non-bound PLL (b → c). Native vesicles are disrupted upon adsorption on (PLL/HA)12/PLL film forming a lipidic bilayer (a → d). Free non-bound PLL is preferably adsorbed on a (PLL/HA)12 film than PLL-covered vesicles (b → e). Liposome-containing film (PLL/HA)12/Lip-PLL/HA/PLL/HA is formed by adsorption of PLL-covered liposomes (Lip-PLL) on (PLL/HA)12 film followed by additional coating with HA/PLL/HA layers (c → f). | ||
In our recent study we have evaluated optimal conditions to prepare PLL-stabilized phospholipid vesicles.47–49 Direct mixing of vesicle and excess of PLL at appropriate conditions led to polymer-coated single vesicles without changing the integrity of the lipid bilayer (Scheme 1, a → b). However, being in contact with the oppositely charged (PLL/HA)12 film, these particles, in the presence of excess PLL from the solution, did not adsorb at all at the multilayer film surface. This is not surprising if one takes into account that adsorption of PLL, which is present in excess, from the vesicle solution, is favoured in comparison with adsorption of larger and less flexible PLL-covered vesicles (Scheme 1, b → e).
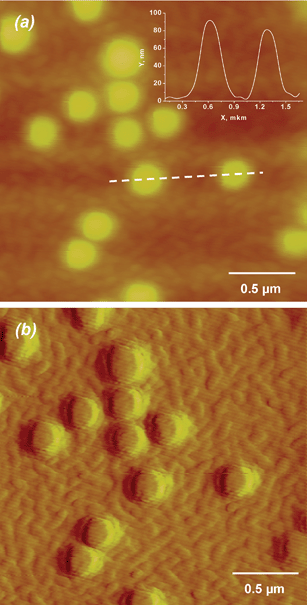
Hence, the PLL-covered vesicles had to be separated from unbound PLL by gel-permeation chromatography (see Fig. S2, ESI†). The obtained vesicles were then used for adsorption on the surface of oppositely charged (PLL/HA)12 films. CLSM imaging showed clearly the presence of green dots on the film surface (Fig. 1(a)) when the unbound vesicles were washed away. The dots represent the adsorbed vesicles filled with the fluorescent marker CF. If the free CF is interacting with the film, the distribution of the dye in the film is homogeneous (Fig. 1(c)). So, the fluorescence is attributed to the adsorbed vesicles containing the entrapped dye. The size of the dots, which is about 0.5–1.0 μm (see inset of Fig. 1(b)), is larger than the diameter of the vesicles (initial vesicles are 133 nm in diameter). This seems to be mainly due to the lower resolution of CLSM than the vesicle size. Individual dots on the film surface demonstrate successful vesicle adsorption.
 | ||
| Fig. 1 CLSM microscopy images. Scanning in XY position (Z position is fixed). The vesicles were adsorbed on the film (PLL/HA)12/Lip-PLL (a) and additionally coated with HA/PLL/HA, yielding a (PLL/HA)12/Lip-PLL/HA/PLL/HA film (b). The inset in image (b) is a fluorescence profile for the section depicted with a dashed line. (c) (PLL/HA)14 film after incubation with CF (6.7 × 10−5 mg mL−1 in Tris-buffer) followed by washing with Tris-buffer. | ||
Simple vesicle adsorption on the film does not give any stabilization/protection for the immobilized vesicles. In addition these vesicles will be directly exposed to cells in experiments aimed to study their behaviour in presence of these films. Hence the adsorbed vesicles were stabilized by additionally coating the assembly with HA/PLL/HA layers in order to anchor them as schematically shown in Scheme 1, c → f. Thus, the formed film has the following architecture: (PLL/HA)12/Lip-PLL/HA/PLL/HA. During the additional coating with the HA/PLL/HA trilayer, considerable vesicle desorption occurs as is apparent when comparing the fluorescence intensity in Fig. 1(b) with that of Fig. 1(a). Indeed, 81 ± 6% of the initially adsorbed vesicles were removed from the surface due to substitution with adsorbing polyelectrolytes. This amount of desorption was calculated by analysis of total CF in the vesicle-containing film. We explain this vesicle replacement upon embedding by formation of the complex between HA and Lip-PLL followed by removal of the complex particles. However, a part of adsorbed vesicles, probably better attached to the outer HA surface layer, stays on the surface in an intact form as was observed by CLSM (Fig. 1(b)).
The cross-section of the film-integrated vesicle (inset in Fig. 1(b)) shows that fluorescence comes only from the vesicle filled with fluorescent dye. In order to prove that the integrated vesicles preserve their integrity and that the encapsulated CF is retained in the vesicle interior, the (PLL/HA)12/Lip-PLL/HA/PLL/HA film was stored one day at room temperature and the amount of CF in the film supernatant was analysed. No CF leakage was found.
To assess the interaction of CF with the polyelectrolytes in the film, a control experiment was performed in which the (PLL/HA)14 film was brought in contact with the same amount of CF as that contained in the adsorbing vesicles and washed away with buffer. Analysis of CF attached to the film after intensive buffer rinse showed that the amount of CF, which remains in the film, is negligible compared to the CF encapsulated in adsorbed liposomes. CF is attached to the multilayers due to electrostatic interactions with amino groups of PLL. The amount of bound CF was the same in the case of incubation time 10 min or 2 h. This means that CF, as a small molecule, can easily permeate into the film. However the amount of attached CF is much lower than that of CF encapsulated in the vesicles. Thus, one can conclude that the measured fluorescence is coming from CF entrapped in the immobilized vesicles which keep their integrity upon immobilization.
3.3 Structure of composite vesicle-containing films
AFM Images (a, b) and (c, d) in Fig. 2 display the morphology of the (PLL/HA)12 film and the same film with integrated vesicles - (PLL/HA)12/Lip-PLL/HA/PLL/HA. The presence of the vesicles is evidenced when comparing the images (a, b) and (c, d). Vesicles represent individual particles and do not form a compact surface layer. This could be explained by insufficient excess of the adsorbing vesicles which was only about two times larger than the amount of vesicles needed to form a close-packed monolayer as well as by removal of the vesicles during HA/PLL/HA deposition on adsorbed vesicles.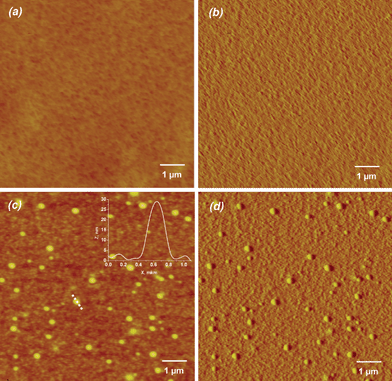 | ||
| Fig. 2 AFM images of (PLL/HA)12 film (a - height and b - deflection images) and liposome-containing film (PLL/HA)12/Lip-PLL/HA/PLL/HA (c - height and d - deflection images). The films were imaged in the dry state and in the contact mode. The inset in (c) corresponds to the height profile of the vesicle along the dotted line (X-axis in μm, Z-axis in nm). | ||
Cross-section analysis of the vesicles (typical profile is presented in the right corner of Fig. 2(c)) shows a typical height profiles of the integrated vesicles. The integrated vesicles have average values of height and width equal to 21 ± 6 and 311 ± 50 nm, respectively. Analysis of height and width of each imaged vesicle showed a quasi linear dependence between height and width values (see Fig. S4, ESI†) which is the expected relationship for spheres emerging from a continuous medium. Taking into account the average diameter of the vesicles in solution (133 ± 2 nm), a first assumption is that the vesicles are flattened out. In order to check the validity of this assumption, films containing non-deformable latex particles integrated by the same way were investigated.
Negatively charged sulfonated PS latex particles having an average diameter of 195 nm, which is close to the vesicle diameter, were used for control experiments instead of the vesicles. The film containing PLL modified PS particles (PLL/HA)12/PS-PLL/HA/PLL/HA was prepared with the same protocol as vesicle-containing films. Hence, the PS particles were also modified with a layer of 28 kDa PLL. Latex particles also formed non-compact monolayers as seen in Fig. 3. Morphological analysis of the entrapped latex particles showed that the average values of particle height emerging from the film surface was equal to 79 ± 12 nm and the width to 393 ± 43 nm, respectively. Typical particle profiles are presented in the inset of Fig. 3.
 | ||
| Fig. 3 AFM images of a (PLL-HA)12/PS-PLL/HA/PLL/HA film (a - height and b - deflection images). The images were acquired in the dry state and in contact mode. The inset corresponds to the height profile of two PS particles along the dotted line (X-axis in μm, Z-axis in nm). | ||
Comparison of the surface morphology of vesicle- and latex-containing films suggests the following film structure (Scheme 2). There are two regimes of additional covering of vesicles or PS-latex particles (yellow sphere) adsorbed on the (PLL/HA)12 film (grey) and covered additionally with HA/PLL/HA layers (green). The additional covering of the particles leads to formation of a thin HA/PLL/HA film on the top of the particle (depicted by the dashed arrow) because the adsorbing polyelectrolytes cannot diffuse through the particle to directly reach the available reservoir of the underlying (PLL/HA)12 film. The thickness of this HA/PLL/HA film on top of the deposited particles could be of the order of a few nanometres if one considers that the thickness of this coverage can be estimated from QCM results (see Fig. S3, ESI†) as if the film build up would proceed as on naked silica.
 | ||
| Scheme 2 Representation of PLL/HA films with incorporated liposomes or PS latex particles both stabilized by PLL coating; X represents the thickness increase over the (PLL/HA)12 film upon deposition of HA/PLL/HA in a location without deposited vesicles and (X + Y) represents the thickness increase upon adsorption of HA/PLL/HA in a location atop the deposited particles assuming that they deposit on the (PLL/HA)12 stratum without penetrating into this architecture. In the graph the particles are depicted on top of the (PLL/HA)12 film. The analysis below, however, reveals that they are embedded about half into this multilayer. | ||
However, the additional coating HA/PLL/HA in between the vesicles (depicted by the bold arrows) allows the polyelectrolytes to diffuse inside the film and this leads to the regular growth of the already formed (PLL/HA)12 film. The increase of the film thickness in this case is much higher. Thus, the additional coverage with HA/PLL/HA layers is not homogeneous over the entire surface of the polyelectrolyte multilayer film. The difference in thickness for the (PLL/HA)12/PLL/HA/PLL/HA and (PLL/HA)12/PLL films was found to be 32 ± 5 nm as measured by AFM using the surface scratching technique. This value corresponds to the thickness of the HA/PLL/HA layers (X) deposited on the (PLL/HA)12 film in a location without adsorbed particles.
Another parameter obtained from AFM imaging is the height of the particles, Y, emerging over the average plane of the film (Scheme 2). For the vesicles and PS latexes the measured Y values are 21 ± 6 and 79 ± 12 nm. According to Scheme 2, the sum of X and Y should give the total diameter of the particles when the thickness of the film formed on the particle top is considered small with respect to X + Y and when the particles do not penetrate in the (PLL/HA)12 support. For the vesicles and latexes the calculated values for X + Y are 53 ± 11 and 111 ± 17 nm, respectively. This is much less than the diameter of these particles in solution (hydrodynamic diameters for vesicles and latexes are 133 ± 2 and 195 ± 5 nm, respectively). However, the difference in these values, which is 58 nm, is very close to the difference in particle diameter in solution, which is 62 nm.
Thus, we suggest that both soft vesicles and stiff latex particles behave in a similar way upon integration into the LbL multilayers. Both kinds of particles are settling down into the bulk of the film when they are covered with additional polyelectrolyte layers but they preserve a spherical shape. This assumption is in agreement with the observation that adenoviral vectors which are about 70 nm in diameter are able to diffuse inside the HA/PLL films.26 At the same time it seems that the gel-like very hydrated PLL/HA matrix can keep the initial structure of even soft vesicles incorporated into this matrix.
3.4 Films with multiple strata of vesicles
Despite removal of the majority of the vesicles when additional coating with HA/PLL/HA layers (see last paragraph), the outstanding feature of the LbL approach allows one to increase the amount of integrated vesicles by simply repeating the procedure of vesicle embedding, thus constructing many vesicle “interlayers”.Fig. 4 shows that the fluorescence coming from the integrated vesicles is progressively increasing with the number of incorporated vesicle “interlayers”. The removal of adsorbed vesicles upon additional coating with HA/PLL/HA layers was always observed for the second and the third “interlayers” and was found to be 60 ± 19% and 74 ± 15%, respectively. Hence, the vesicle desorption upon further embedding under a polyelectrolyte cushion is also a process that does not depend on the number of vesicle immobilization steps. If the additional coating on top of the initially deposited vesicles is continued from HA/PLL/HA up to (HA/PLL)3/HA layers, no further release is observed indicating that the deposition of three layers (HA/PLL/HA) suffices to remove a part of adsorbed vesicles but to immobilize the vesicles left.
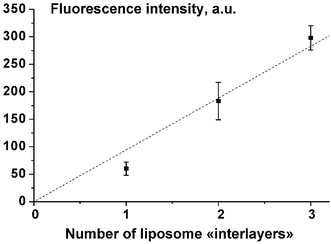 | ||
| Fig. 4 Fluorescence of solutions obtained by solubilisation of liposome-containing films vs. the number of vesicle “interlayers” in the films. Each value is the average of at least three independent experiments. The dotted line is not a fit but is aimed to guide the eye. The error bars correspond to the standard deviation. | ||
Thus, one can conclude that the additional coating of adsorbed vesicles with HA/PLL/HA layers leads to partial substitution of the initially adsorbed vesicles by the polyelectrolytes, but the total amount of integrated vesicles can be easily increased by construction of numerous vesicle “interlayers” (Fig. 4). This allows finally to increase the film load with vesicle encapsulated dye.
3.5 Triggered release of the encapsulated dye
In our previous study, we were not able to control the release of the molecules encapsulated inside the vesicles being adsorbed onto a poly(allylamine hydrochloride)/poly-L-glutamic acid film.45,46 Electrochemistry experiments showed that ferrocyanide, initially encapsulated in the vesicles, was spontaneously liberated in the multilayer film in about 10 h.45 The revealed compact vesicle surface localization rather points towards the presence of vesicle aggregates on the film surface.In the present study the integration of individual vesicles into the polyelectrolyte film is reached. We have shown that it is possible to embed PLL modified and CF entrapped vesicles into PLL-HA multilayer films without spontaneous dye release for at least one day at room temperature. In addition it is possible to increase the load of entrapped vesicles and hence of dye (or drug in future applications), despite the prevalent desorption (70–80%) occurring upon embedding under polyelectrolyte layers, by simply increasing the number of vesicle deposition steps (up to three, Fig. 4). We would now like to trigger the release of the entrapped dye by means of an external physical perturbation. Our previous study47 has shown that CF can be released from PLL coated vesicles in solution when the temperature is increased above the main phase transition temperature of the DPPC/DPPG lipid mixture, namely 41 °C.
When the vesicles are embedded in the (PLL/HA)12/Lip-PLL/HA/PLL/HA architecture, no spontaneous release is observed in the time frame of a day, but as soon as the temperature is increased slightly above the main phase transition temperature of the DPPC/DPPG/CL lipid mixture a very fast dye release is obtained: about 75% of the initially encapsulated dye is released in about 30 min (Fig. 5). Even if the experiments have been made only three times, they show a marked effect of the temperature increase above the main transition temperature of the lipid mixture, there is virtually no release for prolonged time at room temperature but important and fast release above 41 °C. The release kinetics is faster when the vesicles are embedded in the multilayer architecture than in solution (Fig. 5). No difference in the peak intensity corresponding to amino groups of PLL was revealed by FTIR for (PLL/HA)8 film heated from at 25 to 45 °C (data not shown). This means that faster release kinetics of film-embedded vesicles is induced not by a change of the polyelectrolyte film properties but due to destabilization of the lipid bilayer interacting with the polyelectrolyte film network. The temperature increase is a very efficient way to trigger fast and controlled dye release from the embedded vesicles.
 | ||
| Fig. 5 Time evolution of the cumulative CF release from vesicles embedded inside a (PLL/HA)12/Lip-PLL/HA/PLL/HA film architecture, when the film is maintained at ambient temperature (▼) or heated and maintained at 45 °C (▲). These release kinetics are compared to the release kinetics obtained for the same PLL covered vesicles in aqueous solution at 45 °C (●). Each value is the average of at least three (two) independent experiments with its standard deviation for measurements at 45 °C (ambient temperature). | ||
In near future we plan to minimize vesicle replacement upon LbL coverage and try to release the encapsulated dye by means of other physical perturbations for instance ultrasound, which is known to be very efficient in inducing vesicle rupture.56
4 Conclusion
Liposome-containing polyelectrolyte multilayer films could be formed by the LbL technique using polyelectrolytes (HA and PLL) and sterically stabilized PLL-covered liposomes as constituents. Integrity of embedded vesicles is preserved due to highly hydrated environments of the gel-like HA/PLL film. When the lipids are in the solid state, the vesicle-encapsulated dye (CF) is retained inside the vesicle interior, but pronounced dye release can be induced by temperature increase when the lipidic bilayer is liquid. Although 60–70% of the vesicles are desorbed upon polyelectrolyte adsorption following vesicle deposition, the total amount of film-integrated liposomes could be simply increased by repeating the step of vesicle adsorption. This study demonstrates the feasibility of a general strategy aimed to encapsulate solutes in the internal aqueous compartment of vesicles embedded inside highly hydrated polyelectrolyte multilayer films without spontaneous release but with the possibility to trigger the release of hydrophilic compounds with external stimuli tolerated by the environmental tissues.Acknowledgements
The work was supported by the French Ministry of Education, INSERM grant for young scientists, and by the EU6 project BIOCOATING (Marie-Curie fellowship, proposal 039589).References
- Y. Hirano and D. J. Mooney, Adv. Mater., 2004, 16, 17–25 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319–348 CrossRef CAS.
- K. Ariga, J. P. Hill and Q. Li, Phys. Chem. Chem. Phys., 2007, 9, 2319–2340 RSC.
- P. T. Hammond, Curr. Opin. Colloid Interface Sci., 2000, 4, 430–442.
- Z. Tang, Y. Wang, P. Podsiadlo and N. A. Kotov, Adv. Mater., 2006, 18, 3203–3224 CrossRef CAS.
- S. S. Shiratori and M. F. Rubner, Macromolecules, 2000, 33, 4213–4219 CrossRef CAS.
- J. B. Schlenoff, H. Ly and M. Li, J. Am. Chem. Soc., 1998, 120, 7626–7634 CrossRef CAS.
- J. B. Schlenoff and S. T. Dubas, Macromolecules, 2001, 34, 592–598 CrossRef CAS.
- Z. Sui, D. Salloum and J. B. Schlenoff, Langmuir, 2003, 19, 2491–2495 CrossRef CAS.
- M. Salomäki, I. A. Vinokurov and J. Kankare, Langmuir, 2005, 21, 11232–11240 CrossRef.
- N. Laugel, C. Betscha, M. Winterhalter, J.-C. Voegel, P. Schaaf and V. Ball, J. Phys. Chem. B, 2006, 110, 19443–19449 CrossRef CAS.
- S. A. Sukhishvili and S. Granick, J. Am. Chem. Soc., 2000, 122, 9550–9551 CrossRef CAS.
- S. A. Sukhishvili and S. Granick, Macromolecules, 2002, 35, 301–310 CrossRef CAS.
- J. Cho and F. Caruso, Macromolecules, 2003, 36, 2845–2851 CrossRef CAS.
- H. Inoue, K. Sato and J. Anzai, Biomacromolecules, 2005, 6, 27–29 CrossRef CAS.
- Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Am. Chem. Soc., 1995, 117, 6117–6123 CrossRef CAS.
- J. I. Anzai, T. Hoshi and N. Nakamura, Langmuir, 2000, 16, 6306–6311 CrossRef CAS.
- G. Ladam, P. Schaaf, F. J. Cuisinier, G. Decher and J.-C. Voegel, Langmuir, 2001, 17, 878–882 CrossRef CAS.
- N. Jessel, F. Atalar, P. Lavalle, J. Mutterer, G. Decher, P. Schaaf, J.-C. Voegel and J. Ogier, Adv. Mater., 2003, 15, 692–695 CrossRef CAS.
- Y. Lvov, G. Decher and G. B. Sukhorukov, Macromolecules, 1993, 26, 5396–5399 CrossRef CAS.
- G. B. Sukhorukov, M. M. Montrel, A. I. Petrov, L. I. Shabarchina and B. I. Sukhorukov, Biosens. Bioelectron., 1996, 11, 913–922 CrossRef CAS.
- R. Pei, X. Cui, X. Yang and E. Wang, Biomacromolecules, 2001, 2, 463–468 CrossRef CAS.
- J. Zhang, L. S. Chua and D. M. Lynn, Langmuir, 2004, 20, 8015–8021 CrossRef.
- C. M. Jewell, J. Zhang, N. J. Fredin and D. M. Lynn, J. Controlled Release, 2005, 106, 214–223 CrossRef CAS.
- M. Dimitrova, Y. Arntz, P. Lavalle, F. Meyer, M. Wolf, C. Schuster, Y. Haïkel, J.-C. Voegel and J. Ogier, Adv. Funct. Mater., 2007, 17, 233–245 CrossRef CAS.
- J. Chluba, J. C. Voegel, G. Decher, P. Erbacher, P. Schaaf and J. Ogier, Biomacromolecules, 2001, 2, 800–805 CrossRef CAS.
- B. Thierry, P. Kujawa, C. Tkaczyk, F. M. Winnik, L. Bilodeau and M. Tabrizian, J. Am. Chem. Soc., 2005, 127, 1626–1627 CrossRef CAS.
- C. Vodouhê, E. Le Guen, J. Mendez Garza, G. Francius, C. Déjugnat, J. Ogier, P. Schaaf, J.-C. Voegel and P. Lavalle, Biomaterials, 2006, 27, 4149–4156 CrossRef CAS.
- A. Schneider, C. Vodouhê, L. Richert, G. Francius, E. Le Guen, P. Schaaf, J.-C. Voegel, B. Frisch and C. Picart, Biomacromolecules, 2007, 8, 139–145 CrossRef CAS.
- D. V. Volodkin, A. I. Petrov, M. Prevot and G. B. Sukhorukov, Langmuir, 2004, 20, 3398–3406 CrossRef CAS.
- N. Benkirane-Jessel, P. Lavalle, F. Meyer, F. Audouin, B. Frisch, P. Schaaf, J. Ogier, G. Decher and J.-C. Voegel, Adv. Mater., 2004, 16, 1507–1511 CrossRef CAS.
- N. Benkirane-Jessel, P. Lavalle, E. Hübsch, V. Holl, B. Senger, Y. Haikel, J.-C. Voegel, J. Ogier and P. Schaaf, Adv. Funct. Mater., 2005, 15, 648–654 CrossRef CAS.
- Y. Barenholz, Curr. Opin. Colloid Interface Sci., 2001, 6, 66–77 CrossRef CAS.
- R. P. Richter, R. Berat and A. R. Brisson, Langmuir, 2006, 22, 3497–3505 CrossRef CAS.
- I. Reviakine and A. Brisson, Langmuir, 2000, 16, 1806–1815 CrossRef CAS.
- T. Cassier, A. Sinner, A. Offenhauser and H. Mohwald, Colloids Surf., B, 1999, 15, 215–225 CrossRef CAS.
- B. Seantier, C. Breffa, O. Félix and G. Decher, Nano Lett., 2004, 4, 5–10 CrossRef CAS.
- K. Katagiri, R. Hamasaki, K. Ariga and J. Kikuchi, Langmuir, 2002, 18, 6709–6711 CrossRef CAS.
- K. Katagiri, R. Hamasaki, K. Ariga and J. Kikuchi, J. Am. Chem. Soc., 2002, 124, 7892–7893 CrossRef CAS.
- P. Vermette, L. Meagher, E. Gagnon, H. G. Griesser and C. J. Doillon, J. Controlled Release, 2002, 80, 179–195 CrossRef CAS.
- F. Patolsky, A. Lichtenstein and I. Willner, J. Am. Chem. Soc., 2001, 123, 5194–5205 CrossRef CAS.
- C. Yoshina-Ishii, G. P. Miller, M. L. Kraft, E. T. Kool and S. G. Boxer, J. Am. Chem. Soc., 2005, 127, 1356–1357 CrossRef CAS.
- S. M. Christensenn and D. Stamou, Soft Matter, 2007, 3, 828–836 RSC.
- M. Michel, A. Izquierdo, G. Decher, J.-C. Voegel, P. Schaaf and V. Ball, Langmuir, 2005, 21, 7854–7859 CrossRef CAS.
- M. Michel, D. Vautier, J.-C. Voegel, P. Schaaf and V. Ball, Langmuir, 2004, 20, 4835–4839 CrossRef CAS.
- D. Volodkin, H. Mohwald, J.-C. Voegel and V. Ball, J. Controlled Release, 2007, 117, 111–120 CrossRef CAS.
- D. Volodkin, V. Ball, P. Schaaf, J.-C. Voegel and H. Mohwald, Biochim. Biophys. Acta, 2007, 1768, 280–290 CAS.
- D. V. Volodkin, V. Ball, J.-C. Voegel, H. Möhwald, R. Dimova and V. Marchi-Artzner, Colloids Surf., A, 2007, 303, 89–96 CrossRef CAS.
- M. Rodahl and B. Kasemo, Sens. Actuators, A, 1996, 54, 448–456 CrossRef.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59, 391–396 CrossRef CAS.
- C. Picart, P. Lavalle, P. Hubert, F. J. G. Cuisinier, G. Decher, P. Schaaf and J.-C. Voegel, Langmuir, 2001, 17, 7414–7424 CrossRef CAS.
- C. Picart, J. Mutterer, L. Richert, Y. Luo, G. D. Prestwich, P. Schaaf, J.-C. Voegel and P. Lavalle, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12531–12535 CrossRef CAS.
- C. Porcel, P. Lavalle, G. Decher, B. Senger, J.-C. Voegel and P. Schaaf, Langmuir, 2007, 23, 1898–1904 CrossRef CAS.
- D. Collin, P. Lavalle, J. M. Garza, J.-C. Voegel, P. Schaaf and P. Martinoty, Macromolecules, 2004, 37, 10195–10198 CrossRef CAS.
- A. Schroeder, Y. Avnir, S. Weisman, Y. Najajreh, A. Gabizon, Y. Talmon, J. Kost and Y. Barenholz, Langmuir, 2007, 23, 4019–4025 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: Gel-permeation chromatography of 28 kDa PLL. Fig. S2: Separation of PLL-covered liposomes from unbound PLL. Fig. S3: Analysis of the PLL/HA film growth by QCM. Fig. S4: Height and width analysis of PLL/HA films containing incorporated vesicles. See DOI: 10.1039/b713563g |
| This journal is © The Royal Society of Chemistry 2008 |
