Spectroscopic studies of 1,2-diaminoanthraquinone (DAQ) as a fluorescent probe for the imaging of nitric oxide in living cells
Francisco Galindo*a, Nurul Kabirbc, Jelena Gavrilovicb and David A. Russellc
aDepartamento de Química Inorgánica y Orgánica, Unidad Asociada de Materiales Orgánicos Avanzados (UAMOA), Universitat Jaume I/CSIC, Avda. Sos Baynat, s/n, E-12071, Castellón, Spain. E-mail: francisco.galindo@uji.es
bSchool of Biological Sciences, University of East Anglia, Norwich, Norfolk, UK NR4 7TJ
cSchool of Chemical Sciences and Pharmacy, University of East Anglia, Norwich, Norfolk, UK NR4 7TJ
First published on 30th November 2007
Abstract
Spectroscopic analysis of the fluorescent probe 1,2-diaminoanthraquinone (DAQ) provides information about the mechanism of nitric oxide imaging in living cells. Fluorescent aggregates of a reaction product of DAQ are thought to be responsible for the images obtained with confocal fluorescence microscopy.
Nitric oxide (NO) is a free radical that has been the subject of intense research1 following its implication in numerous biological functions.2,3 NO acts as a vasodilator in the cardiovascular system,4 as a neurotransmitter,5 and as effector within the immune system.6 Specific probes for imaging NO in living cells or tissues have been designed and commercialised, which have accelerated rapidly the research in the field. Fluorescence represents one of the most useful tools (together with EPR) to visualise the presence of NO in biological systems7–10 and recent reviews can be found in the literature.11–15 One of the probes commercialised for NO sensing is 1,2-diaminoanthraquinone (DAQ or DAA) (Chart 1). It has been reported that DAQ is not cytotoxic,16 in contrast with other NO probes, and it does not need ester derivatives for cellular uptake.16 It has been reported that the non-fluorescent DAQ reacts with NO in the presence of oxygen to give the triazole DAQ-TZ (Chart 1) which is detectable by means of fluorescence microscopy.16 Further experiments reproduced this observation.17–22 However, in the course of our research on nitric oxide detection systems23–25 we found some apparent inconsistencies about the use of DAQ for the detection of NO. Accordingly we decided to undertake a spectroscopic study of this probe in solution and intracellularly in order to establish the species responsible for the fluorescence observed in the biological samples.
 | ||
| Chart 1 | ||
The original description of the reaction between DAQ and NO was reported by Heiduschka and Thanos:16 a glass slide containing an aqueous solution of DAQ was exposed to commercially available NO gas. The colour of DAQ changed from violet to brownish and a new red fluorescence became visible with a rhodamine filter (above 580 nm). It was reported that the triazole formed was water insoluble and that it precipitated. Similar experiments were performed by us by preparing two air equilibrated solutions: (a) DAQ 50 µM in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (9
DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), pH 7.4, HEPES 10 mM, NaCl 0.1 M, and (b) DAQ 50 µM in DMSO. Pure NO gas (3 ml) was bubbled slowly into each solution. Such a quantity of gas was enough to obtain a saturated solution of NO.11 In both cases the rapid bleaching of the initial pink-red colour to yield a colourless solution was observed as shown in the inset of Fig. 1(a). However, no visible precipitate could be seen with the concentrations used.
1), pH 7.4, HEPES 10 mM, NaCl 0.1 M, and (b) DAQ 50 µM in DMSO. Pure NO gas (3 ml) was bubbled slowly into each solution. Such a quantity of gas was enough to obtain a saturated solution of NO.11 In both cases the rapid bleaching of the initial pink-red colour to yield a colourless solution was observed as shown in the inset of Fig. 1(a). However, no visible precipitate could be seen with the concentrations used.
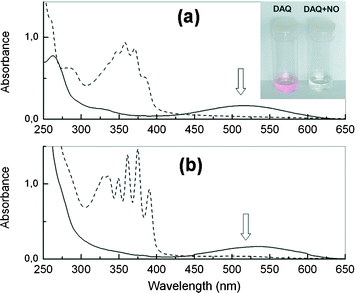 | ||
Fig. 1 Absorption spectra of DAQ before (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and after reaction ( ) and after reaction (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) with gaseous NO. (a) DAQ 50 µM in water ) with gaseous NO. (a) DAQ 50 µM in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (9 DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) pH 7.4. (b) DAQ 50 µM in DMSO. 1) pH 7.4. (b) DAQ 50 µM in DMSO. | ||
In the absorption spectra, the elimination of the band at ca. 450–600 nm in both solutions (a and b) was observed after 15 min (Fig. 1). The same result was also observed in water–DMSO solution at pH 5.9 (data not shown). These absorption changes can be interpreted in terms of reaction of the electron pairs of the free amino groups of DAQ to form the DAQ-TZ triazole (hence eliminating the CT band) responsible for the red colour.26 In fact, the addition of a few drops of concentrated HCl to a solution of DAQ induces bleaching of the red colour due to the protonation of the amino groups.
On the other hand, the weak fluorescence emission of the solutions of DAQ27 was also quenched following reaction with gaseous NO (in oxygenated medium). These observations raised some questions about the use of DAQ for fluorescence microscopy, since previous reports rely on the excitation of a band (centred at ca. 520 nm) that no longer exists when NO is present. It is also apparent that the resulting NO product (DAQ-TZ) does not absorb at wavelengths reported for excitation of DAQ in cells or tissues (>520 nm).
Imaging of NO by means of DAQ in biological samples has been extensively reported and accordingly we attempted the visualization of NO in living cells. Thus, a culture of Raw 264.7 mouse macrophage cells was loaded with DAQ and stimulated to produce NO following standard procedures28 and examined by means of confocal laser fluorescence microscopy. Excitation of the intracellularly loaded DAQ with both λ = 543 and 488 nm gave the results shown in Fig. 2, the excitation of the DAQ at 488 nm affording a brighter image than at 543 nm. The nature of the high fluorescence intensity positive areas is currently being investigated. However, co-localization experiments with the pH probe FG-H50329 suggest a lysosomal identity, and also an important role of acidic pH. No subcellular structures could be visualized using DAQ in non-stimulated cells.
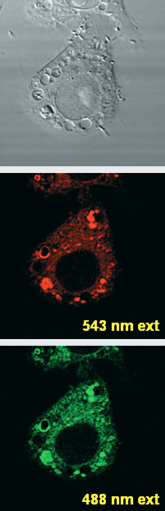 | ||
| Fig. 2 Confocal laser fluorescence microscopy image of Raw 264.7 cells loaded with DAQ and stimulated to produce nitric oxide. Images correspond to DIC channel (top), 543 nm and 488 nm excitation. | ||
To address the question of how the biological experiments exciting at >520 nm could afford fluorescence from DAQ if no absorption is apparent (since DAQ absorption is quenched by NO), a working hypothesis was considered: the conditions of bioimaging are such that a high concentration of DAQ-TZ molecules occurs and that supramolecular aggregates of DAQ-TZ are formed which are able to emit fluorescence. As previously discussed, the original paper16 reported that a brownish precipitate appeared upon exposure of DAQ to NO gas, which supports the possible formation of aggregates. In this regard, it should be noted that the formation of fluorescent aggregates is a common photophysical phenomenon and many examples of aggregation-induced emission can be found in the literature.30
In order to confirm this hypothesis the fluorescence of three solutions containing DAQ at varying concentrations, in DMSO (5, 50 and 500 µM) were studied. To 1 ml of such solutions (air equilibrated), 6 ml of NO gas was bubbled. For the 5 µM and 50 µM DAQ the initial pink-red colour disappeared within a few seconds to yield colourless solutions. With the 500 µM DAQ solution a light brown solution was obtained after addition of the NO gas. The fluorescence of these solutions showed, upon increasing the concentration, a relative rise of the shoulder at ca. 600 nm relative to the band at ca. 440. The fluorescence emission spectra of these three solutions were recorded exciting at 350 nm and the results can be seen in Fig. 3(a). With the dilute solution (5 µM) the emission spectrum exhibited a maximum at 440 nm only. However at 500 µM the DAQ solution showed two well distinguished emission bands at ca. 450 nm and 580 nm. These bands can be tentatively assigned to the emission of the monomer and aggregate of the triazole DAQ-TZ, respectively. The relationship between the monomer/aggregate intensity ratios and probe concentration can be seen in the inset of Fig. 3(a). If no aggregation process had taking place a horizontal line would be expected. However it is clear from Fig. 3(a) (inset) that the ratio of fluorescence emission intensities (F580/F436) increases with increasing DAQ concentration.
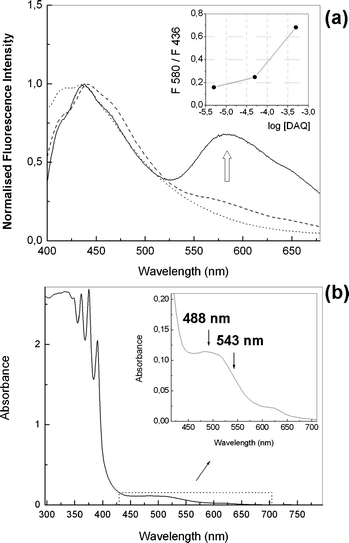 | ||
Fig. 3 (a) Fluorescence spectra after addition of NO gas to varying concentrations of DAQ in DMSO: 5 µM (⋯), 50 µM (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ), 500 µM ( ), 500 µM (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ). Excitation wavelength = 350 nm. Inset: intensity ratios at 580 and 436 nm vs.DAQ concentration. (b) Absorption spectrum of DAQ (500 µM) in DMSO after reaction with gaseous NO. ). Excitation wavelength = 350 nm. Inset: intensity ratios at 580 and 436 nm vs.DAQ concentration. (b) Absorption spectrum of DAQ (500 µM) in DMSO after reaction with gaseous NO. | ||
A careful inspection of the absorption spectrum of the brownish solution (not precipitated) in DMSO after reaction with NO (500 µM in DAQ) afforded some insight. As it can be seen in Fig. 3(b), there is a discernable absorption between 450 and 650 nm which could be attributed to the putative aggregates, which also would impart the brown colour to the DMSO solution.
In another experiment the solvent was changed to tetrahydrofuran (THF) so that the DAQ-TZ aggregates would be soluble enough, as in DMSO, to enable the detection of the fluorescence signal. Analogously, the fluorescence intensity ratios increased with increasing concentration of DAQ between 5 and 180 µM (Fig. 4(a)). Additionally the absorption spectra of such solutions showed a new absorption band at ca. 450–500 nm (Fig. 4(b)) which did not follow the linearity predicted by the Beer law (inset of Fig. 4(b)).
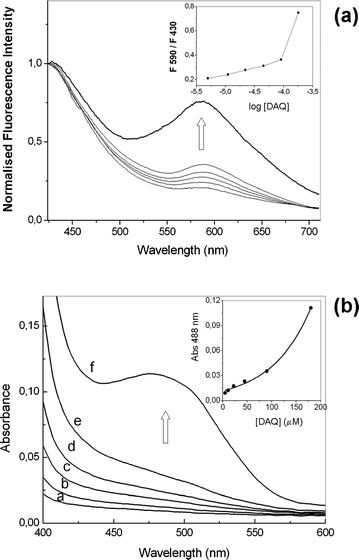 | ||
| Fig. 4 (a) Fluorescence spectra after addition of NO gas to varying concentrations of DAQ in THF (5–180 µM). Excitation wavelength = 350 nm. Inset: intensity ratios at 590 and 430 nm vs.DAQ concentration. (b) Absorption spectrum of DAQ in THF (a = 5, b = 10, c = 20, d = 45, e = 90, f = 180 µM) after reaction with gaseous NO. Inset: absorbance intensity at 488 nm vs. concentration of probe. | ||
A comparison of the recorded fluorescence spectra obtained with three typical wavelengths used in confocal fluorescence microscopy is shown in Fig. 5. As it can be seen, 488 nm excitation yields greater emission intensity than that of both 543 nm and 364 nm, in agreement with the higher absorption at such wavelength.
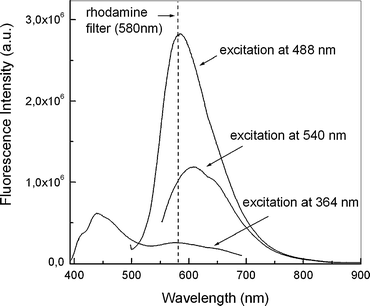 | ||
| Fig. 5 Effect of excitation wavelength on the fluorescence. Addition of NO gas to DAQ (500 µM) in DMSO. The cut-off of the rhodamine filter is shown to illustrate the emission eliminated with its use. | ||
The main practical consequence of the above findings is that knowing the species origin of the emission it is possible not only to excite the fluorophore selectively but also to collect the maximum amount of light from the sample through selection of suitable emission filters. In the original application of DAQ16 it is reported that a rhodamine filter should be used (cutting off 580 nm and below). Moreover in subsequent reports the specific use of 520 nm19 and 543 nm17 excitation was described. However, with consideration of the results reported here it would seem that a better choice would be to excite the samples at a shorter wavelength value and collect the emission even below the rhodamine filter (580 nm). Excitation at the wavelength of the argon-ion laser, typically used in confocal laser scanning microscopy (488 nm), rather than that of the helium–neon laser (543 nm), and collecting the emitted light above 500 nm, would improve the imaging experiments. This fact would explain, in part, the bright image recorded with excitation at 488 nm, in comparison with the one using 543 nm.
In addition to this practical application for improved imaging of NO, a mechanistic implication can be also inferred. Oxygenated solutions of nitric oxide react with DAQ but the product is only detected when the concentration of both are high enough to yield fluorescent aggregates of DAQ-TZ. Consequently those cellular regions not showing fluorescence emission may contain some NO but at lower concentrations. Other probes sensitive to NO should be used complementarily.7,11–15 We are aware of the complexities of the intracellular world and hence we can not rule out any secondary reaction with DAQ leading to a fluorescent product different from DAQ-TZ in the cells.31 However, this seems unlikely as NO inhibitors have been used,17–19 demonstrating that the fluorescent signal correlates well with the presence of NO.
In summary, the spectroscopic analysis of DAQ and DAQ-TZ has provided some insights into the mechanism of NO imaging in live cells. With consideration that (a) DAQ is strongly coloured but practically non-fluorescent, (b) that DAQ-TZ is colourless in dilute solutions (not able to absorb appreciably at >450 nm) and (c) that as DAQ-TZ tends to associate in DMSO and THF it is thought that the aggregates of DAQ-TZ (precipitated into the cells) are the possible origin for the fluorescent images acquired by microscopy, but not the isolated DAQ-TZ. A practical implication of this result is apparent: it is optimal to excite at the maximum absorption of the aggregates, i.e., 488 nm.
Experimental details: DAQ and other reagents were purchased from Sigma-Aldrich. Solvents were of analytical reagent grade or better. Water used was Millipore® quality. UV-vis spectra were recorded using a Hitachi U-3000 spectrophotometer. Steady state emission and excitation spectra were recorded using a FluoroMax-2 instrument. 1 cm path-length quartz cells were used for both the absorption and emission measurements. All the solutions were air-equilibrated prior to reaction with NO (gas). For all the experiments with NO, saturating conditions were obtained in order to attain complete conversion of DAQ to DAQ-TZ. Therefore the corresponding DAQ solution in aqueous or organic solvent was placed in the quartz cell and after measurement of absorption and fluorescence spectra, an excess of NO (gas) was bubbled slowly through the sample (typically 3–6 ml injected by means of a syringe). After a sufficient stabilization period, typically a few minutes, the absorption and fluorescence spectra were again recorded. The macrophage cells were imaged using a confocal laser scanning microscope (Carl Zeiss, LSM 510 meta) with 543 nm and 488 nm laser excitation. Appropriate emission bands were selected for the two fluorescent channels. A 63×, 1.4 NA objective was used to ensure high-resolution images. Details of cell growth have been reported previously.29
Acknowledgements
F.G. acknowledges the financial support of the Spanish M.E.C. (project CTQ2006–26251-E/BQU and Ramón y Cajal Program), of the Generalitat Valenciana (projects GV/2007/277 and ARVIV/2007/079) and of the Fundació Caixa Castelló-Bancaixa/Universitat Jaume I (Project 04I007.25/1). D.A.R. and J.G. acknowledge The Wellcome Trust (Showcase Award 071083/Z/03/Z) for financial support.Notes and references
- D. L. H. Williams, A chemist's view of the nitric oxide story, Org. Biomol. Chem., 2003, 1, 441–449 RSC , and references cited therein.
- E. Culotta and D. E. Koshland, Jr., NO news is good-news, Science, 1992, 258, 1862–1865.
- R. M. Palmer, D. S. Ashton and S. Moncada, Vascular endothelial-cells synthesize nitric-oxide from L-arginine, Nature, 1988, 333, 664–666 CrossRef CAS.
- L. J. Ignarro, G. M. Buga, K. S. Wood, R. E. Byrns and G. Chaudhuri, Endothelium-derived relaxing factor produced and released from artery and vein is nitric-oxide, Proc. Natl. Acad. Sci. USA, 1987, 84, 9265–9269 CAS.
- J. Garthwaite, S. L. Charles and R. Chess-Williams, Endothelium-derived relaxing factor release on activation of nmda receptors suggests role as intercellular messenger in the brain, Nature, 1988, 336, 385–388 CrossRef CAS.
- J. R. Lancaster and J. B. Hibbs, EPR demonstration of iron nitrosyl complex-formation by cytotoxic activated macrophages, Proc. Natl. Acad. Sci. USA, 1990, 87, 1223–1328 CAS.
- M. H. Lim, D. Xu and S. J. Lippard, Visualization of nitric oxide in living cells by a copper-based fluorescent probe, Nat. Chem. Biol., 2006, 2, 375–380 CrossRef CAS.
- M. H. Lim, B. A. Wong, W. H. Pitcock, Jr., D. Mokshagundam, M.-H. Baik and S. J. Lippard, Direct nitric oxide detection in aqueous solution by copper (II) fluorescein complexes, J. Am. Chem. Soc., 2006, 128, 14364–14373 CrossRef CAS.
- E. Sasaki, H. Kojima, H. Nishimatsu, Y. Urano, K. Kikuchi, Y. Hirata and T. Nagano, Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs, J. Am. Chem. Soc., 2005, 127, 3684–3685 CrossRef CAS.
- M. Sato, N. Hida and Y. Umezawa, Imaging the nanomolar range of nitric oxide with an amplifier-coupled fluorescent indicator in living cells, Proc. Natl. Acad. Sci. USA, 2005, 102, 14515–14520 CrossRef CAS.
- T. Nagano and T. Yoshimura, Bioimaging of nitric oxide, Chem. Rev., 2002, 102, 1235 CrossRef CAS.
- O. von Bohlen und Halbach, Nitric oxide imaging in living neuronal tissues using fluorescent probes, Nitric Oxide, 2003, 9, 217–228 CrossRef CAS.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, Use of fluorescence probes for detection of reactive nitrogen species: a review, J. Fluoresc., 2006, 16, 119–139 CrossRef CAS.
- S. Wang, J. F. R. Paton and S. Kasparov, The challenge of real-time measurements of nitric oxide release in the brain, Autonomic Neuroscience-Basic & Clinical, 2006, 126–127, 59–67 Search PubMed.
- P. Wardman, Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects, Free Rad. Biol. Med., 2007, 43, 995–1022 CrossRef CAS.
- P. Heiduschka and S. Thanos, NO production during neuronal cell death can be directly assessed by a chemical reaction in vivo, NeuroReport, 1998, 9, 4051–4057 Search PubMed.
- X. Chen, C. Sheng and X. Zheng, Direct nitric oxide imaging in cultured hippocampal neurons with diaminoanthraquinone and confocal microscopy, Cell. Biol. Int., 2001, 25, 593–598 CrossRef CAS.
- O. von Bohlen und Halbach, D. Albrecht, U. Heinemann and S. Schuchmann, Spatial nitric oxide imaging using 1,2-diaminoanthraquinone to investigate the involvement of nitric oxide in long-term potentiation in rat brain slices, NeuroImage, 2002, 15, 633–639 CrossRef.
- S. Schuchmann, D. Albrecht, U. Heinemann and O. von Bohlen und Halbach, Nitric oxide modulates low-Mg2+-induced epileptiform activity in rat hippocampal-entorhinal cortex slices, Neurobiol. Disease, 2002, 11, 96–105 Search PubMed.
- M. V. Beligni, A. Fath, P. C. Bethke, L. Lamattina and R. L. Jones, Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers, Plant Physiol., 2002, 129, 1642–1650 CrossRef CAS.
- B. Püttmann, E.-M. Gerlach, M. Krüger and D. Blottner, Neuromuscular contacts induce nitric oxide signals in skeletal myotubes in vitro, Neurosignals, 2005, 14, 85–95 Search PubMed.
- C. Stöhr and S. Stremlau, Formation and possible roles of nitric oxide in plant roots, J. Exp. Bot., 2006, 57, 463–470 CrossRef.
- M. Bru, M. I. Burguete, F. Galindo, S. V. Luis, M. J. Marín and L. Vigara, Cross-linked poly(2-hydroxyethylmethacrylate) films doped with 1,2-diaminoanthraquinone (DAQ) as efficient materials for the colorimetric sensing of nitric oxide and nitrite anion, Tetrahedron Lett., 2006, 47, 1787–1791 CrossRef CAS.
- D. H. P. Hedges, D. J. Richardson and D. A. Russell, Electrochemical control of protein monolayers at indium tin oxide surfaces for the reagentless optical biosensing of nitric oxide, Langmuir, 2004, 20, 1901–1908 CrossRef CAS.
- D. J. Blyth, J. W. Alyott, J. W. B. Moir, D. J. Richardson and D. A. Russell, Optical biosensing of nitric oxide using the metalloprotein cytochrome c′, Analyst, 1999, 124, 129–134 RSC.
- M. S. Khan and Z. H. Khan, Electronic absorption spectra of amino substituted anthraquinones and their interpretation using the ZINDO/S and AM1 methods, Spectrochim. Acta, Part A, 2003, 59, 1409–1426 CrossRef.
- The fluorescence quantum yield of DAQ was estimated to be ca. 0.0004 at pH 7.4 and 5.9 using ethidium bromide as standard.
- W. J. Murphy, M. Muroi, C. X. Zhang, T. Suzuki and S. W. Russell, Both basal and enhancer kappa B elements are required for full induction of the Mouse inducible nitric oxide synthase gene, J. Endotoxin Res., 1996, 3, 381–393 Search PubMed.
- F. Galindo, M. I. Burguete, L. Vigara, S. V. Luis, N. Kabir, J. Gavrilovic and D. A. Russell, Synthetic macrocyclic peptidomimetics as tunable pH probes for the fluorescence imaging of acidic organelles in live cells, Angew. Chem., Int. Ed., 2005, 44, 6504–6508 CrossRef CAS.
- H. Tong, Y. Hong, Y. Dong, M. Häußler, J. W. Y. Lam, Z. Li, Z. Guo, Z. Guo and B. Z. Tang, Fluorescent “light up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics, Chem. Commun., 2006, 3705–3707 RSC , and references therein.
- X. Zhang, W.-S. Kim, N. Hatcher, K. Potgieter, L. L. Moroz, R. Gillette and J. V. Sweedler, Interfering with nitric oxide measurements. 4,5-Diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid, J. Biol. Chem., 2002, 277, 48472–48478 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2008 |
