Hot off the press
Robert A.
Hill
and
Andrew
Sutherland
Department of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK. E-mail: bobh@chem.gla.ac.uk; andrews@chem.gla.ac.uk
First published on 15th January 2008
Abstract
A personal selection of 36 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as bolivianine, which has a new skeleton, from Hedyosmum angustifolium.
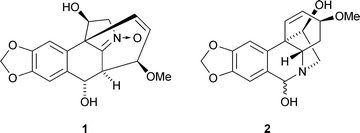
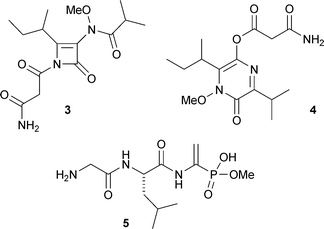
The structure of hostasinine A1, from Hosta plantaginea, was confirmed by X-ray crystallography (X.-J. Hao and co-workers, Org. Lett., 2007, 9, 5279). A biosynthetic pathway to the novel skeleton of hostasinine A 1 from the co-occurring haemanthidine2 has been proposed and a biomimetic conversion of haemanthidine 2 into hostasinine A 1 has been effected. The structure of kasarin, an antibacterial metabolite of a marine Hyphomycetes species, has been revised from the β-lactam 3 to the pyrazinone 4 on the basis of detailed spectroscopic analysis of the natural product and model compounds (D. Uemura and co-workers, Tetrahedron Lett., 2007, 48, 8628). A second revision of the structure of antibiotic A53868, a metabolite of Streptomyces luridus, has been proposed on the basis of synthetic studies (W. A. van der Donk and co-workers, Angew. Chem., Int. Ed., 2007, 46, 9089). The correct structure of antibiotic A53868 is 5, which contains an unusual amino dehydrophosphonic acid.
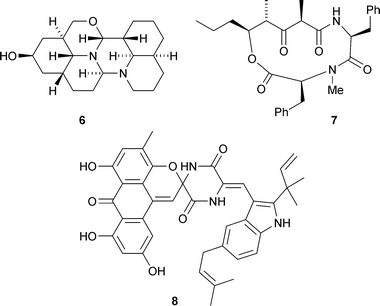
A possible biosynthetic pathway from lysine has been proposed for the hexacyclic alkaloid myrobotinol6 isolated from leaves of Myrioneuron nutans (V. C. Pham et al., J. Org. Chem., 2007, 72, 9826). Stereocalpin A7 is a cyclic depsipeptide from the Antarctic lichen Stereocaulon alpinum that contains an unusual 5-hydroxy-2,4-dimethyl-3-oxooctanoic acid moiety (H. Oh and co-workers, Tetrahedron Lett., 2008, 49, 29). A strain of Aspergillus variecolor found growing in high-salinity conditions is the source of several unusual diketopiperazine metabolites containing an anthopyranoid moiety, including variecolortide A8 whose structure was confirmed by X-ray analysis (Q.-Q. Gu, W.-M. Zhu and co-workers, Chem. Biodiversity, 2007, 4, 2913).
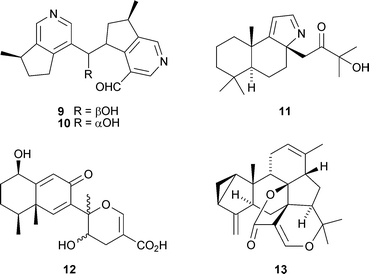
Argutanes A 9 and B 10, the first monoterpene alkaloid dimers of the boschniakine group, have been isolated from the roots of Incarvillea arguta (W.-D. Zhang and co-workers, Helv. Chim. Acta, 2007, 90, 2151). The diterpenoid alkaloid chamobtusin A11, from Chamaecyparis obtusa cv. tetragon, is the first alkaloid to be isolated from this genus (N.-H. Tan and co-workers, Org. Lett., 2007, 9, 4579). The structure of chamobtusin A 11 was confirmed by X-ray analysis. Several extended eremophilane derivatives, including ligulasagitin A12, have been found in Ligularia sagitta (Z.-J. Jia and co-workers, Tetrahedron, 2007, 63, 12665). The authors propose biosynthetic routes to the ligulasagitins involving Diels–Alder-type couplings. The sesterterpenoid bolivianine13, from Hedyosmum angustifolium, is suggested to be formed via geranylation at C-9 of a 1,3-cycloeudesmane sesquiterpenoid (V. Jullian and co-workers, Org. Lett., 2007, 9, 4693).
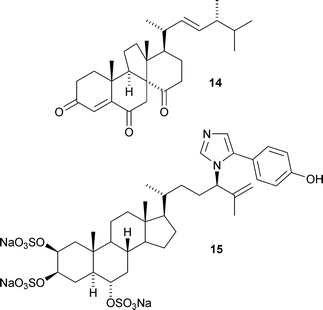
A strain of the fungus Gymnacella dankaliensis derived from a sponge of the Halichondria genus is the source of several unusual steroids including dankasterone A14, the first 13(14→8)-abeo-ergostane derivative (T. Amagata et al., J. Nat. Prod., 2007, 70, 1731). The structure of dankasterone A 14 was confirmed by X-ray analysis. The 24-N-imidazolyl-substituted sulfated steroid amaranzole A15, from the tropical sponge Phorbas amaranthus, is the first of this class (T. F. Molinski and co-workers, Org. Lett., 2007, 9, 5219).
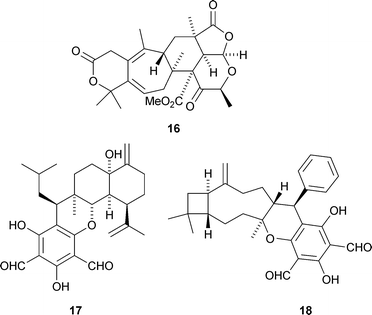
A strain of Penicillium rubrum found in the acidic Berkeley Pit Lake produces several meroterpenoid metabolites including berkeleyacetal A16 (D. B. Stierle et al., J. Nat. Prod., 2007, 70, 1820). Biosynthetic pathways are proposed for these unusual meroterpenoids. Eucalyptal A17, a meroterpenoid from fruits of Eucalyptus globulus, consists of a cadinanesesquiterpenoid linked to a phloroglucinol derivative (J.-M. Yue and co-workers, Org. Lett., 2007, 9, 5549). The structures of both berkeleyacetal A 16 and eucalyptal A 17 were confirmed by X-ray analysis. Guajadial18, from guava leaves (Psidium guajava), is also a meroterpenoid with a phloroglucinol moiety; in this case linked to a caryophylane sesquiterpenoid (J.-K. Liu and co-workers, Org. Lett., 2007, 9, 5135). The authors propose a biosynthetic pathway to guajadial 18.
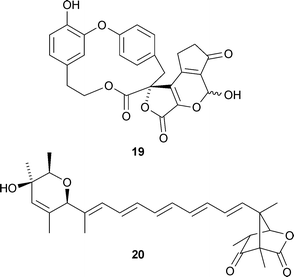
Retipolide A 19 and several related metabolites have been isolated from the mushrooms Retiboletus retipes and Retiboletus ornatipes (W. Steglich and co-workers, Eur. J. Org. Chem., 2007, 5560). Biosynthetic pathways for these spiromacrolactones have been proposed. Labelling studies have been used to establish the polyketide nature of prugosene A120 and related metabolites of the sponge-derived marine fungus Penicillium rugulosum (J. F. Imhoff and co-workers, Tetrahedron, 2007, 63, 11844). A. B. Smith III and B. S. Freeze have reviewed the synthesis of discodermolide and its analogues together with the interesting biological activities of this important group of compounds (Tetrahedron, 2008, 64, 261).
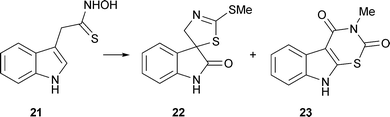
E. Gravel and E. Poupon have pointed out that natural products of considerable complexity may be assembled with minimal assistance of enzymes (Eur. J. Org. Chem., 2008, 27). Labelling studies have demonstrated that indolyl-3-acetothiohydroxamic acid21 is the common precursor to the crucifer phytoalexins such as cyclobrassinin22 and rutalexin23 (M. S. C. Pedras and D. P. O. Okinyo, Org. Biomol. Chem., 2008, 6, 51).
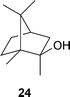
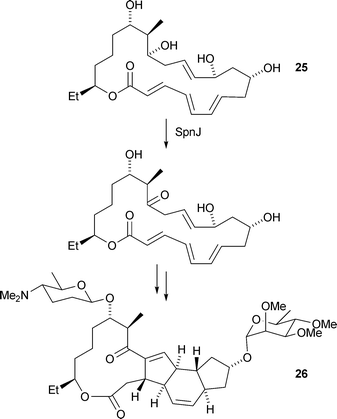
S. Schulz and co-workers have investigated the biosynthesis of 2-methylisoborneol24 by the myxobacterium Nannocystis exedens (Angew. Chem., Int. Ed., 2007, 46, 8287). Feeding studies suggest a pathway involving the methylation of geranyl pyrophosphate followed by ring-closing reactions to complete the biosynthesis. H.-W. Liu and co-workers have synthesised macrolactone 25, a postulated intermediate in the biosynthesis of spinosyns, polyketide -derived macrolides produced by Saccharopolyspora spinosa (J. Am. Chem. Soc., 2007, 129, 14582). Incubation of 25 with SpnJ, an enzyme which exhibits sequence homology to a flavin -dependent dehydrogenase, resulted in the specific oxidation of the C-15 hydroxyl group (Scheme 1). These results confirm the role of SpnJ during spinosyn A26 biosynthesis and 25 as a metabolite of this pathway.
 | ||
| Scheme 1 | ||
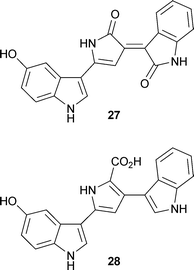
D. O’Hagan and co-workers have reported the mechanism of fluorination by the enzyme 5′-fluoro-5′-deoxyadenosine synthetase (FDAS) in Streptomyces cattleya (J. Am. Chem. Soc., 2007, 129, 14597). A combination of site-directed mutagenesis, structural analyses and isothermal calorimetry have established the key residues for catalysis and the order of substrate binding. Mechanistic studies on the biosynthesis of violacein27, a pigment produced by Chromobacterium violaceum, have allowed the isolation of a genuine intermediate, protoviolaceinic acid28 (T. Hoshino and co-workers, Chem. Commun., 2007, 4140). The isolation of this intermediate from the mixed enzymes of VioABDE has led to a proposed synthesis of this pigment and showed that VioC is required for oxidation of the right-hand indole ring.
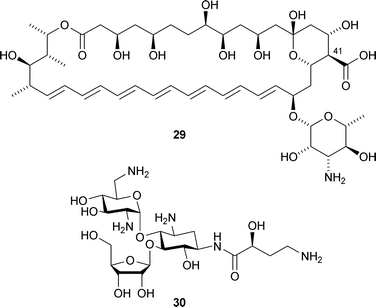
M. D. Burke and co-workers have shown that post-PKS oxidation of the amphotericin B29 skeleton is not required for potent antifungal activity (J. Am. Chem. Soc., 2007, 129, 13804). Synthesis and antifungal testing of various analogues of amphotericin B showed that loss of the carboxylate at C-41, thought to be involved in ion channel formation, still leads to compounds with significant antifungal activity. T. Eguchi and co-workers have chracterised BtrN, an enzyme involved in the biosynthesis of butirosin B30, a 2-deoxystreptamine-containing aminoglycoside with antibiotic activity (J. Am. Chem. Soc., 2007, 129, 15147). Their studies showed that BtrN is a SAM dehydrogenase which catalyses the oxidation of a hydroxyl group by a radical mechanism.
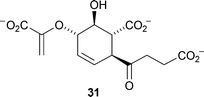
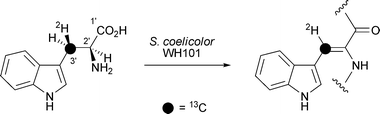
Doubly labelled (2′S,3′R)-[3′-2H,13C]-tryptophan has been used to investigate the stereochemical outcome of the biosynthesis of calcium-dependent antibiotics in Streptomyces coelicolor (J. Org. Chem., 2007, 72, 8950). Incubation of (2′S,3′R)-[3′-2H,13C]-tryptophan with Trp-His auxotrophic Streptomyces coelicolor strain WH101 and subsequent analysis by NMR spectroscopy showed that dehydrogenation occurs with syn stereochemistry with loss of the 2′ and pro-3′S hydrogen atoms (Scheme 2). Z. Guo and co-workers have identified and characterised a key intermediate in the biosynthesis of menaquinone, a lipid -soluble molecule which is used as a vitamin (K2) in the diet of humans and animals (Org. Lett., 2007, 9, 4765). The stereochemistry of this key intermediate, (1R,2S,5S,6S)-2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate31, was determined using NMR spectroscopy and this has allowed the complete elucidation of the menaquinone biosynthetic pathway.
 | ||
| Scheme 2 | ||
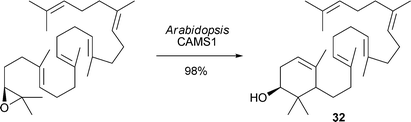
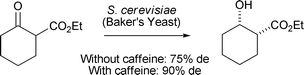
S. P. T. Matsuda and co-workers have used a native oxidosqualene cyclase for the conversion of oxidosqualene to camelliol C32 (Scheme 3) (Org. Lett., 2007, 9, 5223). This is the first example of a native oxidosqualene cyclase that produces an A-ring monocycle as the major product. M. Bertau and co-workers have shown that the addition of caffeine to Baker's yeast reductions of cyclic β-keto esters leads to an increase in diastereoselectivities (Scheme 4) (Org. Biomol. Chem., 2007, 5, 3456). This effect has been ascribed to the fact that caffeine causes cell-death of pre-stressed cells, which have been found to decrease diastereoselectivity.
 | ||
| Scheme 3 | ||
 | ||
| Scheme 4 | ||
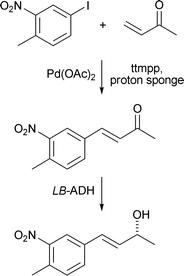
S. Xia and co-workers have screened a number of microorganisms for their ability to prepare chiral α-hydroxy acid derivatives either by α-hydroxylation of an acetic acid derivative or by reduction of the corresponding α-keto ester (Tetrahedron: Asymmetry, 2007, 18, 2537). A one-pot method for the preparation of chiral (R)- and (S)-allylic alcohols has been reported using a palladium-catalysed Heck reaction of aryl iodides with butenone followed by an enzymatic reduction of the resulting α,β-unsaturated ketone (Tetrahedron: Asymmetry, 2007, 18, 2791). For example, reaction of 4-methyl-5-nitroiodobenzene with butenone under Heck conditions followed by reduction of the ketone with an alcohol dehydrogenase from Lactobacillus brevis gave the corresponding (R)-allylic alcohol in 80% yield and >99% ee (Scheme 5). J. C. Moore and co-workers have reviewed recent progress in the enzymatic reduction of ketones (Acc. Chem. Res., 2007, 40, 1412).
 | ||
| Scheme 5 | ||
U. Kragl and co-workers have reported the first example of an (R)-selective hydroxynitrile lyase with an α/β-hydrolase fold. This enzyme from Arabidopsis thaliana has broad substrate specificity, producing cyanohydrins in excellent yields and enantioselectivites (Scheme 6) (Angew. Chem., Int. Ed., 2007, 46, 8679). C. J. Moody and co-workers have shown that benzimidazole- and benzothiazole-quinones are excellent substrates for two-electron reduction by recombinant human NAD(P)H:quinone oxidoreductase (NQO1) (Org. Biomol. Chem., 2007, 5, 3665).
 | ||
| Scheme 6 | ||
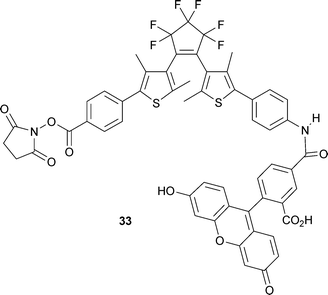
A novel fluorescent photochromic compound (33) has been developed for labelling proteins (M. Irie and co-workers, Chem. Commun., 2007, 5206). The compound, which is composed of diarylethene, fluorescein and succinimidyl ester units, has been shown to control the “off/on” state of fluorescence in the protein of interest. E. Kato et al. have developed a synthetic approach for the preparation of ginkgolide photoaffinity–biotin probes (Org. Biomol. Chem., 2007, 5, 3758). The introduction of biotin and benzophenone onto the ginkgolide scaffold have produced a compound that the authors suggest can be used to study ginkgolide–receptor interactions.
| This journal is © The Royal Society of Chemistry 2008 |