A study of ground-level ozone pollution, ozone precursors and subtropical meteorological conditions in central Taiwan
Dai-Hua
Tsai
a,
Jia-Lin
Wang
b,
Chieh-Heng
Wang
b and
Chang-Chuan
Chan
*a
aInstitute of Occupational Medicine and Industrial Hygiene, College of Public Health, National Taiwan University, No. 17, Xu-Zhou Rd., Taipei 100, Taiwan. E-mail: ccchan@ntu.edu.tw; Fax: +886-2-23222362; Tel: +886-2-33228082
bDepartment of Chemistry, National Central University, Chungli 320, Taiwan
First published on 23rd November 2007
Abstract
Hourly concentrations of ozone (O3), 55 volatile organic compounds (VOCs, ozone precursors) and nitrogen oxides (NOx) were measured at an upwind urban site, a downwind suburban site, and a rural site in central Taiwan, from January 2003 to December 2006. VOC and NOx mean concentrations showed a gradient from high to low across the urban (56 ppb and 34 ppb), suburban (38 ppb and 27 ppb) and rural sites (25 ppb and 21 ppb) but a reverse gradient in ozone across these sites (24, 27, and 29 ppb, respectively). Although there was about twice the difference in VOC concentrations between the urban and rural sites, nearly 65% ozone formation potential was contributed to by the same 9 VOCs. Seasonal patterns showed peak ozone levels in autumn and minima in summer at the urban site, but minima in winter at the downwind suburban and rural sites. Ozone precursor levels, on the other hand, were lowest in summer and highest in winter. The diurnal pattern showed that ozone levels peaked one hour later at the rural site than at the urban site. The ethylbenzene to m,p-xylene ratio, an indicator of the age of the air mass, increased from 0.4 at the urban site to 0.6 at the suburban site and 0.8 at the rural site during daily peak ozone times. This finding suggests the transport of ozone and precursors from upwind to downwind producing elevated ozone levels in the suburban and rural areas. Ozone episodes occurred mostly in days with a mean midday UV index of 6.5 (1 UV index = 100 J m–2) and wind speed at 1.3 m s–1 at all three sites.
1. Introduction
Ground-level ozone has received considerable attention in the past years1 because short-term ozone exposure is associated with decrement in lung function among young and old humans with a history of respiratory diseases and workers in many epidemiological studies;2–6 and it increases rhinoconjunctival symptoms in seasonal allergies.7 It is a secondary pollutant, produced mainly from its precursors of volatile organic compounds (VOCs), and nitrogen oxides (NOx), via photochemical reactions in sunlight.8 Meteorological factors, such as sunny and warm weather conditions and stagnant wind patterns, favor ozone formation.9 In many areas of the world, ozone standards are defined and monitored along with its precursors. Despite the progress in understanding ozone formation processes, strategies of ozone containment are highly area dependent, as source types, distribution of sources, climate, and terrain all vary from one place to another. Consequently, rules that are effective in one given area may not be applicable to other places. Nevertheless, establishing the capabilities to measure ozone precursors with adequate time span, resolution, and area coverage is of primary importance in all places plagued by a high ozone problem.In the European Union (EU), the number of people exposed to ozone levels above the EU target 8 hour average value of 120 µg m–3 (i.e. 60 ppb) is estimated to be about 18 million.10Ozone episodes occur mostly in southern and central Europe, including the Mediterranean countries, the Po Valley, south-eastern France and southern Germany. The maximum number of exceedance per station is about 11 days per year in southern Europe.11 In the United States, the number of exceedance days per year has changed from an average of 218 days in 1980–1982 to an average of 124 days in 1997–1999.12 The number of people living in counties with ozone exposure levels exceeding the 1 hour standard was 37 million and 100 million lived in counties exceeding the 8 hour standard, which is 80 ppb, in 2003. In order to investigate the nature of ozone precursors in ozone non-attainment areas, the US Environmental Protection Agency (EPA) has established photochemical assessment monitoring sites (PAMS) to monitor ozone precursors of 55 C2–C12 VOCs.13 As of 1998, there were 78 operating PAMS sites in the USA.14 Likewise, the co-operative programme for monitoring and evaluation of the long-range transmission of air pollutants in Europe set up a European VOC monitoring network in 1992 to evaluate the distribution and trends of 30 C2–C9 VOCs and ozone.15 While most of the above-mentioned studies were conducted in temperate areas, high ozone levels also occur in subtropical regions, such as Mexico City, Mexico, southern China, Taiwan, and Hong Kong.16–19 The ozone formation processes in these subtropical areas may have their own unique patterns controlled by characteristic meteorology. The weather pattern of the subtropical island of Taiwan is affected by daily and seasonal changes in sunlight and land–sea breezes, which result in large variations in UV intensity, wind speed, and precipitation over days and seasons.20
Based on measurements of ozone, NOx and non-methane hydrocarbon compounds (NMHCs) from air quality monitoring stations, past studies have identified that NOx and NMHCs are decreasing but ozone has been increasing in the past decade, especially in rural central Taiwan.18,21 However, a lack of speciated VOC measurements with detailed spatial and temporal resolution has impeded an understanding of the formation and transport mechanism for ozone and its precursors between urban and downwind rural areas in central Taiwan. In order to unravel this unknown but important photochemical pollution problem, we used long-term hourly measurements of ozone, VOC and NOx, and meteorological parameters at these monitoring sites in this study. Our strategy was to use air pollution and meteorological data at one upwind urban station and two sites at the downwind suburban and rural areas to characterize spatial and temporal patterns and the upwind–downwind transport of ozone and its precursors. By observing the spatial, seasonal and diurnal patterns of ozone and its precursors between urban and rural areas in central Taiwan, we tried to elucidate the general mechanism of ozone formation and regional transportation in the subtropical area. Furthermore, the maximum increment reactivity (MIR) of individual VOCs was used to calculate the ozone formation potential (OFP) in order to determine responsible VOC species for ozone pollution in this area.
2. Experimental
2.1 Study sites
The studied subtropical area comprises a city and its surrounding suburban and rural areas in central Taiwan, where a PAMS network of three stations was established. The urban site (Chonglun station; 24.07°N, 120.39°E) is located in Taichung City with approximately 1 million inhabitants (population density: 6391 people per km2). The two downwind sites, i.e., the suburban site (Caotun station; 23.58°N, 120.39°E) and the further downwind rural site (Jhushan station; 23.45°N, 120.40°E), are located in Nantou County with a population of 535![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (population density: 130 people per km2). The distances from the upwind urban site to the downwind suburban site and to the downwind rural site are 16.7 and 40.8 km, respectively. Taichung City is a densely populated metropolis with a vehicle density of 5600 vehicles per km2 and about 3300 registered factories. To avoid direct contamination by close-by point sources, the urban site is placed in a public park and the sampling inlet is about 5 m above the surface. By contrast, Nantou County is considered a rural area with a limited density of 121 vehicles per km2 and about 1300 registered factories.22,23 The sampling inlets of the suburban and rural sites are installed on the roof of a two-story building, which is about 13 m above the surface. Fig. 1 shows the satellite image of terrestrial features of the studied area. The urban site is located at a populous and busy downtown in the urban core of Taichung city. The suburban and rural sites are closer to Central Mountain Range. Therfore, the sea breezes during daytime can sweep high level of ozone precursors from the urban area to the downwind mountaneous surburban and rural areas forming ozone along the transport route.
000 (population density: 130 people per km2). The distances from the upwind urban site to the downwind suburban site and to the downwind rural site are 16.7 and 40.8 km, respectively. Taichung City is a densely populated metropolis with a vehicle density of 5600 vehicles per km2 and about 3300 registered factories. To avoid direct contamination by close-by point sources, the urban site is placed in a public park and the sampling inlet is about 5 m above the surface. By contrast, Nantou County is considered a rural area with a limited density of 121 vehicles per km2 and about 1300 registered factories.22,23 The sampling inlets of the suburban and rural sites are installed on the roof of a two-story building, which is about 13 m above the surface. Fig. 1 shows the satellite image of terrestrial features of the studied area. The urban site is located at a populous and busy downtown in the urban core of Taichung city. The suburban and rural sites are closer to Central Mountain Range. Therfore, the sea breezes during daytime can sweep high level of ozone precursors from the urban area to the downwind mountaneous surburban and rural areas forming ozone along the transport route.
 | ||
| Fig. 1 Location of the urban site (Chonglun station, as the red balloon indicates), the suburban site (Caotun station, as the upper green arrow indicates), and the rural site (Jhushan station, as the lower green arrow indicates) in central Taiwan. The wind roses show the wind distribution at the urban site (left figure) and at the rural site (right figure).46 | ||
According to the 2005 emission inventory of the Taiwan Environmental Protection Administration (TEPA), 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544 tons of VOCs and 16
544 tons of VOCs and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 tons of NOx per year were emitted in Taichung City (urban site). In Nantou County (suburban and rural sites), 17
490 tons of NOx per year were emitted in Taichung City (urban site). In Nantou County (suburban and rural sites), 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 396 tons of VOCs and 12
396 tons of VOCs and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 tons of NOx per year were estimated.24
093 tons of NOx per year were estimated.24
To facilitate data interpretation, the three PAMS stations were placed next to the air quality monitoring stations operated by TEPA, which provide hourly meteorological and key pollutant data. The data include ozone, NOx, wind speed, wind direction, temperature, relative humidity (RH), and ultraviolet (UV) radiation. The yearly averaged temperature is 24 ± 6 °C, and relative humidity is about 70 ± 10% at the three sites from 2003 to 2006.
2.2 Sampling instruments
2.3 Statistical analysis
The 4 year VOC data from January 2003 to December 2006 were used for the analyses in this study because hourly VOC concentrations were continuously monitored at all three PAMS stations since 2003. The total effective measurement length is 48 months per site. However, the urban site has 47 effective months due to 1 month of system malfunction. The 4 year hourly concentrations of VOCs were statistically transformed into hourly and monthly averages in order to investigate their diurnal and seasonal variations. Statistics were summarized over all available VOC data. Missing data due to instrumental malfunction were not included in the data analyses. Pearson’s correlation coefficient was used to evaluate the relations among ozone, ozone precursors and meteorological factors. All descriptive computations and statistical analyses were made using SPSS software (version 11.0; SPSS Inc., Chicago, IL, USA).3. Results and discussion
3.1 Concentration of VOCs, NOx, and ozone at urban, suburban and rural sites
Table 1 summarizes the 4 year averaged concentrations of the 55 VOCs, NOx, and ozone measured during 2003–2006 at the three sites. There is a gradient in the total VOC (TVOC) and NOx mean concentrations from high to low across the urban (56 ppb and 34 ppb), suburban (38 ppb and 27 ppb) and rural site (25 ppb and 21 ppb). The concentrations of TVOC and most speciated VOC (50 of 55 VOCs) at the urban site were about 1 to 3 times higher than those at the suburban site and 1 to 6 times than those at the rural site. NOx concentrations in the urban site were about 1.3 to 1.6 times higher than those in the suburban and rural sites. Although higher VOC and NOx levels were observed at the urban site, a reverse gradient in ozone was observed across these three sites with 24 ppb at the urban site, 27 ppb at the suburban site, and 29 ppb at the rural site, respectively. A similar gradient also exists for the number of ozone episode days with one hour maximum greater than 100 ppb over four years, which were 103 days at the urban site, 253 days at the suburban site, and 334 days at the rural site.| Urban | Suburban | Rural | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (ppb) | OFPb | Concentration (ppb) | OFPb | Concentration (ppb) | OFPb | |||||||
| Mean | SD a | Mean | SD a | Mean | SD a | Mean | SD a | Mean | SD a | Mean | SD a | |
| a Standard deviation. b Unit of OFP is µg(O3) g(VOC)–1. | ||||||||||||
| Alkanes | ||||||||||||
| Ethane | 3.95 | 2.63 | 1.22 | 0.81 | 3.17 | 2.68 | 0.97 | 0.82 | 2.29 | 1.47 | 0.71 | 0.45 |
| Propane | 4.28 | 3.31 | 3.70 | 2.87 | 2.76 | 2.08 | 2.39 | 1.80 | 2.35 | 1.76 | 2.03 | 1.52 |
| Iso-butane | 1.69 | 1.16 | 4.87 | 3.35 | 1.10 | 0.99 | 3.16 | 2.84 | 0.98 | 0.68 | 2.83 | 1.95 |
| n-Butane | 3.08 | 2.05 | 7.46 | 4.97 | 2.03 | 1.79 | 4.92 | 4.34 | 1.70 | 1.18 | 4.12 | 2.86 |
| Cyclopentane | 0.33 | 0.22 | 2.30 | 1.54 | 0.44 | 0.97 | 3.06 | 6.65 | 0.20 | 0.38 | 1.35 | 2.64 |
| Iso-pentane | 0.83 | 0.92 | 3.39 | 3.74 | 1.98 | 1.70 | 8.06 | 6.93 | 0.03 | 1.11 | 0.12 | 4.54 |
| n-Pentane | 0.28 | 0.31 | 0.86 | 0.96 | 0.92 | 0.97 | 2.82 | 2.97 | 0.11 | 0.89 | 0.34 | 2.74 |
| 2,2-Dimethylbutane | 0.24 | 0.17 | 0.68 | 0.50 | 0.27 | 1.38 | 0.79 | 3.98 | 0.06 | 0.05 | 0.16 | 0.15 |
| 2,3-Dimethylbutane | 0.36 | 0.25 | 1.37 | 0.96 | 0.33 | 1.40 | 1.24 | 5.30 | 0.09 | 0.08 | 0.34 | 0.31 |
| 2-Methylpentane | 1.37 | 1.02 | 9.61 | 7.14 | 0.70 | 0.84 | 4.91 | 5.89 | 0.35 | 0.32 | 2.47 | 2.24 |
| 3-Methylpentane | 1.07 | 0.99 | 7.50 | 6.93 | 0.64 | 1.63 | 4.46 | 11.42 | 0.34 | 0.31 | 2.39 | 2.14 |
| n-Hexane | 1.13 | 0.85 | 3.92 | 2.94 | 0.64 | 0.72 | 2.23 | 2.47 | 0.52 | 0.66 | 1.80 | 2.28 |
| Methylcyclopentane | 0.65 | 0.45 | 6.26 | 4.38 | 0.28 | 0.21 | 2.73 | 2.03 | 0.23 | 0.17 | 2.18 | 1.63 |
| 2,4-Dimethylpentane | 0.21 | 0.16 | 1.28 | 1.01 | 0.10 | 0.07 | 0.59 | 0.45 | 0.06 | 0.04 | 0.37 | 0.28 |
| Cyclohexane | 0.33 | 0.28 | 1.44 | 1.23 | 0.19 | 0.20 | 0.83 | 0.88 | 0.12 | 0.14 | 0.54 | 0.63 |
| 2-Methylhexane | 0.66 | 0.52 | 2.93 | 2.31 | 0.28 | 0.20 | 1.24 | 0.91 | 0.23 | 0.20 | 1.00 | 0.88 |
| 2,3-Dimethylpentane | 0.28 | 0.22 | 1.50 | 1.21 | 0.13 | 0.10 | 0.69 | 0.52 | 0.09 | 0.08 | 0.49 | 0.42 |
| 3-Methylhexane | 0.69 | 0.55 | 3.94 | 3.18 | 0.27 | 0.20 | 1.58 | 1.17 | 0.25 | 0.21 | 1.41 | 1.23 |
| 2,2,4-Trimethylpentane | 0.41 | 0.31 | 1.78 | 1.34 | 0.19 | 0.13 | 0.82 | 0.56 | 0.12 | 0.10 | 0.53 | 0.45 |
| n-Heptane | 0.58 | 0.45 | 1.91 | 1.51 | 0.23 | 0.18 | 0.76 | 0.60 | 0.18 | 0.18 | 0.60 | 0.59 |
| Methylcyclohexane | 0.25 | 0.18 | 1.83 | 1.31 | 0.12 | 0.11 | 0.84 | 0.78 | 0.08 | 0.08 | 0.59 | 0.59 |
| 2,3,4-Trimethylpentane | 0.16 | 0.12 | 1.17 | 0.93 | 0.07 | 0.07 | 0.55 | 0.51 | 0.04 | 0.03 | 0.31 | 0.25 |
| 2-Methylheptane | 0.37 | 0.39 | 1.24 | 1.31 | 0.24 | 0.25 | 0.83 | 0.85 | 0.10 | 0.12 | 0.32 | 0.42 |
| 3-Methylheptane | 0.27 | 0.23 | 0.95 | 0.79 | 0.11 | 0.10 | 0.39 | 0.36 | 0.08 | 0.07 | 0.28 | 0.24 |
| n-Octane | 0.31 | 0.29 | 0.86 | 0.80 | 0.13 | 0.13 | 0.35 | 0.36 | 0.07 | 0.12 | 0.20 | 0.33 |
| n-Nonane | 0.15 | 0.15 | 0.44 | 0.44 | 0.06 | 0.06 | 0.17 | 0.18 | 0.04 | 0.05 | 0.11 | 0.15 |
| n-Decane | 0.16 | 0.14 | 0.42 | 0.37 | 0.08 | 0.07 | 0.20 | 0.20 | 0.05 | 0.07 | 0.14 | 0.18 |
| n-Undecane | 0.19 | 0.20 | 0.52 | 0.53 | 0.11 | 0.16 | 0.28 | 0.42 | 0.06 | 0.10 | 0.16 | 0.26 |
| n-Dodecane | 0.23 | 0.23 | 0.62 | 0.61 | 0.12 | 0.19 | 0.31 | 0.51 | 0.07 | 0.54 | 0.18 | 1.43 |
| Alkenes | ||||||||||||
| Ethylene | 2.60 | 2.06 | 22.08 | 17.51 | 1.84 | 1.95 | 15.62 | 16.59 | 1.40 | 1.13 | 11.89 | 9.60 |
| Propylene | 1.65 | 1.25 | 26.67 | 20.15 | 0.96 | 1.21 | 15.51 | 19.64 | 0.70 | 0.60 | 11.28 | 9.67 |
| t-2-Butene | 0.68 | 0.40 | 15.70 | 9.23 | 0.39 | 0.86 | 9.02 | 19.69 | 0.25 | 0.19 | 5.73 | 4.29 |
| 1-Butene | 0.41 | 0.29 | 8.39 | 5.92 | 0.32 | 1.02 | 6.59 | 20.86 | 0.19 | 0.15 | 3.96 | 3.16 |
| cis-2-Butene | 0.40 | 0.24 | 9.13 | 5.52 | 0.31 | 1.17 | 7.06 | 26.93 | 0.15 | 0.13 | 3.50 | 2.92 |
| t-2-Pentene | 0.39 | 0.32 | 9.91 | 8.16 | 0.19 | 0.78 | 4.70 | 19.63 | 0.07 | 0.08 | 1.82 | 2.04 |
| 1-Pentene | 0.32 | 0.26 | 5.69 | 4.58 | 0.16 | 0.65 | 2.84 | 11.48 | 0.05 | 0.05 | 0.91 | 0.81 |
| cis-2-Pentene | 0.22 | 0.30 | 5.55 | 7.53 | 0.18 | 0.96 | 4.50 | 24.30 | 0.04 | 0.04 | 1.01 | 0.99 |
| Isoprene | 0.47 | 0.54 | 11.91 | 13.75 | 0.24 | 0.29 | 6.10 | 7.41 | 0.36 | 0.44 | 9.05 | 11.11 |
| Alkyne | ||||||||||||
| Acetylene | 2.41 | 1.15 | 1.28 | 0.61 | 2.84 | 2.85 | 1.51 | 1.52 | 1.29 | 0.50 | 0.69 | 0.27 |
| Aromatics | ||||||||||||
| Benzene | 1.15 | 0.75 | 1.54 | 1.01 | 0.72 | 0.44 | 0.97 | 0.59 | 0.63 | 0.51 | 0.84 | 0.69 |
| Toluene | 11.42 | 11.42 | 116.23 | 116.15 | 8.01 | 9.83 | 81.49 | 100.00 | 6.07 | 8.66 | 61.80 | 88.08 |
| Ethylbenzene | 0.99 | 0.76 | 11.66 | 8.89 | 0.54 | 0.51 | 6.31 | 5.92 | 0.34 | 0.35 | 4.01 | 4.15 |
| m,p-Xylene | 2.78 | 2.32 | 89.32 | 74.47 | 1.32 | 1.38 | 42.41 | 44.30 | 0.90 | 0.99 | 29.05 | 31.88 |
| Styrene | 0.24 | 0.23 | 2.27 | 2.11 | 0.11 | 0.14 | 1.04 | 1.28 | 0.07 | 0.10 | 0.69 | 0.92 |
| o-Xylene | 1.04 | 0.82 | 29.44 | 23.12 | 0.52 | 0.49 | 14.57 | 13.97 | 0.35 | 0.36 | 9.76 | 10.04 |
| Isopropylbenzene | 0.07 | 0.05 | 0.74 | 0.55 | 0.04 | 0.06 | 0.45 | 0.68 | 0.03 | 0.03 | 0.28 | 0.31 |
| n-Propylbenzene | 0.18 | 0.14 | 1.89 | 1.48 | 0.08 | 0.08 | 0.82 | 0.85 | 0.06 | 0.06 | 0.58 | 0.60 |
| m-Ethyltoluene | 0.64 | 0.53 | 25.75 | 21.64 | 0.23 | 0.21 | 9.29 | 8.47 | 0.17 | 0.21 | 7.02 | 8.54 |
| p-Ethyltoluene | 0.37 | 0.30 | 11.98 | 9.64 | 0.17 | 0.29 | 5.61 | 9.32 | 0.13 | 0.12 | 4.03 | 3.93 |
| 1,3,5-Trimethylbenzene | 0.34 | 0.28 | 16.77 | 13.71 | 0.12 | 0.12 | 6.13 | 5.93 | 0.10 | 0.11 | 4.95 | 5.31 |
| o-Ethyltoluene | 0.30 | 0.25 | 9.43 | 7.86 | 0.13 | 0.13 | 4.05 | 4.01 | 0.10 | 0.10 | 3.02 | 3.18 |
| 1,2,4-Trimethylbenzene | 1.20 | 0.99 | 52.12 | 42.92 | 0.43 | 0.41 | 18.50 | 17.69 | 0.35 | 0.39 | 15.25 | 17.04 |
| 1,2,3-Trimethylbenzene | 0.41 | 0.33 | 18.12 | 14.43 | 0.17 | 0.13 | 7.22 | 5.90 | 0.14 | 0.13 | 5.96 | 5.57 |
| m-Diethylbenzene | 0.08 | 0.07 | 2.08 | 1.95 | 0.04 | 0.06 | 0.96 | 1.62 | 0.03 | 0.03 | 0.70 | 0.84 |
| p-Diethylbenzene | 0.21 | 0.18 | 5.47 | 4.64 | 0.07 | 0.07 | 1.91 | 1.93 | 0.06 | 0.08 | 1.59 | 2.02 |
| TVOC | 55.50 | — | 587.12 | — | 37.81 | — | 327.36 | — | 24.91 | — | 227.45 | — |
| NOx | 33.8 | — | — | — | 27.2 | — | — | — | 20.8 | — | — | — |
| Ozone | 24.2 | — | — | — | 26.5 | — | — | — | 29.1 | — | — | — |
| 1 h ozone >100 ppb days | 103 | — | — | — | 253 | — | — | — | 334 | — | — | — |
| 1 h ozone >120 ppb days | 24 | — | — | — | 61 | — | — | — | 108 | — | — | — |
| 8 h ozone >80 ppb days | 106 | — | — | — | 186 | — | — | — | 293 | — | — | — |
When grouping the species into alkanes, alkenes, alkynes (with acetylene as the only compound) and aromatics, the percentage contributions of these groups were similar at the three sites, corresponding to 44–47%, 12–13%, 4–8%, and 34–39% of TVOC, respectively. At all three sites, propane, ethane and n-butane were the three most abundant alkanes. Among the alkenes, ethylene and propylene were the two most abundant compounds. Toluene and m,p-xylene had the highest levels among the aromatics, and toluene was the most abundant compound of all VOCs at all three sites. The concentrations of these two compounds fall in the ranges of VOC measurements reported in two previous studies, indicating that mobile sources remain a major source of VOC emissions in Taiwan.27,28
The C2–C4alkane levels at our urban site were similar to those reported for other Asian cities.17,29–31 This indicated that Asian urban areas have similar emission sources of alkanes.32 Despite VOC concentrations that differ between urban and rural areas, the general similarity of VOC compositions in percentage terms among the 55 VOCs suggested a monotonic nature of emission sources across all three areas. Transport of VOCs from urban areas to the leeward suburban and rural areas may be responsible for the observed decreasing concentrations with similar composition profiles across these three areas. The slight difference in VOC composition among the three monitoring sites seems to arise from minor contributions from local emissions and photochemical reactions during transportation. In addition, the year-to-year concentrations of VOC and NOx showed a decreasing gradient which could have implications on ozone control strategies.
3.2 Ozone formation potential
MIR was employed in the assessment of OFPs for various VOC compounds,33,34 as listed in Table 1. The total OFP of all VOCs combined for the urban site (587.12 µg(O3) g(VOC)–1) was more than twice higher than for the rural site (227.45 µg(O3) g(VOC)–1). The proportion of total OFP in alkane, alkene, alkyne, and aromatic groups was about 12–16%, 20–22%, 0.2–0.5%, and 62–67%, respectively, for the urban, suburban and rural sites. About 65% ozone formation potential is contributed to by the same 9 VOCs at all three sites, which are toluene, m,p-xylene, 1,2,4-trimethylbenzene, o-xylene, propylene, m-ethyltoluene, ethylene, 1,2,3-trimethylbenzene, and t-2-butene. Furthermore, toluene, xylenes, and trimethylbenzenes of the 16 aromatics, and ethylene and propylene of the 9 alkenes are the five VOC species with the greatest OFPs. Even though aromatics accounted for 34–39% of TVOC concentrations, they contributed as much as 62–67% to the total OFP at each site. Similar findings are also found in other urban areas in Germany and Spain.35,36 Such results imply that ozone precursor concentrations and OFPs both are equally important factors in determining ground-level ozone pollution.3.3 Seasonal variation of ozone and ozone precursors
Fig. 2 shows the average monthly concentrations of TVOC, NOx and ozone during the four year measurement period. The levels of TVOC and NOx reach a minimum in summer (from June to August) and a maximum in winter (from December to February). In winter, the average TVOC concentration was 64 ppb at the urban site, 42 ppb at the suburban site, and from 38 ppb at the rural site. In summer, it was 41 ppb at the urban site, 22 ppb at the suburban site, and 12 ppb at the rural site. The NOx concentrations in summer and in winter were 24 ppb and 43 ppb at the urban site, 15 and 39 ppb at the suburban site, and 10 and 30 ppb at the rural site. Ozone had a different seasonal pattern from TVOC and NOx. The maximum ozone levels occurred in autumn, and the second peak occurred in spring. The lowest ozone levels are found to be in summer at the urban site and in winter at the suburban and rural sites. The high average ozone concentration in autumn was 32 ppb at the urban site, 34 ppb at suburban site, and 38 ppb at the rural site.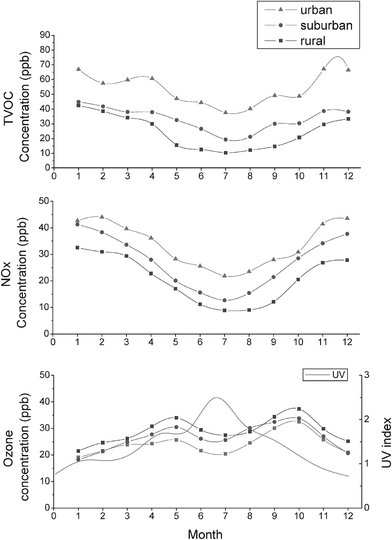 | ||
| Fig. 2 Gradients in the monthly average concentration of (a) TVOC, (b) NOx and (c) ozone at upwind urban, downwind suburban and downwind rural sites. | ||
Atmospheric dispersion and mixing height can explain the coherent annual cycles of TVOC, NOx, and ozone levels. The elevated mixing height and strong dispersion in summer keeps pollutants at lower levels. In contrast, the low mixing height and weak dispersion in winter are prone to accumulate pollutants to higher levels. On the other hand, the increased photochemical reaction via stronger solar radiation can explain the high ozone seasons of autumn and spring in these areas. It should be noted that strong solar radiation did not compensate for the efficient dispersion to lower ozone levels in summer. By examining month-to-month data of ozone, precursors and UV radiation simultaneously, we noticed that ozone peaked at the urban (32 ppb), suburban (34 ppb) and rural (37 ppb) sites in October when UVI was greater than 1.2 and TVOC : NOx ratios were 1.6, 1.1, and 1.0 : 1, respectively. By contrast, ozone concentrations were not elevated in winter (less than 25 ppb) when the TVOC : NOx ratios were comparatively higher than those in autumn but UVI was lower than 1.2.
The urban–rural difference in yearly averaged TVOC and NOx levels was consistent with the difference in traffic and population density between these two areas. There are two possible explanations for the fact that ozone levels increased with distance from the urban center to the rural area. The more abundant NOx in the urban center resulted in stronger suppression of ozone by NOx titration in the urban center than in the rural area. Furthermore, the ozone at the rural site was not only locally produced by photochemical reaction but also supplied by transport from the upwind urban area.37
3.4 Diurnal variation of ozone and ozone precursors
Fig. 3 shows the daily concentration variation of TVOC, NOx and ozone at the three sites. The diurnal patterns of both TVOC and NOx display double peaks occurring in the morning around 07:00 to 09:00 and in the evening after 18:00, which are presumably linked to rush hour traffic. The temperature inversion emerging near the surface at night also helped augmenting and prolonging the evening humps,38 until the traffic flow subsided at late night, causing the concentration to decrease accordingly. Similar to yearly concentration averages, diurnal TVOC and NOx levels also displayed a concentration gradient from the urban site to the rural site. This, again, is further evidence of traffic emissions dominating ozone precursors in this region.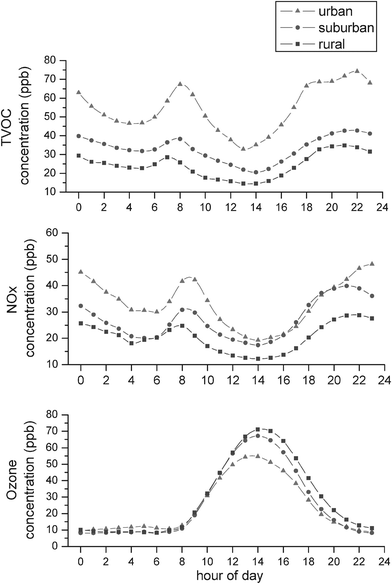 | ||
| Fig. 3 Daily concentration variation of (a) TVOC, (b) NOx and (c) ozone at upwind urban, downwind suburban and downwind rural sites. | ||
By contrast, ozone had one single peak in the midday, which was generally in agreement with the diurnal cycle of solar radiation. The ozone peak concentration occurred at 13:00 at the upwind urban site, but was delayed by at least one hour between 14:00 to 15:00 at the downwind suburban and rural sites. The time lag in the ozone peaks provides further evidence of regional transportation of air mass carrying ozone and precursors from urban to rural mountainous areas.
3.5 Photochemical reaction of regionally transported air mass
Ozone is a secondary pollutant, and its formation depends on the complexities of photochemistry and meteorology. The photochemical process can be revealed by examining the ratios of a compound pair with a common origin but a considerable difference in reactivity.39 For instance, by taking the ratio of a less reactive species to a more reactive species, the ratio tends to be constant in the source area, but gradually increases as an air parcel is subjected to photochemical processes while it is transported to the leeward areas. This is because the more reactive species tends to react more rapidly than its pairing species, which gradually leads to an increase in the ratio along the path of transport. Continuous measurement with adequate time resolution at a receptor site is particularly suited to studying the effects of photochemistry as the aging process can be dynamically observed on any given day. Because the atmospheric lifetime is about 3 days for ethylbenzene and about 0.96 days for m,p-xylene,40 the ethylbenzene to m,p-xylene ratio (E : X ratio) can be used to indicate the age of an air parcel along the transport path. Because iso-butane and n-butane have similar atmospheric lifetimes of 2.9 days and 2.8 days, respectively, and are mainly from the same mobile emission sources,41 the iso-butane : n-butane ratio can be used to verify whether VOCs are continuously emitted from anthropogenic sources.42As shown in Fig. 4, the hourly E : X ratio remains around 0.4 at all three sites during 18:00–07:00 when there is no photochemical reaction in a daily cycle. The hourly E : X ratio starts increasing after 07:00, peaking at midday and finally decreasing back to 0.4 at 18:00 for urban and suburban sites. Their peak E : X value is 0.6 at the suburban site and 0.8 at the rural site. By contrast, the peak E : X value remains around 0.4 at the rural site. Because the peak E : X values differ by a factor of 1.5 to 2 between upwind and downwind areas, the aged air parcels at the downwind suburban and rural sites are likely to be transported from the upwind urban site. The difference in the peak size of the indicator among the three sites could substantiate the difference in ozone levels observed at those sites. The consistency in time and average amplitude between ozone and the age indicator provides strong evidence to tie ozone production to the photochemistry of VOCs. Ozone is produced along the path of transport and increases by the time the air masses arrive at the downwind sites in the afternoon. The curves of iso-butane : n-butane ratios in Fig. 4 show that their ratios are close to 0.6 : 1 across all three sites. The relatively constant ratios of iso-butane : n-butane as opposed to significant diurnal variation of the E : X ratios imply that photochemically reactive VOCs contribute significantly to ozone formation in the air mass regionally transported from urban to rural areas.
 | ||
| Fig. 4 Ratios of ethylbenzene : m,p-xylene (E : X) and iso-butene : n-butane at upwind urban, downwind suburban and downwind rural sites. | ||
3.6 Relationship between ozone precursors and meteorological conditions
The meteorological conditions are mostly the same at the three sites with slight differences in wind speed. The wind speed (WS) at the urban site (1.8 to 2.2 m s–1) is higher than at the suburban site (1.4 to 1.6 m s–1) and the rural site (1.0 to 1.3 m s–1). The temperatures at the three sites all display a similar temperature range of 17.3 to 28.9 °C at the urban site, 18.1 to 28.7 °C at the suburban site and 18.1 to 28.1 °C at the rural site. The urban site has the lowest RH (67% to 70%), while the rural site has the highest RH (73% to 76%). However, the year-to-year temperature and relative humidity did not vary at each site from 2003 to 2006.The relationship between ozone precursors, ozone and those meteorological factors can be used to explore possible ozone formation mechanisms. It is reported that a negative correlation between ozone, TVOC, NOx and RH indicates that TVOC and NOx reactions can enhance the formation of ozone under low humidity conditions.43 In addition, the positive correlation between ozone and temperature indicates that high temperature conditions favor ozone formation. In general, low humidity and high temperature are important meteorological factors for ozone formation.
We noticed that there is an increase in correlation between various gas species shown in Table 2 on moving further away from the urban center. For instance, correlation of TVOC with NOx is noticeably higher at the two remote sites (R2 > 0.8) than at the urban site (R2 < 0.8). Furthermore, although alkanes and aromatics have different lifetimes, e.g., from a few hours to several days,44 the correlation between alkanes to aromatics increases from 0.66 at the urban site, to 0.70 at the suburban site, to 0.87 at the rural site. It should be noted that these different types of gas species are usually multi-sourced, resulting in poorer correlation in concentration if monitored in the source area.45 Air parcels subject to more vigorous mixing and less source influence as moving downwind could explain the improved correlation, which again is in support of the argument that transport is responsible for the elevated ozone levels in rural environments.
| (a) Upwind urban site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TVOC | Alkanes | Alkenes | Aromatics | Ozone | NOx | WS | Temp | RH | |
| TVOC | 1.000 | — | — | — | — | — | — | — | — |
| Alkanes | 0.975 | 1.000 | — | — | — | — | — | — | — |
| Alkenes | 0.847 | 0.816 | 1.000 | — | — | — | — | — | — |
| Aromatics | 0.804 | 0.660 | 0.622 | 1.000 | — | — | — | — | — |
| Ozone | –0.173 | –0.113 | –0.195 | –0.261 | 1.000 | — | — | — | — |
| NOx | 0.798 | 0.715 | 0.622 | 0.833 | –0.144 | 1.000 | — | — | — |
| WS | 0.299 | 0.303 | 0.144 | 0.198 | –0.223 | 0.276 | 1.000 | — | — |
| Temp | –0.680 | –0.638 | –0.453 | –0.643 | 0.265 | –0.856 | –0.523 | 1.000 | — |
| RH | –0.240 | –0.274 | –0.306 | –0.044 | –0.393 | –0.158 | –0.410 | 0.074 | 1.000 |
| (b) Downwind suburban site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TVOC | Alkanes | Alkenes | Aromatics | Ozone | NOx | WS | Temp | RH | |
| TVOC | 1.000 | — | — | — | — | — | — | — | — |
| Alkanes | 0.966 | 1.000 | — | — | — | — | — | — | — |
| Alkenes | 0.851 | 0.775 | 1.000 | — | — | — | — | — | — |
| Aromatics | 0.855 | 0.700 | 0.763 | 1.00 | — | — | — | — | — |
| Ozone | –0.362 | –0.356 | –0.459 | –0.230 | 1.000 | — | — | — | — |
| NOx | 0.871 | 0.866 | 0.753 | 0.662 | –0.490 | 1.000 | — | — | — |
| WS | –0.481 | –0.382 | –0.547 | –0.627 | 0.062 | –0.272 | 1.000 | — | — |
| Temp | –0.787 | –0.749 | –0.704 | –0.648 | 0.628 | –0.897 | 0.262 | 1.000 | — |
| RH | –0.164 | –0.178 | –0.336 | –0.090 | –0.028 | –0.115 | 0.453 | 0.025 | 1.000 |
| (c) Downwind rural site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TVOC | Alkanes | Alkenes | Aromatics | Ozone | NOx | WS | Temp | RH | |
| TVOC | 1.000 | — | — | — | — | — | — | — | — |
| Alkanes | 0.984 | 1.000 | — | — | — | — | — | — | — |
| Alkenes | 0.919 | 0.936 | 1.000 | — | — | — | — | — | — |
| Aromatics | 0.944 | 0.872 | 0.784 | 1.000 | — | — | — | — | — |
| Ozone | –0.415 | –0.378 | –0.448 | –0.421 | 1.000 | — | — | — | — |
| NOx | 0.913 | 0.892 | 0.794 | 0.887 | –0.336 | 1.000 | — | — | — |
| WS | 0.004 | –0.041 | –0.125 | 0.086 | –0.093 | –0.024 | 1.000 | — | — |
| Temp | –0.891 | –0.895 | –0.816 | –0.816 | 0.471 | –0.895 | –0.084 | 1.000 | — |
| RH | –0.401 | –0.384 | –0.384 | –0.41 | –0.175 | –0.437 | 0.316 | 0.313 | 1.000 |
3.7 Comparisons of ozone episode and non-episode days
We defined the ozone episode days as the days with the maximum 1 hour ozone concentrations exceeding 100 ppb. The number of episode days was 103 (7%) at the urban site, 253 (18%) at the suburban site, and 334 (23%) at the rural site over the 4 year observation in central Taiwan. These episode days occurred mostly in autumn.Fig. 5 shows the daily variation of ozone, TVOC, NOx, and the meteorological factors, including wind speed and solar radiation on the ozone episode and non-episode days by monitoring site. The mean wind speed during episode days was lower than that of non-episode days for both urban and suburban sites, which were 1.4 m s–1vs. 2.0 m s–1 and 1.3 m s–1vs. 1.5 m s–1, respectively. By contrast, mean wind speed was not different between episode and non-episode days at the rural site, which was 1.1 m s–1vs. 1.2 m s–1.
 | ||
| Fig. 5 Daily variation of TVOC, ozone, NOx and wind speed during episode days and non-episode days at upwind urban, downwind suburban and downwind rural sites. | ||
Both TVOC and NOx concentrations on ozone episode days are higher than those on non-episode days at urban and suburban sites. At the urban site, there are about 23–28 ppb increments of TVOC and 12–15 ppb increments of NOx during the morning rush hours (06:00–09:00) on episode days. At the suburban site, the increments are 1–11 ppb of TVOC and 1–2 ppb of NOx. There is little difference in TVOC (1–2 ppb) and NOx levels (2–3 ppb) between episode and non-episode days at the rural site. At all three sites, the UVI on episode days (6.5) is about 0.8 higher than that on non-episode days (5.7). The wind speed on episode days (1.4 m s–1) is about 0.6 m s–1 lower than on non-episode days (2.0 m s–1). Our findings of low wind speed and high solar radiation on episode days in central Taiwan agree with a previous study in Hong Kong.19
4. Conclusions
The PAMS network in central Taiwan identifies a common source of ozone precursors, VOCs and NOx, mainly from traffic emissions. Among the 55 VOCs, aromatics exerted the major OFPs. A decreasing concentration gradient in VOC and NOx across the urban, suburban and rural site but a reverse gradient in ozone levels was observed. The diurnal pattern shows that ozone peaks one hour later at the rural site (14:00) than the urban site (13:00). The gradient of midday ethylbenzene : m,p-xylene ratios over the three sites indicates there is an upwind-to-downwind transport of air mass carrying ozone and precursors from the urban area to its suburban and rural areas. The repetitive diurnal and seasonal concentration variations based on the 4 year continuous hourly measurements of 55 VOCs from 2003 to 2006 can be used to assess the progress of emission controls in the future. Our analysis concludes that the transport of ozone precursors is an important meteorological factor responsible for ozone formation in general in this area. Two additional meteorological conditions, high solar radiation (UVI = 6.5) and low wind speed(1.4 m s–1), are needed to cause ozone episodes, which occur mostly in autumn in central Taiwan.Acknowledgements
The authors would like to express their greatest gratitude to the Department of Air quality control at EPA of Taiwan for their constant assistance and financial support. This research is under the continuous project with contract number: EPA-94-FA11-03-A225, EPA-95-FA11-03-D088 and EPA 96-FA11-03-D060.References
- R. Vingarzan, A Review of Surface Ozone Background Levels and Trends, Atmos. Environ., 2004, 38, 3431–3442 CrossRef CAS.
- R. T. Burnett, M. Smith-Doiron, D. Stieb, M. E. Raizenne, J. R. Brook, R. E. Dales, J. A. Leech, S. Cakmak and D. Krewski, Association between Ozone and Hospitalization for Acute Respiratory Diseases in Children Less Than 2 Years of Age, Am. J. Epidemiol., 2001, 153, 444–452 CrossRef CAS.
- P. C. Chen, Y. M. Lai, C. C. Chan, J. S. Huang, C. Y. Yang and J. D. Wang, Short-Term Effect of Ozone on the Pulmonary Function of Children in Primary School, Environ. Health Perspect., 1999, 107, 921–925 CrossRef CAS.
- P. Höppe, A. Peters, G. Rabe, G. Praml, J. Lindner, G. Jakobi, G. Fruhmann and D. Nowak, Environmental Ozone Effects in Different Population Subgroups, Int. J. Hyg. Environ. Health, 2003, 206, 505–516 Search PubMed.
- B. B. Jalaludin, T. Chey, B. I. O'Toole, W. T. Smith, A. G. Capon and S. R. Leeder, Acute Effects of Low Levels of Ambient Ozone on Peak Expiratory Flow Rate in a Cohort of Australian Children, Int. J. Epidemiol., 2000, 29, 549–557 Search PubMed.
- C. C. Chan and T. H. Wu, Effects of Ambient Ozone Exposure on Mail Carriers’ Peak Expiratory Flow Rates, Environ. Health Perspect., 2005, 113, 735–738 CAS.
- M. Riediker, C. Monn, T. Koller, W. A. Stahel and B. Wuthrich, Air Pollutants Enhance Rhinoconjunctivitis Symptoms in Pollen-Allergic Individuals, Ann. Allergy, Asthma, Immunol., 2001, 87, 311–8 Search PubMed.
- J. H. Seinfeld, Urban Air-Pollution—State of the Science, Science, 1989, 243, 745–752 CAS.
- W. L. Chameides, F. Fehsenfeld, M. O. Rodgers, C. Cardelino, J. Martinez, D. Parrish, W. Lonneman, D. R. Lawson, R. A. Rasmussen, P. Zimmerman, J. Greenberg, P. Middleton and T. Wang, Ozone Precursor Relationships in the Ambient Atmosphere, J. Geophys. Res., [Atmos.], 1992, 97(D5), 6037–6055 CAS.
- DMU, Assessment of the Effectiveness of European Air Quality Policies and Measures, Final Report, B4-3040/2003/365967/MAR/C1, European Commission's Directorate-General Environment, 2004.
- EEA, Air Pollution by Ozone in Europe in Summer 2006, European Environment Agency and Office for Official Publications of the European Communities, 2007.
- FCAP, National Ozone Trends, retrieved on 2007/9/19, available from: http://www.cleanairprogress.org/national_ozone/index.asp.
- US EPA, Pams Implementation Manual, EPA-454/B-93-051, 1994.
- US EPA, Pams Networks and Sites, retrieved on 2007/9/19, available from: http://www.epa.gov/air/oaqps/pams/network.html.
- EMEP, Background Document with Justification and Specification of the EMEP Monitoring Programme 2004–2009, Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutants in Europe (EMEP), United Nations Economic Commission for Europe, 2003.
- ESPIS, Gulf of Mexico Air Quality Study, Final Report, The Environmental Studies Program Information System (ESPIS), 1995, available from: http://www.gomr.mms.gov/homepg/espis/espismaster.asp?appid=1.
- H. Guo, T. Wang, D. R. Blake, I. J. Simpson, Y. H. Kwok and Y. S. Li, Regional and Local Contributions to Ambient Non-Methane Volatile Organic Compounds at a Polluted Rural/Coastal Site in Pearl River Delta, China, Atmos. Environ., 2006, 40, 2345–2359 CrossRef CAS.
- K. S. Chen, Y. T. Ho, C. H. Lai, Y. A. Tsai and S. J. Chen, Trends in Concentration of Ground-Level Ozone and Meteorological Conditions During High Ozone Episodes in the Kao-Ping Airshed, Taiwan, J. Air Waste Manage. Assoc., 2004, 54, 36–48 CAS.
- Y. C. Lee, G. Calori, P. Hilis and G. R. Carmichael, Ozone Episodes in Urban Hong Kong 1994–1999, Atmos. Environ., 2002, 36, 1957–1968 CrossRef CAS.
- I. L. Lai, S. C. Chang, P. H. Lin, C. H. Chou and J. T. Wu, Climatic Characteristics of the Subtropical Mountainous Cloud Forest at the Yuanyang Lake Long-Term Ecological Research Site, Taiwan, Taiwania, 2006, 51, 317–329 Search PubMed.
- C. K. Chou, S. C. Liu, C. Y. Lin, C. J. Shiu and K. H. Chang, The Trend of Surface Ozone in Taipei, Taiwan, and Its Causes: Implications for Ozone Control Strategies, Atmos. Environ., 2006, 40, 3898–3908 CrossRef CAS.
- TEPA, Taiwan Environmental Protection Administration, available from: http://www.epa.gov.tw/b/b0100.asp?Ct_Code=05X0004801X0002205&L=2 (last accessed 2006/2/5).
- DOS, Department of Statistics, Ministry of the Interior, Taiwan, available from: http://www.ris.gov.tw/ch4/static/st10-6.xls (last accessed 2006/2/5).
- TEPA, Taiwan Environmental Protection Administration, Annual VOC Emission Report (in Chinese), 2006.
- J. L. Wang, G. Z. Din and C. C. Chan, Validation of a Laboratory-Constructed Automated Gas Chromatograph for the Measurement of Ozone Precursors through Comparison with a Commercial Analogy, J. Chromatogr., A, 2004, 1027, 11–18 CrossRef CAS.
- TEPA, Taiwan Environmental Protection Administration, 2006, available from: http://www.epa.gov.tw.
- C. C. Chang, T. Y. Chen, C. Y. Lin, C. S. Yuan and S. C. Liu, Effects of Reactive Hydrocarbons on Ozone Formation in Southern Taiwan, Atmos. Environ., 2005, 39, 2867–2878 CrossRef CAS.
- C. H. Lai, K. S. Chen, Y. T. Ho and M. S. Chou, Characteristics of C2–C15 Hydrocarbons in the Air of Urban Kaohsiung, Taiwan, Atmos. Environ., 2004, 38, 1997–2011 CrossRef CAS.
- T. Wang, C. H. Wong, T. F. Cheung, D. R. Blake, R. Arimoto, K. Baumann, J. Tang, G. A. Ding, X. M. Yu, Y. S. Li, D. G. Streets and I. J. Simpson, Relationships of Trace Gases and Aerosols and the Emission Characteristics at Lin’an, a Rural Site in Eastern China, During Spring 2001, J. Geophys. Res., [Atmos.], 2004, 109 DOI:10.1029/2003JD004119.
- A. Monod, B. C. Sive, P. Avino, T. Chen, D. R. Blake and F. S. Rowland, Monoaromatic Compounds in Ambient Air of Various Cities: A Focus on Correlations between the Xylenes and Ethylbenzene, Atmos. Environ., 2001, 35, 135–149 CrossRef CAS.
- K. Na, Y. P. Kim, K. C. Moon, I. Moon and K. Fung, Concentrations of Volatile Organic Compounds in an Industrial Area of Korea, Atmos. Environ., 2001, 35, 2747–2756 CrossRef CAS.
- A. L. Swanson, N. J. Blake, E. Atlas, F. Flocke, D. R. Blake and F. S. Rowland, Seasonal Variations of C2–C4 Nonmethane Hydrocarbons and C1–C4 Alkyl Nitrates at the Summit Research Station in Greenland, J. Geophys. Res., [Atmos.], 2003, 108, 4065 CrossRef.
- A. G. Russell, J. B. Milford, M. S. Bergin, S. McBride, L. McNair, Y. Yang, W. R. Stockwell and B. E. Croes, Urban Ozone Control and Atmospheric Reactivity of Organic Gases, Science, 1995, 269, 491–495 CrossRef CAS.
- W. P. L. Carter, Development of Ozone Reactivity Scales for Volatile Organic Compounds, J. Air Waste Manage. Assoc., 1994, 44, 881–899 CAS.
- N. Durana, M. Navazo, M. C. Gomez, L. Alonso, J. A. Garcia, J. L. Ilardia, G. Gangoiti and J. Iza, Long Term Hourly Measurement of 62 Non-Methane Hydrocarbons in an Urban Area: Main Results and Contribution of Non-Traffic Sources, Atmos. Environ., 2006, 40, 2860–2872 CrossRef CAS.
- B. Rappenglück and P. Fabian, Nonmethane Hydrocarbons (Nmhc) in the Greater Munich Area/Germany, Atmos. Environ., 1999, 33, 3843–3857 CrossRef CAS.
- B. Rappenglück, P. Oyola, I. Olaeta and P. Fabian, The Evolution of Photochemical Smog in the Metropolitan Area of Santiago De Chile, J. Appl. Meteorol., 2000, 39, 275–290 Search PubMed.
- K. L. Yang, C. C. Ting, J. L. Wang, O. W. Wingenter and C. C. Chan, Diurnal and Seasonal Cycles of Ozone Precursors Observed from Continuous Measurement at an Urban Site in Taiwan, Atmos. Environ., 2005, 39, 3221–3230 CrossRef CAS.
- R. Atkinson, Gas-Phase Tropospheric Chemistry of Organic Compounds, J. Phys. Chem. Ref. Data, 1994, Monograph 2, pp. 1–216 Search PubMed.
- R. G. Prinn, R. F. Weiss, B. R. Miller, J. Huang, F. N. Alyea, D. M. Cunnold, P. J. Fraser, D. E. DHartley and P. G. Simmonds, Atmospheric Trends and Lifetime of CH3CCl3 and Global OH Concentrations, Science, 1995, 269, 187–192 CrossRef CAS.
- S. R. Arnold, J. Methven, M. J. Evans, M. P. Chipperfield, A. C. Lewis, J. R. Hopkins, J. B. McQuaid, N. Watson, J. D. Lee, R. M. Purvis, E. Atlas, D. R. Blake and B. Rappenglueck, Statistical Inference of OH Concentrations and Air Mass Dilution Rates from Successive Observations of Nonmethane Hydrocarbons in Single Air Masses, J. Geophys. Res., [Atmos.], 2007, 112 DOI:10.1029/2006JD007594.
- S. Kato, Y. Kajii, R. Itokazu, J. Hirokawa, S. Koda and Y. Kinjo, Transport of Atmospheric Carbon Monoxide, Ozone, and Hydrocarbons from Chinese Coast to Okinawa Island in the Western Pacific During Winter, Atmos. Environ., 2004, 38, 2975–2981 CrossRef CAS.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics from Air Pollution to Climate Change, Wiley, New York, 1998 Search PubMed.
- D. D. Parrish, A. Stohl, C. Forster, E. L. Atlas, D. R. Blake, P. D. Goldan, W. C. Kuster and J. A. de Gouw, Effects of Mixing on Evolution of Hydrocarbon Ratios in the Troposphere, J. Geophys. Res., [Atmos.], 2007, 112 DOI:10.1029/2006JD007583.
- H. L. A. Wong, T. Wang, A. Ding, D. R. Blake and J. C. Nam, Impact of Asian Continental Outflow on the Concentrations of O3, Co, Nmhcs and Halocarbons on Jeju Island, South Korea During March 2005, Atmos. Environ., 2007, 41, 2933–2944 CrossRef CAS.
- Google Maps, retrieved in 2007/9/19, available from http://maps.google.com/maps.
| This journal is © The Royal Society of Chemistry 2008 |
