NixCuy/Al2O3 based catalysts for hydrogen production
Loredana
De Rogatis
,
Tiziano
Montini
,
Barbara
Lorenzut
and
Paolo
Fornasiero
*
Department of Chemical Sciences, Center of Excellence for Nanostructured Materials, INSTM-Trieste Research Unit, CNR-ICCOM Trieste Research Unit, University of Trieste, via L. Giorgieri 1, 34127, Trieste, Italy. E-mail: pfornasiero@units.it; Fax: +39 040 5583903; Tel: +39 040 5583973
First published on 20th June 2008
Abstract
Ni(x wt.%)Cu(y wt.%)/Al2O3 samples were investigated as active and thermally stable catalysts for methanol and ethanol steam reforming. XRD data clearly evidenced the formation of a NiCu alloy under the adopted preparation procedure. The bimetallic systems exhibited improved activity in the methanol steam reforming with respect to the monometallic ones. The introduction of copper in the catalyst formulation showed a positive effect inhibiting the formation of methane, an undesirable by-product. On the other hand, in the ethanol steam reforming, the catalytic performance was less promising. Furthermore, the Ni : Cu ratio did not seem to affect the product distribution. However, enhanced stability was observed in the two subsequent run-up experiments, indicating the positive role played by the bimetallic systems.
A Introduction
The finding of new energy sources is an imperative challenge today. The crucial energy problem is mainly related to the decrease in fossil fuel reserves due to world population growth, technological developments and increasing energy demands. The damaging environmental effect of continuous fossil fuel usage is another factor which has to be considered. There are many different kinds of alternative fuels which have been proposed, like methanol, dimethylether, ethanol, biodiesel, synfuel and hydrogen.1 Among these options, hydrogen is expected to play an important role in future energy scenarios, both in mobile and stationary applications.2Methanol as a hydrogen carrier has received great attention due to its high hydrogen-to-carbon ratio and its molecular simplicity resulting in relatively low reforming temperatures (250–350 °C).3 The most feasible process for on-board production of hydrogen from methanol for fuel cells applications is the steam reforming.4 Unlike methanol, ethanol is completely renewable. It can be produced in large amounts from biomass such as agricultural wastes and forestry residues. Nevertheless, the ethanol steam reforming requires higher operative temperatures (T > 500 °C) due to the presence of the C–C bond.5 This actually makes the effective integration of the ethanol reformer with fuel cells more difficult.
Ni-based catalysts represent the systems which are currently more widely used in steam reforming processes both at laboratory and industrial scales.6 Unfortunately, the main problem with Ni is that the rate of C–C bond formation is relatively high, leading to the rapid growth of carbon deposits which poison the catalysts.7 To overcome this problem, industrial plants add excess quantities of steam to the feedstock. Notably, a high steam concentration is not desirable, since additional energy is required to heat and vaporize water with, consequently, higher economic costs. Therefore, several attempts have been made to develop Ni-based catalysts with higher carbon tolerance and higher stability against metal sintering.8 In this respect, it was reported that the introduction of copper in catalyst formulation suppresses carbon formation, as well as the sintering of the active phase, in methane steam reforming and in hydrocarbon decomposition reactions.9
In this article we report an explorative study of the catalytic activity of a series of NixCuy/Al2O3catalysts prepared by wet-impregnation method. The role and the effect of Ni : Cu ratio on the chemical–physical properties and the catalytic performance were examined. The catalysts were tested towards both methanol and ethanol steam reforming (MeOH-SR and EtOH-SR, respectively) and they were characterized by means of surface area measurements, X-ray diffraction (XRD), CO chemisorption and temperature programmed reduction (TPR).
B Experimental
B.1 Catalyst preparation
Ni/Cu were supported on a commercial Al2O3 (Sasol HP 14-150 pre-calcined at 900 °C for 24 h with surface area of 97 m2 g−1) by the impregnation method using Ni(NO3)2·6H2O (puriss. Fluka) and Cu(NO3)2·3H2O (puriss. Fluka) as metal precursors.Briefly, appropriate amounts of Ni(NO3)2 or/and Cu(NO3)2 were dissolved in ethanol. Aluminium oxide was added to the metals solution under continuous stirring. The obtained slurry was dried under vacuum until nearly all the alcohol was evaporated and the solid residue was further dried overnight at 120 °C in air. The material was calcined in a static oven at 600 °C in air for 5 h (heating/cooling rate of 3 °C min−1). The resulting powders were pelletized, crushed and sieved to collect the fraction smaller than 250 μm. All the catalysts have a total nominal metal loading of 10 wt.%.
The final materials obtained are hereafter designated as NixCuy-Al, where x and y represent the nickel and copper loading (wt.%), respectively.
B.2 Textural and structural characterization
The BET surface area, pore volume and average pore diameter of the catalysts were measured by N2 adsorption at liquid nitrogen temperature using a Micromeritics ASAP 2020. Approximately 100 mg of catalyst, previously degassed overnight at 350 °C, were used for each analysis.CO chemisorption experiments were performed at 25 °C on a Micromeritics ASAP 2020 after cleaning pretreatment at 500 °C for 1 h under O2 (5%) in an Ar flow followed by reduction at 750 °C in H2(5%)/Ar for 2 h and evacuation at 400 °C for 4 h. Typically, 0.5 g of samples were used and an equilibration time of 10 min was employed. Adsorbed volumes were determined by extrapolation to zero pressure of the linear part of the adsorption isotherm (100–400 Torr) after elimination of the so-called reversible adsorption. A chemisorption stoichiometry CO : M = 1 : 1 and a spherical geometry were assumed.
Powder X-ray diffraction (XRD) patterns of the samples after calcination and reduction in H2(5%)/Ar at 750 °C for 2 h were recorded with a computer-controlled Philips X'Pert diffractometer using Cu Kα radiation (λ = 0.154 nm). The data were collected at 0.02° in the (2θ) range from 10° to 100°.
B.3 Temperature programmed reduction (TPR)
Temperature programmed reduction (TPR) experiments were performed on ∼30 mg of the calcined materials. The samples were pre-treated at 500 °C for 1 h by pulsing of O2 in an Ar flow every 75 s, then cooled to room temperature (RT). The O2 pulses were stopped when the sample temperature was lower than 150 °C. H2(5%)/Ar was admitted into the reactor at RT and the flow allowed to stabilise for 30 min before increasing the temperature to 900 °C at 10 °C min−1. After TPR, the samples were outgassed under Ar flow at 900 °C for 15 min and cooled to 427 °C, at which temperature oxidation was carried out with pulses of diluted O2 for 1 h. The TPR/oxidation procedure was repeated another 2 times. The re-oxidation step of the second cycle was conducted at 600 °C while the third cycle was performed at 900 °C and prolonged for 5 h in order to evaluate the effect of more severe oxidizing conditions. H2 uptake was monitored using a thermal conductivity detector (TCD).B.4 Catalytic activity measurements
All catalytic tests were conducted at atmospheric pressure in a conventional U-shaped quartz microreactor with internal diameter of 4 mm. The temperature of the catalyst was measured with a K-type thermocouple. Before testing the catalytic activity, the calcined materials were pre-treated under O2(5%)/Ar at 500 °C for 1 h (40 mL min−1, 10 °C min−1) and activated by reduction in H2(5%)/Ar at 750 °C for 2 h (40 mL min−1, 10 °C min−1).Approximately 120 mg of catalyst were typically used in the alcohol steam reforming experiments. EtOH/H2O 1 : 5 and MeOH/H2O 1 : 4 mixtures were injected into an Ar flow with a Hamilton Gastight syringe using a INSTECH Model 2000 syringe pump. All the transfer lines between syringe, reactor and GC were heated at 120 °C. Gas flow rates were ∼31 mL min−1 to ensure GHSV values of ∼16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. On-line GC analysis was performed using a Hewlett Packard 5890 Series II gas chromatograph. A Molsieve 5A column, with Ar as carrier, was connected to a TCD to analyse H2, O2, N2, CH4 and CO. A Select Permanent Gases/CO2PLOT column (parallel PoraPLOT 50 m × 0.53 mm ID and Molsieve 5A 10 m × 0.32 mm ID columns) with He as carrier and connected in series to a methanator and to a flame ionization detector (FID) was used to analyze the carbon-containing compounds.
000 mL g−1 h−1. On-line GC analysis was performed using a Hewlett Packard 5890 Series II gas chromatograph. A Molsieve 5A column, with Ar as carrier, was connected to a TCD to analyse H2, O2, N2, CH4 and CO. A Select Permanent Gases/CO2PLOT column (parallel PoraPLOT 50 m × 0.53 mm ID and Molsieve 5A 10 m × 0.32 mm ID columns) with He as carrier and connected in series to a methanator and to a flame ionization detector (FID) was used to analyze the carbon-containing compounds.
The gaseous mixture was first introduced in the reactor at 150 °C, before ramping the furnace temperature to 700 °C at 0.7 °C min−1.
H2 yield was calculated on the basis of the stoichiometry of the following reactions (eqn (1) for MeOH-SR; eqn (2) for EtOH-SR), which include the water gas shift (WGS):
CH3OH + H2O = CO2 + 3H2
 | (1) |
CH3CH2OH + 3H2O = 2CO2 + 6H2
 | (2) |
C Results and discussion
C.1 Sample characterization
Table 1 summarizes the main textural properties of the calcined materials.| Sample | S BET a/m2 g−1 | d M b/nm | Cumulative pore volume/mL g−1 |
|---|---|---|---|
| a BET surface area. b Pore diameter: maximum of the pore distribution obtained from the desorption branch of the adsorption isotherm. c Reference alumina calcined at 900 °C for 24 h. | |||
| Al2O3c | 97 | 47 | 0.93 |
| Ni10-Al | 84 | 53 | 0.73 |
| Ni7Cu3-Al | 78 | 58 | 0.77 |
| Ni5Cu5-Al | 82 | 55 | 0.71 |
| Ni3Cu7-Al | 78 | 58 | 0.78 |
| Cu10-Al | 80 | 56 | 0.77 |
All samples present Type IV isotherms with hysteresis loops, typical of mesoporous materials.10 The t-plot analysis indicates that the microporous volume is always negligible, while the BJH analysis reveals that the materials have a pore distribution centred approximately at 55 nm. All the supported metal catalysts have a specific surface area of ∼80 m2 g−1. The lower surface area and pore volume of the impregnated samples with respect to bare Al2O3 is consistent with the relatively high metal loading (10 wt.%). Furthermore, Ni/Cu particles might block pores of Al2O3 during metal deposition.
XRD patterns of the samples after calcination and after standard activation in H2 (750 °C for 2 h) are presented in Fig. 1. Overlapping of the XRD peaks of the transitional aluminas complicates phase attribution, as does the low crystallinity of the support. However, the presence of γ-Al2O3 and θ-Al2O3 is evident, consistent with the low calcination temperature.11 Notably, α-Al2O3 is absent, in accordance with the relative high surface area of the samples.11
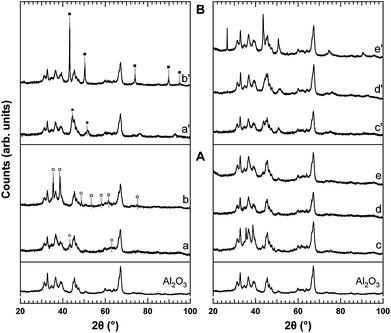 | ||
| Fig. 1 XRD powder diffraction profiles of Ni10-Al (a, a′), Cu10-Al (b, b′), Ni3Cu7-Al (c, c′), Ni5Cu5-Al (d, d′) and Ni7Cu3-Al (e, e′), after calcination at 600 °C for 5 h (A) and reduction at 750 °C for 2 h (B). The XRD profile of the starting Al2O3 is reported for comparison. (○) NiO, (□) CuO, (●) Ni, (■) Cu. | ||
The XRD patterns of the calcined Ni10-Al and Cu10-Al samples (Fig. 1 A, a and b) show the characteristic peaks of NiO (JCPDS 14-0481) and CuO (JCPDS 80-1268), respectively, in addition to those of the support. Average crystallite diameters of 8 nm for NiO and 26 nm for CuO were calculated by means of the Scherrer's equation applied to the main reflections of the two oxides. Other phases related to Ni and Cu were not identified.
In the XRD pattern of the calcined Ni3Cu7-Al sample (Fig. 1 A, c), it is possible to recognize a monoclinic phase and a cubic one (minor extent). The position of the diffraction peaks related to these phases indicates that they do not correspond to CuO (monocline structure) and NiO (cubic structure). Since the ionic radius of Ni2+ is bigger than that of Cu2+, a partial substitution between the two cations is possible. The cubic phase will be rich in Ni2+, while the monocline phase will be rich in Cu2+, according to the different ability of the two ions to enter in the two crystallographic structures. The crystallites have an average diameter of 14 nm for the cubic phase and 28 nm for the monoclinic one. On the other hand, in the case of the Ni5Cu5-Al (Fig. 1 A, d) and Ni7Cu3-Al (Fig. 1 A, e) samples, the XRD patterns indicate the formation of a solid solution of NixCu1−xO with a cubic structure (as for NiO). In fact, the addition of Cu leads to a shift towards higher angles of all the NiO diffraction peaks. An average crystallite diameter of 7 nm was obtained for both samples.
After activation in H2 at 750 °C, in the XRD patterns of all samples (Fig. 1 B) it is possible to identify only the peaks related to γ and θ aluminas and those of the metallic phases. No other Ni or Cu species were detected, although the presence of other phases (such as oxides or aluminates) with a high dispersion or below the detection limit can not be excluded.
Specifically, the XRD patterns of the reduced Ni10-Al and Cu10-Al (Fig. 1 B, a′ and b′) exhibit features due to the presence of the metallic phase with cubic structure (JCPDS 04-0850 and JCPDS 04-0836, respectively). The reflection width indicates average crystallites diameter of 10 nm for Ni and 44 nm for Cu.
In the case of the bimetallic samples, a progressive shift of the metallic phase peaks is observed (Fig. 2A). In fact, the XRD pattern shows diffraction peaks in an intermediate position between that attributable to metallic Ni and to metallic Cu (Fig. 2 A), indicating the formation of a Ni–Cu alloy. This fact was previously reported also by Lee et al.12 The composition (e.g. Cu-rich or Ni-rich alloy) and the number of alloys, which can be generated, depend on the Ni : Cu ratio.
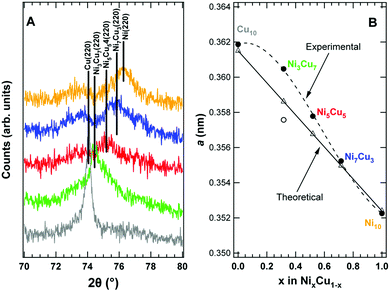 | ||
| Fig. 2 (A) Detail of the range 2θ = 70–80° of the XRD patterns of the reduced samples, showing the shift of the (220) reflection with the composition; (B) trend of the experimental cell parameter a with the composition of the metal phase, in comparison with the theoretical value. | ||
Average crystallite diameters of 9–10 nm for Ni7Cu3-Al and Ni5Cu5-Al, and of 31 nm for Ni3Cu7-Al were obtained.
Fig. 2 B presents the comparison between the experimental cell parameter a of the metallic phases (obtained from the position of the (220) reflection), the tabulated values for Ni and Cu, and the theoretical value calculated using Vegard's law for intermediate compositions.
For Cu and Ni, there is a good agreement between the two values. This is also observed in the case of the reduced Ni7Cu3-Al sample, where the metallic phase consists of an alloy with the desired composition. Otherwise, the cell parameter of Ni3Cu7 and Ni5Cu5 is appreciably different with respect to the theoretical values. When the Ni : Cu molar ratio decreases (from 2.33 of Ni7Cu3-Al to 0.42 of Ni3Cu7-Al), the lattice parameter increases, and the catalyst with copper content of 7 wt.% shows the presence of two metal phases: a Cu rich alloy, which is the main component, and a Ni-rich alloy. For the Ni5Cu5-Al sample, only one cubic structure is detectable for the metal phase. It is not possible to identify a second metal phase, which is probably beyond the detection limit of the XRD technique. The discrepancy between the observed and theoretical cell parameters suggests that also in this case two metal alloys can be present. Notably, a similar trend was previously described in the literature.13
Selective CO chemisorption experiments were performed with the aim of further investigating the accessibility of the metal nanoparticles and finally to evaluate the TOFs. The data indicates that Ni10-Al shows a rather low metal dispersion (4.4%) which corresponds to an exposed metallic surface of 2.87 m2 g−1. An average particle diameter of 23 nm was estimated by assuming a spherical geometry. This is significantly higher with respect to the crystallite size obtained from the XRD suggesting that the Ni particles are composed of more crystallites and by the fact that part of the particles is in contact with the support. Cu10-Al shows even lower metal dispersion (2.4%) and exposed metallic surface (1.55 m2 g−1). The estimated Cu particle diameter is around 43 nm assuming a spherical geometry. The Ni5Cu5-Al system shows a metal dispersion slightly lower (4.1%) with respect to that of Ni10-Al. Unfortunately, considering the relative small differences between the chemisorption data of the investigated systems, the relative error bar and the fact that we are facing a complex combination of various possible reactions, the extrapolation of the TOFs would appear as speculation more than as a real quantitative analysis.
C.2 Temperature programmed reduction (TPR)
The TPR spectra of the investigated samples are shown in Fig. 3.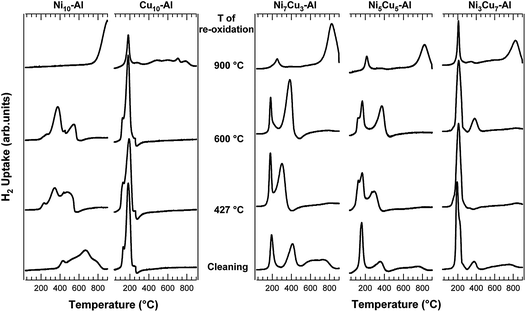 | ||
| Fig. 3 Series of consecutive TPR of calcined Ni10-Al, Cu10-Al, Ni7Cu3-Al, Ni5Cu5-Al and Ni3Cu7-Al samples after a standard cleaning procedure and re-oxidation at different temperatures. | ||
Calcined Ni10-Al displays a broad multipeak reduction profile in the temperature 400–800 °C range. Vice versa, unsupported bulk NiO shows a sharp reduction peak at about 380 °C (data not shown), as previously reported in the literature.14 This suggests the presence of a mixture of NiOx species, originated by the different interaction of NiO particles with the Al2O3 support. Furthermore, the different reduction temperature could be partially ascribed to a different size of NiO particles. Generally, on Ni-based catalysts, the low temperature H2 uptakes are attributed to the reduction of the NiO particles weakly interacting with the support, while the high temperature ones are assigned to the reduction of NiO species in intimate contact with the support and/or forming new species such as NiAl2O4.15
For this system, there is a strong dependence of the reduction behaviour on the thermal treatments. Indeed, the first TPR followed by mild oxidation (O2 at 427 °C or 600 °C for 1 h) leads to pronounced downward shifts of the reduction peaks, indicating significant changes in the Ni chemical environment. Notably, only the relative intensity of different components changes increasing the oxidation temperature from 427 to 600 °C. Finally, the severe re-oxidation at 900 °C for 5 h results in the formation of stable species (e.g.Ni-aluminate spinel species), formed by diffusion of NiOx into the Al2O3. These species are difficult to be reduced.16
Calcined Cu10-Al shows two reduction peaks at 130 and 180 °C. These two components are shifted towards lower temperature with respect to pure bulk CuO, which exhibits two reduction peaks centred at 240 and 315 °C (data not reported). In the case of pure bulk Cu2O, a single component is present at 350 °C (data not reported). The TPR profile of the calcined Cu10-Al indicates the absence of Cu2O, consistently with the XRD data. No appreciable effect of the thermal oxidative treatments on the reduction of the Cu was detected. Cu species appear to interact with the support more weakly than Ni species. New broad peaks (400–900 °C temperature range) are observed in the TPR spectra only after the re-oxidation at 900 °C. These new features can be attributed either to CuOx crystallites with different dimensions, or to the CuOx species which interact differently with the support: the stronger the interaction, the higher the reduction temperature.
The comparison of the TPR profile of NixCuy-Al samples with that of Ni10Al clearly indicates that the addition of Cu strongly promotes Ni reduction. This effect is more evident when increasing the copper content. Notably, even a physical mixture of bulk NiO and CuO shows a significant promotion of the NiO reduction (data not shown). Consistently, the supported bimetallic systems present a sharp reduction peak below 200 °C (160 °C for Ni5Cu5-Al and 190 °C for Ni3Cu7-Al and Ni7Cu3-Al), which can be associated with the reduction of Cu in well dispersed mixed oxide species. Furthermore, a broad peak at intermediate temperature (410 °C for Ni7Cu3-Al, 355 °C for Ni5Cu5-Al and 375 °C Ni3Cu7-Al) is observed. This latter process can be associated with the reduction of Ni-based species promoted by the presence of metallic Cu. Thus, the very strong interaction found between the Ni and Al2O3 with respect to the interaction of Cu-Al2O3, does not seem to limit the strong catalytic effect of Cu on the Ni species. However, a minor reduction contribution, for all bimetallic systems, is observed at high temperature (above 450 °C), which could be either related to the presence of traces of Ni oxides species strongly interacting with the Al2O3 or to some kind of buoyancy effect.
A clear attribution of all the reduction peaks is complicated by the presence of more than one NixCu1−xO phase on the Cu-rich samples. The subsequent oxidative treatment at 427 °C does not influence the peaks related to the reduction of Cu, while the features at 350–400 °C temperatures are shifted towards lower temperatures (ΔT ∼ 115 °C for Ni7Cu3-Al and ΔT ∼ 60 °C for Ni5Cu5-Al). Noteworthy, in the case of the Ni3Cu7-Al sample, no signals are detected between 300 and 400 °C. A similar behaviour is observed after oxidation at 600 °C. Finally, under strong oxidation conditions (at 900 °C) a reduction peak at 820 °C grows in the TPR spectra, which can be attributed to NiO and/or CuO and/or NixCu1−xO species strongly interacting with the support, such as Ni aluminates.
C.3 Catalytic activity measurements
Fig. 4 shows the results of the steam reforming of methanol over pre-reduced NixCuy-Al. On bare Al2O3, methanol conversion starts around 230 °C, producing essentially dimethylether (CH3OCH3) up to 400 °C. The formation of this product is related to the acidic characteristics of the support. Above 400 °C, H2, CO and CH4 are observed. CO2 is also detected at 700 °C in minor concentrations.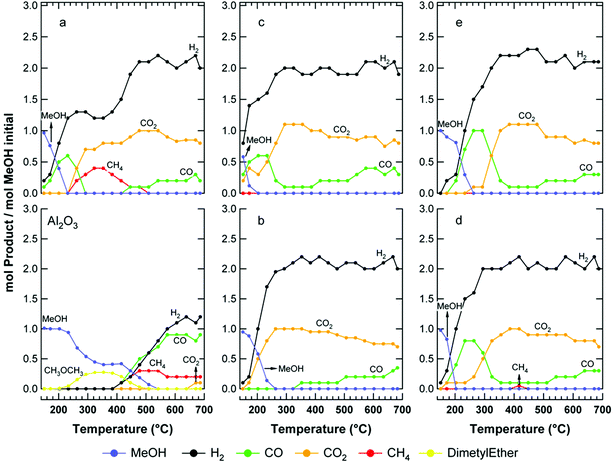 | ||
Fig. 4
Methanol steam reforming over bare Al2O3 and pre-reduced at 750 °C for 2 h: (a) Ni10-Al, (b) Cu10-Al, (c) Ni3Cu7-Al, (d) Ni5Cu5-Al, (e) Ni7Cu3-Al. Conditions: MeOH (1.0%) + H2O (4.0%) in Ar, GHSV = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. 000 mL g−1 h−1. | ||
Over the Ni10-Al sample, methanol begins to be converted above 150 °C (Fig. 4 a). Below 250 °C, the main products are CO and H2, which derive from methanol decomposition. The formation of CO2 is observed for temperatures higher than 230 °C, indicating that the WGS reaction becomes operative only above this temperature. Significant amounts of CH4 are also detected in the 230–510 °C temperature range, as a product of CO and CO2 hydrogenation. This parallel reaction decreases the H2 yield. The maximum for H2 yield is reached above 510 °C, where the evolution of CH4 is inhibited.
The behaviour of the Cu10-Al catalyst (Fig. 4 b) is different. In this case, the WGS is already operative at low temperature (T < 230 °C), consistent with the well know ability of Cu to promote the WGS activity. The formation of small quantities of CO is observed only above 350 °C. It has to be noted that, on increasing the temperature, the WGS equilibrium shifts towards the reactants. Therefore, the conversion of CO to CO2 due to the WGS reaction slightly decreases above 450 °C. The catalytic performance observed in this work is in agreement with data reported by Liu et al. on Cu(15 wt.%)/Al2O3.17
Copper-containing catalysts are well known as showing particularly high activity and selectivity for the MeOH-SR. They are active at low temperature and, for this reason, they are preferred to other catalysts. In fact Ni-based systems possess high activity in carbon oxide hydrogenation and, consequently, promote the formation of undesirable by-products (e.g. CH4). This makes the use of such metals an unattractive option for processes where hydrogen is desired. Consistently, to the best of our best knowledge there have been no investigations on MeOH-SR performed over Ni/Al2O3 samples.
The catalytic performance of the bimetallic samples is interesting (Fig. 4 c, d and e). The conversion of methanol starts above 150 °C on Ni7Cu3-Al and Ni5Cu5-Al, while it is active already at this temperature over Ni3Cu7-Al. The complete conversion is reached around 260 °C for Ni7Cu3-Al and around 205 °C for Ni3Cu7-Al and Ni5Cu5-Al.
The composition of the bimetallic systems does not seem to influence the product distribution above 300 °C. On the other hand, at temperatures below 300 °C, some differences are evident. Indeed, when the Ni : Cu ratio increases, the CO yield increases while the CO2 yield follows the opposite trend.
By comparing these activity profiles with those of the monometallic systems, it is clear that the relative amounts of carbon oxides are related with the copper relative concentration in the catalysts, which influences the WGS equilibrium. Notably, CH4 production was not observed during the run-up experiment on the samples, suggesting that it is possible to decrease significantly the CO/CO2 hydrogenation activity of nickel catalysts by copper alloying. It is well established, and reported in several reviews, that Cu does not easily dissociate CO.18 It is reasonable to infer that this low reactivity towards CO dissociation yields the inhibition of CO activation on the nickel–copper alloy due to dilution of the active nickel sites by the inactive copper atoms. The influence of copper on carbon deposits cannot be also excluded. In this respect, it is known that Cu segregates to the surface of NiCu particles just at room temperature, driven by the lower surface energy of Cu compared with Ni. According to the alloy particle size, the surface-segregated Cu atoms can occupy preferentially either flat terrace sites, or edge and kink Ni sites which are responsible for carbon formation.19
No significant deactivation of the catalyst activity for all samples was observed in the two consecutive run-up experiments.
Fig. 5 presents typical activity profiles for Ni/Cu-based catalysts under the ethanol steam reforming reaction. On bare Al2O3, ethanol conversion starts above 250 °C, producing essentially ethylene through the dehydration reaction. Only above 600 °C, some reforming products are detected, obtained mainly from the cracking of the carbonaceous compounds.
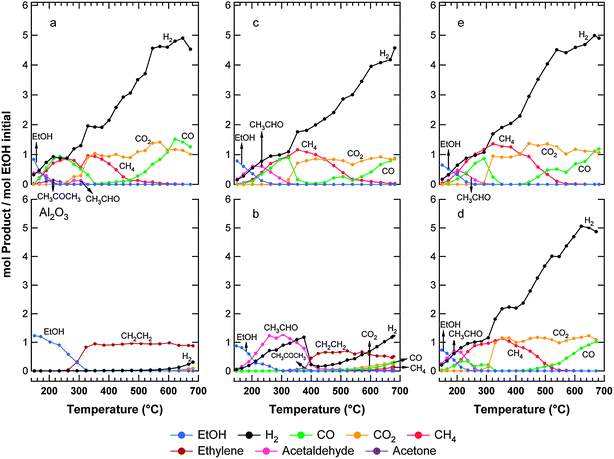 | ||
Fig. 5
Ethanol steam reforming over bare Al2O3 and pre-reduced: (a) Ni10-Al, (b) Cu10-Al, (c) Ni3Cu7-Al, (d) Ni5Cu5-Al, (e) Ni7Cu3-Al. Conditions: EtOH (1.0%) + H2O (5.0%) in Ar, GHSV = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. 000 mL g−1 h−1. | ||
On the Ni10-Al catalyst (Fig. 5 a) ethanol begins to be converted at 200 °C. At this temperature, acetaldehyde and H2 are the main products. Increasing the temperature, the acetaldehyde yield decreases while CO and CH4 start to be produced. The CO yield reaches a maximum around 250 °C, after which it drops to zero at 350 °C. As already observed for MeOH-SR, above 230–250 °C, the WGS reaction becomes operative as confirmed by the presence of CO2 among the products. The formation of CH4 is strongly favored between 250 and 450 °C, while the evolution of ethylene is appreciably inhibited. For higher temperatures, the product distribution is dictated by the equilibrium of the WGS and the methanation reactions. The reforming reactions are predominant only above 500 °C, where only H2, CO and CO2 are detected as products. No modification of the catalytic activity was observed after three run-up experiments.
It is interesting to note that the dehydration of ethanol to ethylene is well known to be strongly active on Al2O3, due to the acidic characteristics of this support. In the case of the Ni10-Al sample, the inhibition in the ethylene production can be related to the partial coverage of the alumina surface by the metal, with consequent reduction of the acidic sites. Furthermore, the activity of nickel in the reforming of ethylene cannot be excluded from these considerations.
EtOH-SR was studied over the Ni/Al2O3 system by several research groups.20 Comas et al.21 studied the reaction in the 300–500 °C temperature range and they did not find evidence for the water gas shift reaction even though the product distribution is perfectly consistent with our results. Notably, other authors suggest that Ni can promote WGS.22 Furthermore, recently we reported that even bare alumina has some WGS activity above 300 °C.23 Akande et al.24 investigated the effects of the catalyst synthesis method, Ni loading and temperature on the catalytic activity of Ni/Al2O3catalysts for ethanol reforming. Three types of preparation methods, namely, coprecipitation, precipitation and impregnation, were evaluated. Optimal Ni loading of 15% was found for maximum ethanol conversion using Ni/Al2O3catalysts prepared by coprecipitation and precipitation methods. For comparison, the Ni loading did not show noticeable effects on the activity when the impregnation method was used. Regarding hydrogen production, the catalyst prepared by coprecipitation with 15% Ni loading showed the best performance. In addition, Ni/Al2O3 prepared by coprecipitation showed the highest selectivity to hydrogen. These results emphasize the importance of the synthesis method, which affects the nature of the active species generated on the catalyst surface. These different species show different redox properties, thus different reactivity under the same reaction conditions.
The catalytic performance of the Cu10-Al catalyst is strongly different (Fig. 5 b). Ethanol conversion becomes significant above 200 °C. Up to 375 °C, the main products are acetaldehyde and hydrogen. Above this temperature, ethylene is observed, while acetaldehyde is not detected. The H2 yield, which reaches a minimum around 450 °C, grows again by increasing the temperature. Traces of CO, CO2 and CH4 are also observed at high temperatures (T > 600 °C). Significant changes are observed in the two subsequent run-up experiments. The catalyst is characterized by a progressive worsening of its catalytic performance. The complete conversion is reached only at high temperatures (T > 600 °C), producing essentially ethylene and H2. Furthermore, the formation of acetaldehyde is strongly inhibited, suggesting that the observed deactivation is mainly due to metal sintering as confirmed by XRD data (see below). Moreover, considerable coke deposition on the catalyst can not be excluded, as indicated by some deficits in the carbon balance during the first run-up experiment. The coke deposits could at least partially cover the Cu particles, reducing the ability of the catalyst to dehydrogenate ethanol and leading to a complete dehydration on the Al2O3 sites.
Cu-based catalysts have also received particular attention. Cavallaro and Freni25 investigated steam reforming of ethanol over CuO/ZnO/Al2O3 and found that the catalyst exhibit good activity with CO, CO2 and H2 as the main products above 350 °C. Amphlett et al.26 suggested that CuO/ZnO, CuO/SiO2, CuO/Cr2O3 or CuO/NiO/SiO2 might prove promising for reforming of ethanol–water mixtures at 350–450 °C. To our best knowledge, no work on the catalytic performance of Cu/Al2O3 is present in the literature.
Taking into account our results, it is clear that neither copper nor nickel alone supported on alumina appear as appropriate catalysts for ethanol steam-reforming under low temperature (T < 500 °C) conditions for hydrogen production. Over the copper sample, dehydrogenation of ethanol into acetaldehyde occurs but the reforming reaction does not proceed further to yield H2 and COx. On the other hand, on the nickel sample, the decomposition reaction of ethanol to CH4 and COx is favored. Only at high temperature (T > 550 °C) methane production is limited due its consumption by steam-reforming processes.
The activity of the bimetallic catalysts, during the first run-up experiment, is not very different (Fig. 5 c, d and e) from that of the monometallic Ni system. Indeed, the product distribution is similar and the Ni : Cu ratio does not seem to affect it. Small differences in the relative amounts of acetaldehyde and CO are observed only at low temperature (T < 300 °C). While the introduction of copper in the catalyst formulation has a positive effect in methanol steam reforming inhibiting the formation of methane, in the case of ethanol, the situation seems to be different. However, it has to be taken into account that methane can be generated not only through carbon oxide hydrogenation, as in the methanol reforming, but also by acetaldehyde decomposition. Thus, it is clear that competitive reaction pathways are involved and the global result will depend on the kinetics of these steps related to the different activity of the metals. Despite their similar activity, these samples present different stability during the two subsequent run-up experiments. The Ni3Cu7-Al catalyst shows the lowest stability, probably due to the sintering of the active phase. By contrast, no appreciable deactivation of the catalytic performance was observed in the case of Ni5Cu5-Al and Ni7Cu3-Al.
It has been previously reported that Cu addition enhances the performance of Ni in EtOH-SR.27,28Ni has limited WGS activity21 but shows high hydrogenation activity and hence it may help in combining adsorbed H atoms on the catalyst surface to form molecular hydrogen.29 By contrast, Cu has limited steam reforming activity, but is a good dehydrogenation catalyst30 and shows high WGS activity.
Marino et al.27 tested at 300 °C a series of Ni–Cu–K/Al2O3catalysts, where potassium was added in the catalyst formulation to neutralize the acidic sites of the support. The activity, selectivity and stability of these materials were correlated to the different species formed during the thermal/chemical treatments (e.g.reduction) before the catalytic test. The selectivity for CH4 was found to decrease as the calcination temperature increases. This fact was attributed to Ni-support interaction, which becomes stronger as the calcination temperature increases (from 450 °C to 800 °C), in parallel with a decrease of the nickel reducibility. Considering that metallic nickel is able to promote ethanol dehydrogenation to acetaldehyde, the decrease in reducibility of this metal as temperature increases explains the observed slight reduction in the catalytic activity. The reduced Ni(6 wt.%)Cu(6 wt.%)–K/Al2O3 sample showed the best performance among the studied samples.27 Notably, in the present work, all samples were calcined at intermediate temperature (600 °C) leading to the formation of species which are completely reduced at 750 °C in H2, as confirmed by TPR data.
The present data suggest an interesting way to combine the different catalytic activity of Ni and Cu, using a two-layer fixed bed reactor. This approach has been recently indicated as very promising31. At low temperature (300–400 °C), ethanol can be first converted by dehydrogenation over Cu into acetaldehyde. The resulting mixture, which primarily consists of acetaldehyde, H2 and excess of water, can then be fed at temperatures around 500–550 °C over a second bed containing Ni catalyst, where it will undergo steam reforming. After this dual-bed reactor, the gaseous mixture has to be treated as usual for syn-gas (WGSR to adjust the H2/CO/CO2 ratio and Preferential Oxidation (PROX) in the case of high-purity H2 production).
The stability of the catalytic activity under isothermal conditions was tested for Ni10-Al, Cu10-Al and Ni5Cu5-Al at 700 °C after 3 run-up experiments. XRD data suggest that a change in the oxidation state of the active phase does not occur. However, a partial sintering of the metal particles is observed. Average crystallite diameters of 11 nm and 50 nm were calculated for Ni10-Al and Cu10-Al, respectively. In the case of Ni5Cu5-Al, a segregation of NiO, with crystallites of 7 nm, from the alloy takes place. Thus, the remaining metal phase, with crystallites of 25 nm, becomes rich in Cu.
Conclusions
NixCuy-Al catalysts with different Ni and Cu contents were synthesized.The interaction with the support and the concomitant presence of the two metals strongly promoted the reducibility of the material.
The catalytic performance of bimetallic systems was promising in the methanol steam reforming. Indeed, the introduction of copper in the catalyst formulation showed a positive effect. The formation of methane, an undesirable by-product, was significantly inhibited. Furthermore, no appreciable deactivation for all samples was observed during two consecutive run-up experiments.
Neither copper nor nickel alone supported on alumina appear as suitable catalysts for ethanol steam-reforming at low temperatures (T < 500 °C). Over the Cu10-Al sample, dehydrogenation of ethanol into acetaldehyde occurred, however the reforming reaction did not proceed to further yield H2 and COx. On the other hand, on the Ni10-Al catalyst, the decomposition reaction of ethanol to CH4 and COx was favoured. Methane production was inhibited only at high temperature (T > 550 °C). Although the activity of the bimetallic systems, during the first run-up experiment, was not very different from that of the monometallic Ni system, they presented improved stability during two subsequent run-up experiments according to the different stability of the NiCu alloy.
Acknowledgements
Prof. M. Graziani is acknowledged for helpful discussions. The University of Trieste, INSTM, FISR2002 “Nanosistemi inorganici ed ibridi per lo sviluppo e l’innovazione di celle a combustibile” are acknowledged for financial support.References
- L. De Rogatis and P. Fornasiero, Catalysis Design for Reforming of Oxygenates, in Catalysis for Sustainable Energy Production, Wiley-VCH, 2008, ch. 8 and references therein Search PubMed.
- R. M. Navarro, M. A. Peňa and J. L. G. Fierro, Chem. Rev., 2007, 107, 3952 CrossRef CAS; N. Hickey, P. Fornasiero and M. Graziani, Hydrogen-based Technologies for Mobile Applications, in Renewable Resources and Renewable Energy: a Global Challenge, ed. M. Graziani and P. Fornasiero, CRC Press (Francis and Taylor Group), 2006, ch. 11 and references therein Search PubMed.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, Beyond Oil and Gas: The Methanol Economy, Wiley-VCH, 2006 Search PubMed.
- D. R. Palo, R. A. Dagle and J. D. Holladay, Chem. Rev., 2007, 107, 3992 CrossRef CAS.
- D. K. Liguras, K. Goundani and X. E. Verykios, J. Power Sources, 2004, 130, 30 CrossRef CAS.
- M. A. Peňa, J. P. Gómez and J. L. G. Fierro, Appl. Catal., A, 1996, 144, 7 CrossRef CAS.
- G. F. Froment, Catal. Rev., 2008, 50, 1 CrossRef CAS; Q. Miao, G. Xiong, S. Sheng, W. Cui, L. Xu and X. Guo, Appl. Catal., A, 1997, 154, 17 CrossRef CAS; R. Jin, Y. Chen, W. Li, W. Cui, Y. Ji, C. Yu and Y. Jiang, Appl. Catal., A, 2000, 201, 71 CrossRef CAS; T. Sperle, D. Chen, R. Lodeng and A. Holmen, Appl. Catal., A, 2005, 282, 195 CrossRef CAS.
- D. K. Kim, K. Stöwe, F. Müller and W. F. Maier, J. Catal., 2007, 247, 101 CrossRef CAS; Z. Boukha, M. Kacimi, M. F. R. Pereira, J. L. Faria, J. L. Figueiredo and M. Ziyad, Appl. Catal., A, 2007, 317, 299 CrossRef CAS; M. Rezaei, S. M. Alavi, S. Sahebdelfar and Z.-F. Yan, Energy Fuels, 2006, 20, 923 CrossRef CAS; J.-S. Choi, K.-I. Moon, Y. G. Kim, J. S. Lee, C.-H. Kim and D. L. Trimm, Catal. Lett., 1998, 52, 43 CrossRef CAS; Z. Zhang, X. E. Verykios, S. M. MacDonald and S. Affrossman, J. Phys. Chem., 2006, 100, 744 Search PubMed; Y.-H. Wang, H. Wang, Y. Lia, Q.-M. Zhu and B.-Q. Xu, Top. Catal., 2005, 32, 109 CrossRef CAS; N. Sahli, C. Petit, A. C. Roger, A. Kiennemann, S. Libs and M. M. Bettahar, Catal. Today, 2006, 113, 187 CrossRef CAS; Z. Hou and T. Yashima, Appl. Catal., A, 2004, 261, 205 CrossRef CAS; B. Pawelec, S. Damyanova, K. Arishtirova, J. L. G. Fierro and L. Petrov, Appl. Catal., A, 2007, 323, 188 CrossRef CAS.
- F. Besenbacher, I. Chorkendorff, B. S. Clausen, B. Hammer, A. M. Molenbroek, J. K. Norskov and I. Stensgaard, Science, 1998, 279, 1913 CrossRef CAS; D. L. Trimm, Catal. Today, 1997, 37, 233 CrossRef CAS; J. R. Rostrup-Nielsen and Ib. Alstrup, Catal. Today, 1999, 53, 311 CrossRef CAS; L. B. Avdeeva, O. V. Goncharova, D. I. Kochubey, V. I. Zaikovskii, L. M. Plyasova, B. N. Novgorodov and Sh.K. Shaikhutdinov, Appl. Catal., A, 1996, 141, 117 CrossRef; C. A. Bernardo, I. Alstrup and J. R. Rostrup-Nielsen, J. Catal., 1985, 96, 517 CrossRef CAS; Y. Li, J. Chen, Y. Qin and L. Chang, Energy Fuels, 2000, 14, 1188 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- R. S. Zhou and R. L. Snyder, Acta Crystallogr., Sect. B, 1991, 47, 617 CrossRef.
- J. H. Lee, E. G. Lee, O. S. Joo and K. D. Jung, Appl. Catal., A, 2004, 269, 1 CrossRef CAS.
- C. A. Bernardo, I. Alstrup and J. R. Rostrup-Nielsen, J. Catal., 1985, 96, 517 CrossRef CAS; T. V. Reshetenko, L. B. Avdeeva, Z. R. Ismagilov, A. L. Chuvilin and V. A. Ushakov, Appl. Catal., A, 2003, 247, 51 CrossRef CAS.
- D. A. Monti and A. Baiker, J. Catal., 1983, 83, 323 CrossRef CAS; S. D. Robertson, J. Catal., 1975, 37, 424 CrossRef CAS.
- H. S. Roh, K. W. Jun, S. C. Baek and S. E. Park, Bull. Korean Chem. Soc., 2002, 23, 1166 CAS; H. S. Roh, K. W. Jun, W. S. Dong, J. S. Chang, S. E. Park and Y. I. Joe, J. Mol. Catal. A, 2002, 181, 137 CrossRef CAS; J. Ye, Z. Li, H. Duan and Y. Liu, J. Rare Earth, 2006, 24, 302 Search PubMed.
- A. J. Akande, R. O. Idem and A. K. Dalai, Appl. Catal., A, 2005, 287, 159 CrossRef CAS; O. S. Joo and K. D. Jung, Bull. Korean Chem. Soc., 2002, 23, 1149 CAS.
- Y. Liu, T. Hayakawa, T. Tsunoda, K. Suzuki, S. Hamakawa, K. Murata, R. Shiozaki, T. Ishii and M. Kumagai, Top. Catal., 2003, 22, 205 CrossRef CAS.
- V. Ponec, New Trends in CO activation, ed. L. Guczi, Elsevier, New York, Studies in Surface Science and Catalysis, 1991, vol. 64, p. 117 Search PubMed; V. Ponec, New Trends in CO activation, ed. L. Guczi, Elsevier, New York, Studies in Surface Science and Catalysis, 1991, vol. 64, p. 225 Search PubMed; V. Ponec and G. C. Bond, Catalysis by Metals and Alloys, Elsevier Science BV, Amsterdam, Studies in Surface Science and Catalysis, 1995, vol. 95 Search PubMed.
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845 CrossRef CAS.
- A. N. Fatsikostas and X. E. Verykios, J. Catal., 2004, 225, 439 CrossRef CAS; S. Freni, S. Cavallaro, N. Mondello, L. Spadaro and F. Frusteri, J. Power Sources, 2002, 108, 53 CrossRef CAS; A. Carrero, J. A. Calles and A. J. Vizcaino, Appl. Catal., A, 2007, 327, 82 CrossRef CAS; H. V. Fajardo and L. F. D. Probst, Appl. Catal., A, 2006, 306, 134 CrossRef CAS; F. Aupretre, C. Descorme and D. Duprez, Catal. Commun., 2002, 3, 263 CrossRef CAS; J. W. C. Liberatori, R. U. Ribeiro, D. Zanchet, F. B. Noronha and J. M. C. Bueno, Appl. Catal., A, 2007, 327, 197 CrossRef CAS; A. L. Alberton, M. M. V. M. Souza and M. Schmal, Catal. Today, 2007, 123, 257 CrossRef CAS; M. S. Batista, R. K. S. Santos, E. M. Assaf, J. M. Assaf and E. A. Ticianelli, J. Power Sources, 2003, 124, 99 CrossRef CAS; J. Llorca, P. R. de la Piscina, J. A. Dalmon, J. Sales and N. Homs, Appl. Catal., B, 2003, 43, 355 CrossRef CAS.
- J. Comas, F. Marino, M. Laborde and N. Amadeo, Chem. Eng. J., 2004, 98, 61 CrossRef CAS.
- M. Nagai, A. MdZahidul and K. Matsuda, Appl. Catal., A, 2006, 313, 137 CrossRef CAS; T.-J. Huang, T.-C. Yu and S.-Y. Jhao, Ind. Eng. Chem. Res., 2006, 45, 150 CrossRef CAS; Y. Li, Q. Fu and M. Flytzani-Stephanopoulos, Appl. Catal., B, 2000, 27, 179 CrossRef CAS.
- T. Montini, L. De Rogatis, V. Gombac, P. Fornasiero and M. Graziani, Appl. Catal., B, 2007, 71, 125 CrossRef CAS.
- A. Akande, R. O. Idem and A. K. Dalai, Appl. Catal., A, 2005, 287, 159 CrossRef CAS.
- S. Cavallaro and S. Freni, Int. J. Hydrogen Energy, 1996, 21, 465 CrossRef CAS.
- B. A. Peppley, J. C. Amphlett, L. M. Kearns and R. F. Mann, Appl. Catal., A, 1999, 179, 21 CrossRef CAS; B. A. Peppley, J. C. Amphlett, L. M. Kearns and R. F. Mann, Appl. Catal., A, 1999, 179, 31 CrossRef CAS.
- F. Marino, M. Boveri, G. Baronetti and M. Laborde, Int. J. Hydrogen Energy, 2004, 29, 67 CrossRef CAS; F. Marino, G. Baronetti, M. Jobbagy and M. Laborde, Appl. Catal., A, 2003, 238, 41 CrossRef CAS.
- F. Marino, M. Boveri, G. Baronetti and M. Laborde, Int. J. Hydrogen Energy, 2001, 26, 665 CrossRef CAS; F. Marino, E. G. Cerrella, S. Duhalde, M. Jobbagy and M. A. Laborde, Int. J. Hydrogen Energy, 1998, 23, 1095 CrossRef CAS.
- J. Kugai, S. Velu and C. Song, Catal. Lett., 2005, 101, 255 CrossRef CAS.
- Y. J. Tu and Y. W. Chen, Ind. Eng. Chem. Res., 1998, 37, 2618 CrossRef CAS.
- P. D. Vaidya, Chem. Eng. J., 2006, 117, 39 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |

