Theoretical studies on the photochemical reaction mechanisms of o-xylylene. Effects of phenyl group for electrocyclic reaction mechanism
Abstract
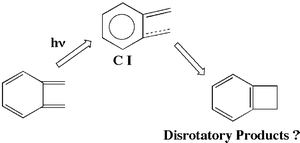
The potential energy surfaces (PESs) of the electrocyclic reactions of o-xylylene at the ground and the lowest excited states are calculated by CASSCF molecular orbital and MRMP2 methods. The lowest excited state geometry of o-xylylene has C2v symmetry and is about 65 kcal mol−1 in energy above the ground state. The PESs in the vicinity of the conical intersection are different from those of the electrocyclic reaction of

 Please wait while we load your content...
Please wait while we load your content...