Deuteron quadrupole coupling in benzene: librational corrections using a temperature-dependent Einstein model, and summary. The symmetries of electric field gradients and conditions for η = 1†
Abstract
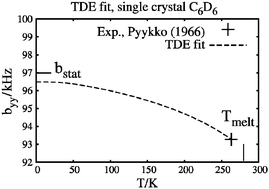
Librational corrections are added to previous single-crystal and polycrystalline measurements of the

 Please wait while we load your content...
Please wait while we load your content...