Interpenetrating three-dimensional coordination networks with a rare 4-connected (65.8) topology and unusual geometrical features†
M. John
Plater
*a,
Thomas
Gelbrich
*b,
Michael B.
Hursthouse
b and
Ben M.
De Silva
a
aDepartment of Chemistry, University of Aberdeen, Meston Walk, Aberdeen, UK AB24 3UE. E-mail: m.j.plater@abdn.ac.uk; Fax: +44(0)1224 272921; Tel: +44(0)1224 272927
bSchool of Chemistry, University of Southampton, Highfield, Southampton, UK SO17 1BJ. E-mail: gelbrich@soton.ac.uk; Fax: +44(0)2380596723; Tel: 44(0)2380594137
First published on 23rd November 2007
Abstract
The reactions of Cu(NO3)2, Co(NO3)2 and Ni(NO3)2 with bp_pen = 1,5-bis(4-pyridyl)pentane yield three-dimensional coordination polymers [M(bp_pen)2(NO3)2] with M = Cu (1), Co (2), Ni (3). These are four-connected uninodal 3D nets exhibiting a rare (65.8) topology defined by metal centres (nodes) and bipyridyl ligands (edges). The reaction of CuNO3 with bp_hep = (1,7-bis(4-pyridyl)heptane yielded a similar net in the structure of [Cu2(bp_hep)4(NO3)3](NO3)(H2O) (4). A characteristic of the networks of 1–4 is that their shortest edge is by far (50%) longer than the shortest distance between two neighbouring nodes. This unusual geometrical feature is facilitated by the long and flexible bp_pen and bp_hep spacers. All four investigated crystal structures are composed of three interpenetrating (65.8) nets.
1. Introduction
Coordination networks formed by crystallisation of bidentate ligands with metal ions are of current interest due to their potential use as functional materials. They can exist in many different topologies and architectures,1,2 and are therefore attractive objects for structural studies. We have previously investigated coordination polymers containing 4-bis(bipyridyl) ligands whose two pyridyl rings are separated by a flexible (CH2)n spacer group.3,4b,4d,4k This ligand type was chosen for its conformational flexibility, a characteristic which may facilitate the formation of novel and unusual network topologies and the occurrence of supramolecular isomerism.54-Bis(bipyridyl) ligands with longer aliphatic chains have a general tendency for disorder in the solid state, but our previous studies have shown that this does not necessarily compromise the ability to determine key structural features. The use of long spacers may give rise to open 3D frameworks containing wide channels. However, the potential for applications may be limited by the fact that a crystal structure is usually composed of several interpenetrating networks. This is a predictable and defining characteristic of the behaviour of flexible bipyridyl ligands with long spacer units.Coordination networks are typically formed by two principle methods, namely solvothermal synthesis and solution layering. Hydrothermal methods work well for aromatic polycarboxylic acids and metal ions where thermal activation energy is needed to drive the formation of a crystalline network often from an amorphous insoluble mixture of starting acid and metal ion. However, bipyridyl ligands crystallise well under milder conditions.
Table 1 lists a group of coordination polymers with [ML2] composition from the current version (5.28, November 2006) of the Cambridge Structural Database.6 All metal centres, M = Cu(II), Co(II) or Ni(II), in each structure are coordinated by the four nitrogen atoms of four flexible 4-bis(bipyridyl) ligands py-(CH2)n-py (n ≥ 2). The most frequent topologies are the 1D ribbon chain and the 2D (4,4) net. These simple topologies are observed in 22 of the 26 listed CSD examples. By contrast, 3D coordination polymers are more elusive. Two three- and four-fold interpenetrating CdSO4-like nets of the composition [Cu(bp_eth)2] and [Cu(bp_hex)2], respectively, have been reported by Power et. al.4o and ourselves.4k To date, no accounts have emerged of two other prototypical square net topologies,2,7 the NbO (64.82) net and the “dense net” (75.9), in this class of compounds. However, Carlucci et al. described a structure with four interpenetrating diamondoid nets [Cu(bp_pro)2].4i This proves that square planar metal centres M are able to serve effectively as tetrahedral nodes in a coordination network, provided that the connecting spacer units L possess sufficient geometric flexibility. Another 3D structure, reported by Carlucci et al., consists of a single binodal [Cu(bp_eth)2] net with unique (64.82) (6.85) topology.4v Two structures with M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios of nearly 1
L ratios of nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 are also noteworthy. The first is a 2D five-level chiral layer [Cu5(bp_eth)9] cut out of the “dense net”,8 and the second is a structure composed of 1D ribbon chains entangled with 1D single stranded chains with one dangling L per M and a [Cu(bp_hex)2]n [Cu(bp_hex)3]n composition.4k
2 are also noteworthy. The first is a 2D five-level chiral layer [Cu5(bp_eth)9] cut out of the “dense net”,8 and the second is a structure composed of 1D ribbon chains entangled with 1D single stranded chains with one dangling L per M and a [Cu(bp_hex)2]n [Cu(bp_hex)3]n composition.4k
| L | M | Refcode |
|---|---|---|
| a Abbreviations: bp_eth = 1,2-bis(4-pyridyl)ethane, bp_pro = 1,3-bis(4-pyridyl)propane, bp_pen = 1,5-bis(4-pyridyl)pentane, bp_hex = 1,6-bis(4-pyridyl)hexane, bp_hep = 1,7-bis(4-pyridyl)heptane. | ||
| 1D: ribbon chain | ||
| bp_eth | Co | FIRCIS01, HUDWOS, LEFYIF, UCUYIA |
| bp_pro | Co | CEHPAM, IBODAE, OFOYIR, |
| OLOKIJ, REDSEZ01, TAHJES | ||
| bp_pro | Ni | IBODEI, WOJRAO |
| 2D: (4, 4) net | ||
| bp_eth | Cu | PAKSOK |
| bp_eth | Co | XACYEF |
| bp_eth | Ni | EJAXOC |
| bp_pro | Cu | CUHZOU, CUHZOU01, CUMZIO, MIJFEQ |
| bp_pro | Co | OBEZAX, REDSEZ, SAZSOC |
| 3D: diamondoid net | ||
| bp_pro | Cu | MIJFIU |
| 3D: CdSO4 net (65.82) | ||
| bp_eth | Cu | PUJQEQ |
| bp_hex | Cu | ODAHIK |
| 3D: (64.82) (6.85) net | ||
| bp_eth | Cu | XASFAY |
| 3D: rare (65.82) net | ||
| bp_pen | Cu, Co, Ni | This work (1, 2, 3) |
| bp_hep | Cu | This work (4) |
Herein we present four crystal structures obtained from M(NO3)2 (M = Cu, Co or Ni) and (1,5-bis(4-pyridyl)pentane) or (1,7-bis(4-pyridyl)heptane): [Cu(bp_pen)2(NO3)2] 1, [Co(bp_pen)2(NO3)2] 2, [Ni(bp_pen)2(NO3)2] 3 and [Cu2(bp_hep)4(NO3)3](NO3)(H2O) 4 which belong to the class listed in Table 1. All are built up of 3D nets with a rare topology (65.82) which is unprecedented in this group.
2. Experimental
2.1. Syntheses
The synthesis of 1,5-bis(4-pyridyl)pentane has been reported previously by us,3a and 1,7-bis(4-pyridyl)heptane was made by a similar method using sodium amide in liquid ammonia.2.2. X-Ray crystallography
Crystallographic data and details of data collection and structure determination are summarized in Table 2. Intensity data were collected using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) on a Bruker-Nonius KappaCCD diffractometer with a Bruker-Nonius FR591 rotating anode.9 The data were corrected for absorption using SADABS.10 The structures were all solved by direct methods and refined on F2 by least-squares procedures.11 Non-hydrogen atoms were refined using anisotropic displacement parameters. All H atoms were located in difference maps and then treated as riding. Displacement parameters for H atoms bonded to C were refined freely for 1 and 3 and with Uiso (H) = 1.2 Ueq(C) in the case of 2 and 4. The correctness of the space group assignment was tested with an alternative refinement in the respective centrosymmetric space group, Pnma (1–3) and P21/m (4). The lattice geometry of structure 4 is pseudo-orthorhombic. The introduction of a twin matrix (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00 010 001) reduced the R1 from 17% to 9%. The final volume fraction for the minor twin component was 0.357(2). The quality of the dataset for structure 4 was not sufficient to locate the position of the H atoms of the water molecule.
00 010 001) reduced the R1 from 17% to 9%. The final volume fraction for the minor twin component was 0.357(2). The quality of the dataset for structure 4 was not sufficient to locate the position of the H atoms of the water molecule.
CCDC reference numbers 654583–654586.
For crystallographic data in CIF or other electronic format see DOI: 10.1039/b711047b
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| a Refined as racemic twin with a minor component fraction of 0.21(10). | ||||
| Formula | C30H36CuN6O6 | C30H36CoN6O6 | C30H36NiN6O6 | C68H90Cu2N12O13 |
| M | 640.19 | 635.58 | 635.36 | 1410.60 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | Pna21 | Pna21 | Pna21 | P21 |
| a/Å | 15.8279(3) | 15.7512(2) | 16.0791(2) | 10.1288(6) |
| b/Å | 10.5692(2) | 10.29690(10) | 10.1956(2) | 21.0031(14) |
| c/Å | 18.4677(5) | 18.7712(2) | 18.9341(3) | 16.3702(10) |
| β/° | 90.961(2) | |||
| U /Å3 | 3089.43(12) | 3044.47(6) | 3103.98(9) | 3482.0(4) |
| Z | 4 | 4 | 4 | 2 |
| Density/g cm–3 | 1.376 | 1.387 | 1.360 | 1.345 |
| µ (Mo-Kα)/mm | 0.759 | 0.616 | 0.677 | 0.681 |
| T/K | 150 | 120 | 120 | 150 |
| Crystal | Blue block | Red block | Colourless block | Blue prism |
| θ range/° | 3–26 | 3–26 | 3–26 | 3–25 |
| Reflections coll. | 15713 | 21102 | 16854 | 22231 |
| Indep. refs./Rint | 5807/0.0489 | 5799/0.0567 | 5361/0.0463 | 10295/0.0826 |
| Parameters | 426 | 389 | 389 | 828 |
| Flack parameter | a | –0.018(8) | –0.038(13) | 0.05(3) |
| R1 (F > 4σ) | 0.0349 | 0.0283 | 0.0324 | 0.0972 |
| w R2 (all data) | 0.0891 | 0.0742 | 0.0842 | 0.1855 |
3. Results and discussion
3.1. [M(bp_pen)2(NO3)2] with M = Cu (1), Co (2) or Ni (3)
Each of the structures in the title consists of three three-dimensional coordination networks. The nets of 1, 2 and 3 have the same topology and show the same type of interpenetration. Thus, the general features discussed in this section for the Cu structure 1, apart from the Jahn–Teller distortion, apply also for the Co (2) and Ni (3) analogues. The asymmetric unit of 1 contains Cu1 in a general position and two bp_pen ligands. Each Cu centre is coordinated in a distorted octahedral fashion by four equatorial pyridyl units and two monodentate axial nitrate anions (Fig. 1). The Cu nodes and bp_pen ligands form a 3D coordination network of M nodes connected by edges L. Despite the square planar coordination of Cu by the four bp_pen nitrogen atoms, the resulting arrangement of nodes in the network deviates from this initial geometry considerably, as illustrated in Fig. 2. The Cu centre X is linked by L to another four Cu nodes, labeled A–D. The resulting planes XABC and XACD are not coplanar but form an angle of 37.2°. This deviation is possible due to the flexibility of the bp_pen ligands. Two of the four connections of X shown in Fig. 2, X⋯A and X⋯B (15.98 Å), utilise a bp_pen ligand with TTTT (all-trans) conformation. The remaining X⋯C and X⋯D linkages (15.80 Å) utilise a TTGT (trans-trans-gauche-trans) ligand. However, the shortest distance between two Cu centres belonging to the same 3D net is just 10.57 Å (along c), and there are two such close neighbours for each Cu node. Networks where some of the shortest distances between two nodes are “non-connecting” can only be observed in some coordination polymers, but are obviously not feasible in inorganic structure types. However, this feature is much more pronounced in the present case, so that the shortest edge of the network is about 50% longer than the shortest distance between two non-connected nodes.![Coordination of Cu1 by four bp_pen ligands and two nitrate anions in the 3D network of [Cu(bp_pen)2(NO3)] (1). Thermal ellipsoids are drawn at the 50% level. Bond distances and angles (Å, °) Cu1–N11 2.036(2), Cu1–N21 2.0315(19), Cu1–N12i 2.0223(19), Cu1–N22ii 2.042(2), Cu1–O2 2.5841(17), Cu1–O4 2.4145(18), O2–Cu1–O4 177.32(6), N11–Cu1–N22ii 178.07(9), N21–Cu1–N12i 176.99(8), N11–Cu1–N12i 89.55(8), N11–Cu1–N21 88.61(8), N12i–Cu1–N22ii 91.61(9), N21–Cu1–N22ii 90.16(8), N11–Cu1–O2 91.19(7), N21–Cu1–O4 85.86(7), N21–Cu1–O2 91.55(7), N11–Cu1–O4 89.39(8), N22ii–Cu1–O2 87.35(8), N22ii–Cu1–O4 92.01(8), N12i–Cu1–O2 86.10(7), N12i–Cu1–O4 96.52(7). Symmetry operations: (i) 1 – x, 1 – y, z – 1/2, (ii) –x – 1/2, y + 1/2, z – 1/2.](/image/article/2008/CE/b711047b/b711047b-f1.gif) | ||
| Fig. 1 Coordination of Cu1 by four bp_pen ligands and two nitrate anions in the 3D network of [Cu(bp_pen)2(NO3)] (1). Thermal ellipsoids are drawn at the 50% level. Bond distances and angles (Å, °) Cu1–N11 2.036(2), Cu1–N21 2.0315(19), Cu1–N12i 2.0223(19), Cu1–N22ii 2.042(2), Cu1–O2 2.5841(17), Cu1–O4 2.4145(18), O2–Cu1–O4 177.32(6), N11–Cu1–N22ii 178.07(9), N21–Cu1–N12i 176.99(8), N11–Cu1–N12i 89.55(8), N11–Cu1–N21 88.61(8), N12i–Cu1–N22ii 91.61(9), N21–Cu1–N22ii 90.16(8), N11–Cu1–O2 91.19(7), N21–Cu1–O4 85.86(7), N21–Cu1–O2 91.55(7), N11–Cu1–O4 89.39(8), N22ii–Cu1–O2 87.35(8), N22ii–Cu1–O4 92.01(8), N12i–Cu1–O2 86.10(7), N12i–Cu1–O4 96.52(7). Symmetry operations: (i) 1 – x, 1 – y, z – 1/2, (ii) –x – 1/2, y + 1/2, z – 1/2. | ||
 | ||
| Fig. 2 One Cu centre (X) linked by four L to another four Cu centres (A, B, C, D) in the network of 1. The deviation of the network from square planar geometry is illustrated by one interplanar angle. Cu⋯Cu distances (Å): XA and XB 15.98, XC and XD 15.80. Cu⋯Cu⋯Cu angles (°): AXB 70.6, BXC 108.9, CXD 84.7, CXA 96.7. | ||
A single 3D net of 1 is depicted in Fig. 3. It consists of edge-fused M6L6 rings which adopt a marked envelope conformation, illustrated in more detail in Fig. 4. This uninodal four-connected net has the same short Schläfli symbol1 as the CdSO4-like net (65.8). However, it can be distinguished2a,b,12 from the latter by its vertex symbol, 6.6.6.6.62.83, whereas the vertex symbol for the CdSO4 net is 6.6.6.6.62.∞. Blatov et al. have described how this rare topology can be derived from the WC structure type.13 To the best of our knowledge, there are only two previous accounts of coordination networks of this kind. Bourne et al. reported two isostructures with three interpenetrating nets of the composition [ZnL1L2] with L1 = m-isophthalate, L2 = 1,2-bis(4-pyridyl)ethane.14 More recently, Liet al. described a similar structure of three interpenetrating [ZnL1L2] nets with L1 = 1,2-bis(imidazol-1′-yl)ethane and L2 = 5-nitroisophthalic acid.15 The structures of the present study are distinct from these two previous examples in two important points. Firstly, they represent the first cases where this rare topology is obtained with uniform ligands L, so that all edges are of approximately equal length. Secondly, the M6L6 rings in the earlier examples of the WC related (65.8) net adopt the chair conformation, making their nodes tetrahedral. This net was therefore called “pseudo-diamondoid” by Bourne et al.14 However, the pronounced envelope conformation observed for the M6L6 rings in the structures reported here imposes a geometry on the network which is distinct from the other examples. In the nets of Bourne et al.14 and Liet al.15 the shortest separation between non-bonded nodes is always in the same range, but actually somewhat longer than the separation between bonded nodes. As discussed above, the novel envelope geometry of the M6L6 rings in the networks of 1–3 is associated with non-bonded separations that are much shorter (in 1: 10.57 Å) than the shortest separation between any two bonded nodes (in 1: 15.80 Å). Thus, one important consequence of the conformational alteration of the M6L6 rings seems to be it enables a relative “squeeze” of distances between “non-bonded” neighbouring nodes. This may also be interpreted as a means to reduce potential open space in networks with long spacers.
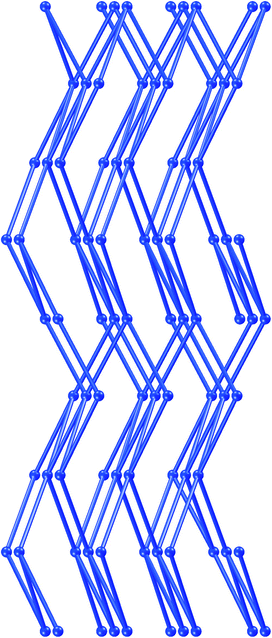 | ||
| Fig. 3 The (65.82) net of structures 1–4. Nodes (M = Cu, Co or Ni) are represented as spheres and edges (L = bp_pen 1–3, L = bp_hep 4) as cylinders. Note that a node is not connected to its two closest neighbours associated with translation along the c-axis in 1–3 and along the a-axis in 4. | ||
 | ||
| Fig. 4 Detailed view of the M6L6 ring in the (65.82) network of 1. Nodes (M = Cu) are depicted as spheres and the edges (L = bp_pen) as broken lines. Note the pronounced envelope conformation of the ring. | ||
The unit cell of a single net of 1 is three times as large as the crystallographic unit cell of the complete crystal structure and has the basis vectors [300], [010] and [001]. Thus, the complete crystal structure comprises three identical nets whose mode of interpenetration is as illustrated in Fig. 5. This arrangement of three networks fills approximately 68% of the available space,16 leaving no space for the inclusion of other molecules. The apparent effectiveness of the space filling in structure 1 can be compared to that of a structure which is based on a CdSO4-like [Cu(bp_hex)2] net.2m The latter structure contains four interpenetrating 3D coordination networks, but there is enough open space for the inclusion of molecules of the free ligand. It is worth mentioning that neither a single net nor the complete structure of 1 contains centres of inversion (space group Pna21).
 | ||
| Fig. 5 Circuits involving a central node (red) and its four coordinating nodes (blue) in the (65.83) net of structures 1–4. Nodes (metal centres) are represented as spheres and edges (bp_pen or bp_hep) as cylinders. | ||
If only the arrangement of the metal centres and pyridyl ligands are considered, then the structure of 1 (M = Cu) is very similar to those of 2 and 3 (M = Co, Ni). However, the two conformations of the bp_pen chain in the Ni compound 3, GTGT and TTGT, differ slightly from those found of 1 and 2 (TTTT and TTGT). Furthermore, as in 1, the two independent distances between connected M nodes are almost equalised (Co⋯Co 16.13 and 16.14 Å) in 2, while the corresponding parameters in 3 differ significantly from one another (Ni⋯Ni 15.52 and 16.30 Å). The metal centres in structures 2 and 3 are octahedrally coordinated, with Co–N 2.15–2.20 Å, Co–O 2.11 Å in 2 and Ni–N 2.10–2.15 Å, Ni–O 2.10–2.11 Å in 3.
3.2. [Cu2(bp_hep)4(NO3)3](NO3)(H2O) (4)
The title structure consists of three-dimensional networks of the composition [Cu2(bp_hep)4(NO3)3], while the remaining nitrate and one water molecule do not coordinate to Cu. The asymmetric unit contains two Cu and four bp_hep. Cu1 is coordinated in a square pyramidal fashion by four pyridyl units (Cu1–N 1.97–2.03 Å) and one apical NO3 (Cu1–O 2.30 Å). The closest contact is along the pyramidal axis opposite to the apex and (nitrate)O–Cu1⋯O(water) = 3.68 Å. By contrast, the octahedral (4 + 2) coordination of Cu2 by two bp_hep and two monodentate nitrate anions is very similar to the Cu coordination in structure 1. The four equatorial Cu2–N distances range between 1.96 and 2.04 Å and both axial Cu2–O lengths are 2.46 Å. The three-dimensional [M(bp_hep)2] net with its two crystallographically independent Cu centres is nevertheless uninodal. It is of the same (65.82) type as the [M(bp_pen)2] networks 1–3 discussed in the previous section. The net of 4 is composed of two independent Cu6L6envelope rings. Each ring type contains two Cu1⋯Cu1 and two Cu2⋯Cu2 edges (16.89 and 16.90 Å) but differ with respect to the remaining two Cu1⋯Cu2 edges. Their values are 17.86 Å in the first ring type and 15.90 Å in the second. Each Cu1 node is bonded by bp_hep to two Cu1 and two Cu2 nodes. However, it is separated from two other non-bonded Cu1 nodes, related to the former via a translation along the a-axis, by just 10.13 Å. A very similar geometry is found around each Cu2 node. Thus, the shortest edge of the network is 57%, and the longest edge is 77% longer than the shortest distance separating neighbouring nodes. The conformation of the aliphatic chain in three of the independent bp_hep units is TTTTGT, and it is TTGGGG in the fourth bp_hep ligand. The base vectors of an individual net of 4 are [010], [10![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ] and [110]. The mode of mutual interpenetration of the three identical (65.82) nets corresponds to that shown in Fig. 6 for the analogous structures 1–3. One nitrate anion and one water molecule per formula unit occupy space between the three interpenetrating networks. The geometry of the lattice is pseudo-orthorhombic (β = 90.961(2)°).
] and [110]. The mode of mutual interpenetration of the three identical (65.82) nets corresponds to that shown in Fig. 6 for the analogous structures 1–3. One nitrate anion and one water molecule per formula unit occupy space between the three interpenetrating networks. The geometry of the lattice is pseudo-orthorhombic (β = 90.961(2)°).
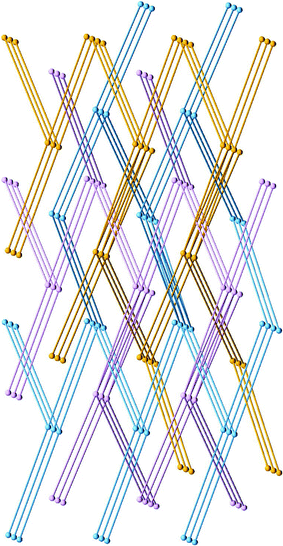 | ||
| Fig. 6 Mode of interpenetration of three (65.82) nets (magenta, blue, orange) in the structure of 1. This scheme is also representative for 2–4. | ||
4. Concluding remarks
The presence of long, flexible spacers is a prerequisite for the formation of the four-connected (65.8) nets presented here. Due to their flexibility it is possible that the net deviates significantly from the underlying planar geometry adopted by the Cu, Co or Ni centre and its surrounding pyridyl ligands. Subtle conformational differences in the ligands of [M(bp_pen)2] M = Cu (1), Co (2) on the one hand and those of [Ni(bp_pen)2] 3 on the other confirm that conformational adaptation of the ligand plays an important role in the geometrical optimisation of the coordination network. The comparison of the [M(bp_pen)2] nets in 1–3 with their [Cu(bp_hep)2] counterpart in 4 suggests that some features, notably the short non-bonding M⋯M distances of 10.13–10.57 Å, are relatively stable. By contrast, there is a difference of 2 Å between different M⋯M edges in the [Cu(bp_hep)2] net, while all corresponding distances are nearly equalised in the bp_pen analogue 1. This discrepancy can be seen as a response to the increased spacer length in 4. The comparison with the two other known examples of the (65.8) net reveals that the latter can be formed in two different ways, so that its fundamental 6-rings adopt either a chair or an envelope conformation. Each conformation imposes a distinct geometry on the 3D network. The longer bp_pen and bp_hep ligands used in our study yield the envelope form. This leads to an unusual network geometry where the two closest neighbouring nodes are not linked to one another, and the shortest edge of the network exceeds the distance between them by approximately 50%. Other new topologies are likely to be discovered owing to the diversity of ligand, metal ion, counterion and solvent that can be used.References
- A. F. Wells, Three-Dimensional Nets and Polyhedra, Wiley, New York, 1977 Search PubMed.
- (a) M. O'Keefe, Z. Kristallogr., 1991, 196, 21; (b) M. O'Keefe and N. E. Brese, Acta Crystallogr., Sect. A, 1992, A48, 663 CrossRef; (c) M. O'Keefe and B. G. Hyde, Crystal Structures I: patterns and symmetry, Mineralogical Society of America, Washington, D.C., 1996 Search PubMed; (d) M. O'Keefe, M. Eddaouhi, H. Li, T. Reinecke and O. M. Yaghi, J. Solid State Chem., 2000, 152, 3 CrossRef CAS.
- (a) M. J. Plater, M. R. St. J. Foreman, T. Gelbrich, S. J. Coles and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 2000, 3065 RSC; (b) M. J. Plater, M. R. St J. Foreman, T. Gelbrich and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 2000, 1995 RSC; (c) M. J. Plater, M. R. St J. Foreman, R. A. Howie and J. M. S. Skakle, Inorg. Chim. Acta, 2000, 318, 175 CrossRef CAS; (d) M. J. Plater, M. R. St J. Foreman and J. M. S. Skakle, Cryst. Eng., 2001, 4, 293 CrossRef.
- (a) (CEHPAH) X. Luan, Y. Chu, Y. Wang, D. Li, P. Liu and Q. Shi, Cryst. Growth Des., 2006, 6, 812 Search PubMed; (b) (CUHZIO), (CUHZOU) M. J. Plater, M. R. St. J. Foreman and A. M. Z. Slawin, J. Chem. Res., 1999, 74, 728 Search PubMed; (c) (CUHZOU01) J. Y. Ryu, C. Kim, D. W. Park, L. O. Park, Y. Kim and A. J. Lough, Acta Crystallogr., Sect. E, 2005, E61, m1489 Search PubMed; (d) (EJAXOC) L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, CrystEngComm, 2003, 5, 190 Search PubMed; (e) (FIRCIS01) C. Merz, M. Desciak, C. O'Brien, R. L. LaDuca, R. C. Finn, R. S. Rarig and J. A. Zubieta, Inorg. Chim. Acta, 2004, 357, 3331 Search PubMed; (f) (HUDWOS) E. Suresh and M. M. Bhadbhade, CrystEngComm, 2001, 3, 50 Search PubMed; (g) (IBODAE), (IBODEI) M. J. Plater, M. R. St. J. Foreman, T. Gelbrich and M. B. Hursthouse, Inorg. Chim. Acta, 2001, 318, 171 Search PubMed; (h) (LEFYIF) N. Seetha Lekshmi and V. R. Pedireddi, Inorg. Chem., 2006, 45, 2400 Search PubMed; (i) (MIJFEQ), (MIJFIU) L. Carlucci, G. Ciani, M. Moret, D. M. Proserpio and S. Rizzato, Chem. Mater., 2002, 14, 12 Search PubMed; (j) (OBEZAX) C. Merz, M. Desciak, C. O'Brien, R. L. LaDuca, R. C. Finn, R. S. Rarig and J. A. Zubieta, Inorg. Chim. Acta, 2004, 357, 3331 Search PubMed; (k) (ODAHEG, ODAHIK) M. J. Plater, M. R. St. J. Foreman, T. Gelbrich and M. B. Hursthouse, Cryst. Eng., 2001, 4, 319 Search PubMed; (l) (OFOYIR) M. T. Bujaci, Xiaotai Wang, Shoujian Li and Chong Zheng, Inorg. Chim. Acta, 2002, 333, 152 Search PubMed; (m) (OLOKIJ) K. J. Nordell, J. D. Hammond and M. D. Smith, Acta Crystallogr., Sect. E, 2003, E59, m852 Search PubMed; (n) (PAKSOK) L. J. May and G. K. H. Shimizu, Chem. Commun., 2005, 1270 Search PubMed; (o) (PUJQEQ) K. N. Power, T. L. Hennigar and M. J. Zaworotko, Chem. Commun., 1998, 595 Search PubMed; (p) (REDSEZ), (REDSEZ01) E.-Q. Gao, Y.-X. Xu, A.-L. Cheng, M.-Y. He and C.-H. Yan, Inorg. Chem. Commun., 2006, 9, 212 Search PubMed; (q) (SAZSOC) M. I. Khan, S. Deb and R. J. Doedens, Inorg. Chem. Commun., 2006, 9, 25 Search PubMed; (r) (TAHJES) T. M. Bujici, X.-T. Wang, S.-J. Li and C. Zheng, Z. Kristallogr. - New Cryst. Struct., 2003, 218, 148 Search PubMed; (s) (UCUYIA) M. L. Hernandez, M. K. Urtiaga, M. G. Barandika, R. Cortes, L. Lezama, N. de la Pinta, M. I. Arriortua and T. Rojo, J. Chem. Soc., Dalton Trans., 2001, 3010 Search PubMed; (t) (WOJRAO) C. V. K. Sharma, R. J. Diaz, A. J. Hessheimer and A. Clearfield, Cryst. Eng., 2000, 3, 201 Search PubMed; (u) (XACYEF) S. H. Park, K. M. Kim, S. Lee and O.-S. Jung, Bull. Korean Chem. Soc., 1998, 19, 79 Search PubMed; (v) (XASFAY) L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, J. Chem. Soc., Dalton Trans., 2000, 3821 Search PubMed.
- T. Hennigar, D. C. MacQuarrie, P. Losier, R. D. Rogers and M. J. Zaworotko, Angew. Chem., Int. Ed., 1997, 36, 972 CrossRef CAS.
- F. H. Allen, Acta Crystallogr., Sect. B, 2002, B58, 380 CrossRef.
- L. Carlucci, G. Ciani, P. Macchi and D. M. Proserpio, Chem. Commun., 1998, 1837 RSC.
- L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, Chem. Commun., 2000, 1319 RSC.
- (a) R. W. W. Hooft, COLLECT data collection software, Nonius B.V., 1998; (b) W. Minor and Z. Otwinowski, Macromol. Crystallogr., 1997, 276, 307 Search PubMed.
- G. M. Sheldrick, SADABS. Version 2.10. Bruker AXS Inc., Madison, Wisconsin, USA, 2003.
- G. M. Sheldrick, SHELXS97 and SHELXL97, University of Göttingen, Göttingen, Germany, 1997.
- Calculations and graphics for Fig. 3, 5 and 6 were carried out using OLEX: O. V. Dolomanov, A. J. Blake, N. R. Champness and M. J. Schröder, Appl. Crystallogr., 2003, 36, 1283 Search PubMed.
- V. A. Blatov, L. Carlucci, G. Gianni and D. M. Proserpio, CrystEngComm, 2004, 6, 377 RSC.
- S. A. Bourne, J. J. Lu, B. Moulton and M. J. Zaworotko, Chem. Commun., 2001, 861 RSC.
- X. Li, R. Cao, Z. Guo, W. Bi and D. Yuan, Inorg. Chem. Commun., 2006, 9, 551 CrossRef CAS.
- Calculation were carried out using Platon: A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 Search PubMed.
Footnote |
| † CCDC reference numbers 654583–654586. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b711047b |
| This journal is © The Royal Society of Chemistry 2008 |
