Iron(III) amine-bis(phenolate) complexes as catalysts for the coupling of alkyl halides with aryl Grignard reagents †
Rajoshree Roy
Chowdhury
,
Angela K.
Crane
,
Candace
Fowler
,
Philip
Kwong
and
Christopher M.
Kozak
*
Memorial University of Newfoundland, Department of Chemistry, Centre for Green Chemistry and Catalysis, St. John's, Newfoundland, Canada A1B 3X7. E-mail: ckozak@mun.ca
First published on 23rd November 2007
Abstract
Catalytic cross-coupling of aryl Grignard reagents with primary and secondary alkyl halides bearing β-hydrogens is achieved using Fe(III) amine-bis(phenolate) halide complexes.
Transition metal-catalyzed Grignard cross-coupling is an important class of C–C bond forming reaction.1 Traditionally, Ni and Pd complexes have been used to perform the Kumada–Corriu coupling of alkyl Grignard reagents with alkyl or aryl halides.2–4 Related iron(III) chloride complexes possessing salen ligands have recently been shown to be capable of cross-coupling alkyl Grignards with aryl halides, as well as coupling aryl Grignards with alkyl halides.5–7 Other iron complexes have shown activity in the cross-coupling of both primary and secondary alkyl halides with aryl Grignard reagents . The use of Fe(acac)3 shows good activity towards the coupling of aryl Grignard reagents with alkyl halides,8 as does FeCl3 in the presence of simple amines .9–11 Also, cross-coupling can be catalyzed by phosphine , phosphite, arsine and carbene ligands with FeCl3 or FeCl2.12 Lower oxidation state iron compounds have also shown cross-coupling activity, including the formally Fe(−II) complex [Li(tmeda)]2[Fe(C2H4)2], the “inorganic Grignard” reagent [Fe(MgX)2] and the Fe(II) ‘ate’ complex [Me4Fe][Li2(OEt2)2].13,14 Iron nanoparticles have also been proposed as active catalysts for the coupling of aryl Grignard reagents with alkyl halides.15 The use of FeCl3 directly in the above reactions, or its use to generate an active catalyst in situ, is often inconvenient on a large scale because it is highly hygroscopic, and yields vary according to its purity and commercial origin.11 Although Fe(acac)3 is a more convenient, less hygroscopic starting material, amine additives must be employed to achieve high conversions and yields of cross-coupled products. An easy to use, non-hygroscopic single component catalyst precursor is therefore highly desirable. Herein, we report the synthesis of iron chloride complexes of amine-bis(phenolate) ether ligands and their use in the catalytic cross-coupling of aryl Grignard reagents with primary and secondary alkyl halides.
The amine-bis(phenolate) ligands in Fig. 1 were prepared using literature procedures by employing a Mannich condensation of the corresponding phenol, amine and formaldehyde.16–19 Single crystals of L1H2 were obtained from a saturated methanol solution and were characterized by X-ray diffraction.‡
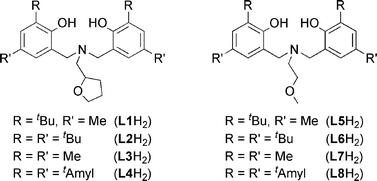 | ||
| Fig. 1 Ligand variations employed in the synthesis of Fe(III) precatalysts. | ||
Eight Fe(III) complexes were prepared by the dropwise addition of a methanol solution of FeCl3 to a methanolic slurry of the ligand at room temperature (eqn. 1). This dark blue solution was neutralized using NEt3 and evaporated to dryness. Extraction into toluene, filtration and removal of the solvent gave analytically pure complexes in good yield.
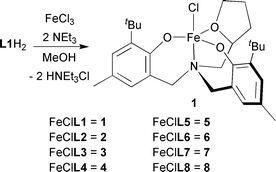 | (1) |
The complexes have magnetic moments of 5.5 to 5.9 µB at room temperature, consistent with high spin d5 configurations. MALDI mass spectrometry using an anthracene matrix can be used to characterize the iron complexes; compounds 1–8 all show molecular ion peaks and characteristic fragment ions. Slow evaporation of a solution of FeClL1 (1) in methanol gave crystals suitable for X-ray diffraction.‡ The structure of 1 is shown in Fig. 2, along with selected bond lengths and angles. The coordination geometry around the iron atom is distorted trigonal bipyramidal. The metal is bonded to two phenolate oxygen atoms and the furfuryl oxygen atom, which define the trigonal plane of the bipyramid. The central nitrogen atom of the ligand and the chloride ion occupy the apical sites. The Fe–O(2) and Fe–O(3) distances of 1.854(2) and 1.850(2) Å, respectively, for the phenolate oxygen donors are similar to those observed in related trigonal bipyramidal and square pyramidal iron(III) complexes possessing diamine bis(phenolate) ligands.20,21 However, the Fe–Cl and Fe–N bond distances of 2.2739(10) and 2.223(3) Å, respectively, are slightly shorter in 1. The iron atom is displaced 0.206 Å above the equatorial plane. The N–Fe–Cl angle of 165.69(8)° is considerably distorted from the ideal linear geometry; it is bent away from the phenolate groups and directed toward the tetrahydrofurfuryl fragment.
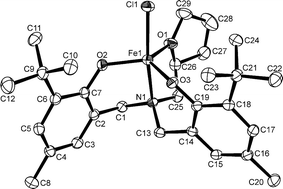 | ||
| Fig. 2 The molecular structure (ORTEP) and numbering scheme for 1. Ellipsoids are shown at 30% probability. Selected bond distances (Å) and angles (°): Fe1–O3 1.850(2), Fe1–O2 1.854(2), Fe1–O1 2.074(3), Fe1–N1 2.223(3), Fe1–Cl1 2.2739(10), O1–C29 1.452(5), O1–C26 1.468(5), O2–C7 1.346(4), O3–C19 1.352(4); O3–Fe1–O2 118.39(10), O3–Fe1–O1 119.00(11), O2–Fe1–O1 119.60(11), O3–Fe1–N1 87.62(10), O2–Fe1–N1 89.37(10), O1–Fe1–N1 75.79(10), O3–Fe1–Cl1 100.81(8), O2–Fe1–Cl1 96.60(8), O1–Fe1–Cl1 89.98(8), N1–Fe1–Cl1 165.69(8). | ||
For the preliminary screening of catalyst performance, the reaction shown in eqn. 2 was chosen. In addition to the desired cross-coupled product, by-products may include the elimination product (cyclohexene), the hydrodehalogenated product (cyclohexane) and two possible homo-coupled products (dicyclohexane and bitoluene).1 Initially, complexes 1 to 8 were examined for their catalytic cross-coupling activity, but little variation was seen among them, thus implying a negligible effect imposed by either the aryl substituents or the pendant donor groups examined. Iron(III) salen complexes displayed a similar behaviour, where ortho and paratBu-modified analogues showed essentially the same activity as systems containing only hydrogen atoms in these positions.5 For subsequent reaction optimization experiments, complex 1 was employed.
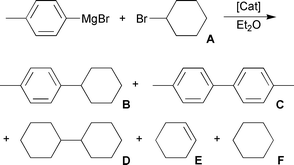 | (2) |
Several sets of reaction conditions were examined to determine their effect on both the conversion to products and the selectivity for cross-coupling. We found that adding the catalyst as a solution in CH2Cl2 and removing the solvent under vacuum consistently gave superior conversions and yields of products compared to when it is added as a solid. This is consistent with what is observed when [FeCl(salen)] complexes were used for this reaction.5 The catalyst loading was maintained at 5.0 mol% throughout. The solvent, temperature and amount of Grignard reagent used were examined for their influence on the conversion and yield (Table 1). Using diethyl ether and conducting the reaction at room temperature gave an excellent yield of cross-coupled product B (Table 1, entry 1). The use of THF (Table 1, entry 2) lead to a lower yield, while conducting the reaction at 0 °C did not affect the yield of the desired product (Table 1, entry 3). Decreasing the Grignard reagent stoichiometry decreased the yield of the desired cross-coupled product (Table 1, entry 4), but increasing the amount of Grignard reagent was only found to generate more biaryl product C. It has been proposed that excess Grignard is required to reduce the Fe(III) precatalyst, thus generating the active catalyst.7,11 During these initial screenings, the presence of products D, E and F were only detected in trace amounts by GC -MS.
| Entry | ArMgBr | Alkyl halide | Product | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: ArMgBr (4.0 mmol), alkyl halide (2.0 mmol), catalyst 1 (5.0 mol%), Et2O, 25 °C, 30 min. b Yield determined by GC using dodecane as the internal standard; average of 2 trials. c Solvent used was THF instead of Et2O. d Reaction conducted at 0 °C. e 2.0 mmol of ArMgBr used. f 0.5 M solution in THF. | ||||
| 1 | Ar = para-tolyl | Bromocyclohexane |
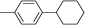
|
99 |
| 2 | Bromocyclohexane c | ″ | 80 | |
| 3 | Bromocyclohexane d | ″ | 99 | |
| 4 | Bromocyclohexane e | ″ | 50 | |
| 5 | Chlorocyclohexane | ″ | 48 | |
| 6 | Iodocyclohexane | ″ | 99 | |
| 7 | Benzyl bromide |
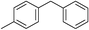
|
50 | |
| 8 | 2-Bromobutane |

|
79 | |
| 9 | 2-Bromopentane |
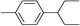
|
99 | |
| 10 | 1-Bromooctane |
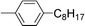
|
90 | |
| 11 | 1-Iodopropane |
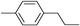
|
54 | |
| 12 | Ar = ortho-tolyl | Bromocyclohexane |
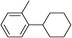
|
99 |
| 13 | Chlorocyclohexane | ″ | 62 | |
| 14 | Iodocyclohexane | ″ | 99 | |
| 15 | Benzyl bromide |
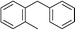
|
68 | |
| 16 | 2-Bromobutane |
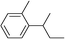
|
50 | |
| 17 | 2-Bromopentane |
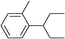
|
64 | |
| 18 | 1-Bromooctane |
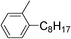
|
99 | |
| 19 | 1-Iodopropane |
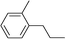
|
53 | |
| 20 | Ar = para-anisylf | Bromocyclohexane |
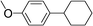
|
99 |
| 21 | Chlorocyclohexane | ″ | 22 | |
| 22 | Iodocyclohexane | ″ | 81 | |
| 23 | Benzyl bromide |

|
0 | |
| 24 | 2-Bromobutane |
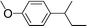
|
37 | |
| 25 | 2-Bromopentane |
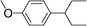
|
35 | |
| 26 | 1-Bromooctane |
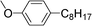
|
30 | |
| 27 | 1-Iodopropane |
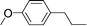
|
42 | |
Subsequently, substrate screening was performed using the conditions shown in Table 1. Under these conditions, conversion to the cross-coupled products of 4-MeC6H4MgBr, 2-MeC6H4MgBr and 4-MeOC6H4MgBr with bromocyclohexane each gave 99% yields. Similarly high yields were observed using iodocyclohexane with 4-MeC6H4MgBr and 2-MeC6H4MgBr (Table 1, entries 6 and 14), but decreased slightly with MeOC6H4MgBr (Table 1, entry 22). Chlorocyclohexane gave very poor yields with all three Grignard reagents (Table 1, entries 5, 13 and 21). Acyclic alkyl halide reagents generally gave poorer yields of cross-coupled products and also showed the highest yields of biaryl products. Benzyl bromide only showed fair cross-coupling activity with tolyl Grignards; no product was observed using MeOC6H4MgBr. Also, using benzyl bromide as the alkyl source generated significant yields of bibenzyl, a product of alkyl halide coupling.
In summary, new Fe(III) chloride complexes with amine-bis(phenolate) ligands have been easily synthesized and show excellent potential as catalysts for the cross-coupling of aryl Grignard reagents with primary and secondary alkyl halides. Further studies evaluating the scope and limitations of this reaction with other aryl Grignard reagents are being pursued. For example, functional group tolerance studies and reaction condition optimization are under way.
We gratefully acknowledge Memorial University and the NSERC of Canada for funding (C. M. K.) and Undergraduate Student Research Awards (A. K. C. and P. K.). We thank Julie Collins for crystallography and C-CART for assistance. P. K. also thanks the Inorganic Chemistry Exchange (ICE) Program.
Notes and references
- Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004 Search PubMed.
- D. J. Cardenas, Angew. Chem., Int. Ed., 2003, 42, 384 CrossRef CAS.
- A. C. Frisch and M. Beller, Angew. Chem., Int. Ed., 2005, 44, 674 CrossRef.
- M. R. Netherton and G. C. Fu, Adv. Synth. Catal., 2004, 346, 1525 CrossRef CAS.
- R. B. Bedford, D. W. Bruce, R. M. Frost, J. W. Goodby and M. Hird, Chem. Commun., 2004, 2822 RSC.
- A. Fürstner and A. Leitner, Angew. Chem., Int. Ed., 2002, 41, 609 CrossRef CAS.
- A. Fürstner, A. Leitner, M. Mendez and H. Krause, J. Am. Chem. Soc., 2002, 124, 13856 CrossRef.
- T. Nagano and T. Hayashi, Org. Lett., 2004, 6, 1297 CrossRef CAS.
- M. Nakamura, K. Matsuo, S. Ito and B. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS.
- R. B. Bedford, D. W. Bruce, R. M. Frost and M. Hird, Chem. Commun., 2005, 4161 RSC.
- G. Cahiez, V. Habiak, C. Duplais and A. Moyeux, Angew. Chem., Int. Ed., 2007, 46, 4364 CrossRef CAS.
- R. B. Bedford, M. Betham, D. W. Bruce, A. A. Danopoulos, R. M. Frost and M. Hird, J. Org. Chem., 2006, 71, 1104 CrossRef CAS.
- R. Martin and A. Fürstner, Angew. Chem., Int. Ed., 2004, 43, 3955 CrossRef CAS.
- A. Fürstner, H. Krause and C. W. Lehmann, Angew. Chem., Int. Ed., 2006, 45, 440 CrossRef.
- R. B. Bedford, M. Betham, D. W. Bruce, S. A. Davis, R. M. Frost and M. Hird, Chem. Commun., 2006, 1398 RSC.
- S. Groysman, I. Goldberg, M. Kol, E. Genizi and Z. Goldschmidt, Inorg. Chim. Acta, 2003, 345, 137 CrossRef CAS.
- E. Y. Tshuva, S. Groysman, I. Goldberg, M. Kol and Z. Goldschmidt, Organometallics, 2002, 21, 662 CrossRef CAS.
- F. M. Kerton, A. C. Whitwood and C. E. Willans, Dalton Trans., 2004, 2237 RSC.
- F. M. Kerton, S. Holloway, A. Power, R. G. Soper, K. Sheridan, J. M. Lynam, A. C. Whitwood and C. E. Willans, Can. J. Chem., 2007 Search PubMed , submitted for publication.
- M. Velusamy, M. Palaniandavar, R. S. Gopalan and G. U. Kulkarni, Inorg. Chem., 2003, 42, 8283 CrossRef CAS.
- P. Mialane, E. Anxaolabéhère-Mallart, G. Blondin, A. Nivorojkine, J. Guilhem, L. Tchertanova, M. Cesario, N. Ravi, E. Bominaar, J. J. Girerd and E. Munck, Inorg. Chim. Acta, 1997, 263, 367 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data for L1H2 and 1, and characterization data (GC and NMR) for the cross-coupled products. See DOI: 10.1039/b713647a |
‡ Crystal data for L1H2: C29H43NO3, M = 453.66, colourless, prism, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2), a = 10.453(4), b = 11.703(4), c = 12.508(4) Å, α = 101.931(6), β = 107.410(5), γ = 104.170(7)°, V = 1349.3(8) Å3, T = 153 K, Z = 2, µ(Mo-Kα) = 0.707 cm−1, Dc = 1.117 g cm−3, 13994 reflections measured, 6808 unique reflections (Rint = 0.019), R[I > 2σ(I)] = 0.0600, wR2 = 0.1659. CCDC 659673. (#2), a = 10.453(4), b = 11.703(4), c = 12.508(4) Å, α = 101.931(6), β = 107.410(5), γ = 104.170(7)°, V = 1349.3(8) Å3, T = 153 K, Z = 2, µ(Mo-Kα) = 0.707 cm−1, Dc = 1.117 g cm−3, 13994 reflections measured, 6808 unique reflections (Rint = 0.019), R[I > 2σ(I)] = 0.0600, wR2 = 0.1659. CCDC 659673.Crystal data for 1: C29H41ClFeNO3, M = 542.95, black prism, monoclinic, P21/n (#14), a = 9.7824(8), b = 25.0778(19), c = 12.3276(9) Å, β = 95.0780(19)°, V = 3012.4(4) Å3, T = 153 K, Z = 4, µ(Mo-Kα) = 6.160 cm−1, Dc = 1.197 g cm−3, 27790 reflections measured, 8185 unique reflections (Rint = 0.049), R[I > 2σ(I)] = 0.0836, wR2 = 0.2400. CCDC 659674. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b713647a. See the ESI for full experimental procedures and characterization.† |
| This journal is © The Royal Society of Chemistry 2008 |
