Assembling metals and clusters around an octaphosphine ligand based on N-substituted bis(diphenylphosphanyl)amines: structural characterization of dendrimer-like Co12 and Co16 branched clusters†
Mireia
Rodriguez-Zubiri‡
a,
Vito
Gallo§
a,
Jacky
Rosé
a,
Richard
Welter
b and
Pierre
Braunstein
*a
aLaboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université Louis Pasteur, 4 rue Blaise Pascal F-67070 Strasbourg, France. E-mail: braunstein@chimie.u-strasbg.fr
bLaboratoire DECOMET, Institut de Chimie (UMR 7177 CNRS), Université Louis Pasteur, 4 rue Blaise Pascal F-67070 Strasbourg, France
First published on 19th October 2007
Abstract
A polypodal ligand based on an aromatic ring decorated by four dppa-type diphosphine moieties has been used to prepare Pt(II) complexes and to assemble four tricobalt or tetracobalt carbonyl clusters, leading to centrosymmetric Co12 and Co16 «clusters of clusters» which were characterized by X-ray diffraction.
Short-bite ligands, i.e. which contain a single atom as spacer between the donor atoms, continue to attract much attention in fundamental and applied coordination chemistry and are particularly useful as assembling ligands in di- and polynuclear chemistry, including cluster chemistry.1 Such bidentate ligands can coordinate to metal centres in monodentate, chelating or bridging modes and relatively minor changes in the ligand substituents or in the coligands may alter their bonding modes or, alternatively, render their interconversions energetically readily accessible. A recent example is shown in eqn (1) where relatively bulky N-substituents result in a higher chelating power of the PNP ligand owing to entropic factors, thus making the energy of the chelate form comparable with that of the bridged form.2
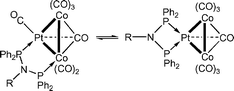 | (1) |
Whereas Ph2PCH2PPh2 (dppm) has been used for decades,1 the analogous ligand Ph2PNHPPh2 (dppa) has been less exploited, although recent results have demonstrated its remarkable properties, and those of its N-substituted derivatives, in the chromium catalyzed selective trimerization or tetramerization of ethylene.3 We have described the first dppa-bridged heterometallic complexes and prepared and characterized, using X-ray diffraction, a number of homo- and heterometallic complexes and clusters with this and related ligands,4 and examined the catalytic properties of some of the Fe-Pd complexes for the dehydrogenative coupling of stannanes.5N-Functionalization of dppa, which is easier than that of dppm owing to the enhanced acidity of the NH proton, allows the synthesis of alkoxysilyl derivatives for the anchoring of clusters into mesoporous alumina or silica supports and the subsequent formation of highly dispersed metallic nanoparticles confined in the matrix.6,7
More complex structures containing interconnected metal centres or clusters each bonded to a dppa-type donor set could be attractive targets in order to examine the possible occurrence of cooperative effects and to generate new steric environments that are susceptible to change of the properties associated with only one of the metal centres or clusters. Furthermore, molecular clusters organised around an organic core could form nano-objects with novel properties and applications.8 We thus turned our attention to multidentate bis(diphenylphosphanyl)amine systems, such as the structurally characterized complexes 19 and 2.10
Our aim was to extend this type of arrangement to a ligand core decorated with four –N(PPh2)2 donors and form metal-richer molecules. Furthermore, introduction of sulfur in the spacer could allow subsequent deposition of the molecule on a Au surface and selective formation of metallic monolayers.
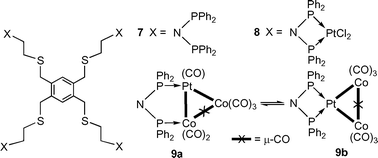
A thioether function has been recently introduced in dppa-type ligands (see 3 and 4) and the Pt(II) complex 5 and Co2Pt cluster 6 have been structurally characterized.2 Our attention was therefore attracted by ligand 79 and we describe here its first cluster derivatives 9–12.
We first set out to coordinate PtCl2 moieties to 7 in order to react them subsequently with [Co(CO)4]– and form four Co2Pt clusters by extension of the procedure used to obtain 6 from 5.2
Reaction of 7 with [PtCl2(cod)] (cod = 1,4-cyclooctadiene) (4 equiv.) in CH2Cl2 resulted in complete consumption of the ligand and rapid precipitation of a white solid whose poor solubility in common organic solvents (THF, CH2Cl2 or acetone) prevented characterization by NMR spectroscopy. However, when a DMF solution of 7 was added dropwise over 1 h to a stirred DMF solution of [PtCl2(cod)] and the reaction mixture further stirred for 1 h, a white solid was obtained after work-up.† It was characterized in the 31P{1H} NMR spectrum by a singlet at δ 17.3 with satellites (1JP–Pt = 3298 Hz) and in the 195Pt{1H} NMR spectrum by a triplet at δ –4028 (1JP–Pt = 3298 Hz). These data, together with the ν(Pt–Cl) IR absorptions at 309 and 292 cm–1, are consistent with the formulation shown for 8. The insoluble species obtained by the former procedure (and during many other attempts) are likely to be polymeric and ionic species (bis-chelation at Pt with displacement of the chloride ligands often results when ligand addition is not slow enough).2
Reaction of 8 with 8 equiv. of Na[Co(CO)4] in CH2Cl2 at room temp. for 3 h afforded after work-up a brown solid whose spectroscopic data are consistent with species 9. Its 31P{1H} NMR spectrum contains a broad signal at δ 100.0 for the Co-bound P nucleus, and a doublet with 195Pt satellites at δ 71.0 (JP–P = 23 Hz, 1JP–Pt = 3629 Hz) and a singlet with 195Pt satellites at δ 56.0 (1JP–Pt = 3010 Hz) for the Pt-bound P in the bridged (a) and chelate forms (b), respectively. Accordingly, the 195Pt{1H} NMR spectrum contains a broad doublet at δ –4129 (1JP–Pt = 3629 Hz) and a triplet at δ –4330 (1JP–Pt = 3010 Hz) for isomers a and b, respectively. Very strong IR absorptions at 2054, 2010, 1966, 1756 cm–1 indicate the presence of terminal and bridging CO groups.†
As an alternative to building the clusters stepwise, we then used [Co3(µ3-CCl)(CO)9] as a precursor, anticipating its stabilization by the short-bite ligand moieties of 7.11 A high yield reaction in toluene yielded, after recrystallization/precipitation from CH2Cl2–toluene–hexane, a fine red powder. Recrystallization from toluene–MeOH–hexane afforded crystals suitable for X-ray diffraction.† Product 10 is characterized by a 31P{1H} NMR singlet at δ 109 and IR absorptions indicating the presence of only terminal CO ligands.† An X-ray diffraction study established its centrosymmetric structure (Fig. 1) with each short-bite site of ligand 7 bridging an edge of a Co3 triangle to form a molecule with four Co3 branches.¶ A related topology has been recently described with a polyalkynylalkene core.12
 | ||
| Fig. 1 View of the molecular structure of 10 in 10·4CH2Cl2·2C6H14. Symmetry operator for equivalent atoms (′): –x, –y, –z + 1. For clarity, only the P-phenyl ipso carbons are represented. | ||
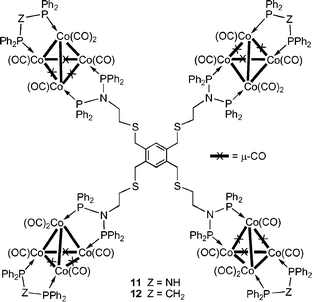
To expand the scope of this approach, we then turned to tetracobalt carbonyl clusters which appeared promising since derivatives of [Co4(CO)12] with dppa and its alkoxysilyl derivatives have been characterized, anchored inside nanoporous alumina membranes,6 and used for the synthesis of Co2P nanoparticles within a SBA-15 matrix.7 Such phosphides are of considerable interest for their electronic, magnetic and catalytic properties.13 The reaction of [Co4(CO)12] with 7 afforded a deep-green, poorly soluble compound which could only be analyzed by IR† and could correspond to a polymeric material because of the possibility for dppa-type units from different molecules of 7 to span two edges of Co4 tetrahedra. We found earlier that short-bite ligand cluster stabilization is achieved in [Co4(CO)10(µ-dppx)] (x = a or m) compared to [Co4(CO)12] and allows a more selective substitution chemistry.14 Thus, in the reaction product of [Co4(CO)10(µ-dppa)] with another dppa-type ligand, two opposite Co–Co edges of the tetrahedron are selectively spanned.6,14 Therefore, 7 was reacted with [Co4(CO)10(µ-dppx)] and purification by column chromatography afforded four fractions.† The third, major fraction was the desired product but we have not been able to assign all the broad resonances in the 31P{1H} NMR spectrum (Co quadrupolar broadening). Its colour is consistent with tetrahedral tetracobalt carbonyl clusters substituted by two short-bite ligands, as shown in 11 and 12.†
The crystal structure of 11·2CH2Cl2¶ established that four Co4 clusters have indeed been branched around the organic core (Fig. 2). As anticipated, the ancillary dppa ligand has prevented further, uncontrolled CO substitution and played the role of a stopper.
 | ||
| Fig. 2 View of the molecular structure of 11 in 11·2CH2Cl2. Symmetry operator for equivalent atoms (′): –x, –y – 1, –z + 1. For clarity, only the P-phenyl ipso carbons are represented. | ||
Future objectives are to deposit such dendrimer-like nano-objects on surfaces,15 prepare metal particles and compare their physical properties with those of nanoparticles obtained from the isolated tetrahedral carbonyl clusters.16
In conclusion, we have reported a stepwise strategy to assemble dendrimer-like Co12 and Co16 metal clusters around a polypodal ligand. To the best of our knowledge, 11 is the largest molecule of its kind to have been characterized by X-ray diffraction. Clusters 10 and 11 form disk-like molecules with a thickness of ca. 11 Å and a diameter of ca. 23 and 30 Å, respectively.
We are grateful to the CNRS (post-doctoral grant to MRZ), the Ministère de la Recherche (Paris) and COST D-30 for funding. We also thank the International Research Training Group (Graduiertenkolleg 532) for support.
Notes and references
- Selected review articles: R. J. Puddephatt, Chem. Soc. Rev., 1983, 12, 99 Search PubMed; M. Witt and H. W. Roesky, Chem. Rev., 1994, 94, 1163 RSC; M. S. Balakrishna, Y. Sreenivasa Reddy, S. S. Krishnamurthy, J. F. Nixon and J. C. T. R. Burckett St. Laurent, Coord. Chem. Rev., 1994, 129, 1 CrossRef CAS; J. T. Mague, J. Cluster Sci., 1995, 6, 217 CrossRef CAS; P. Bhattacharyya and J. D. Woollins, Polyhedron, 1995, 14, 3367 CAS; P. Braunstein, M. Knorr and C. Stern, Coord. Chem. Rev., 1998, 178–180, 903 CrossRef CAS; T. Appleby and J. D. Woollins, Coord. Chem. Rev., 2002, 235, 121 CrossRef CAS.
- V. Gallo, P. Mastrorilli, C. F. Nobile, P. Braunstein and U. Englert, Dalton Trans., 2006, 2342 RSC.
- J. T. Dixon, M. J. Green, F. M. Hess and D. H. Morgan, J. Organomet. Chem., 2004, 689, 3641 CrossRef CAS; M. J. Overett, K. Blann, A. Bollmann, J. T. Dixon, D. Haasbroek, E. Killian, H. Maumela, D. S. McGuinness and D. H. Morgan, J. Am. Chem. Soc., 2005, 127, 10723 CrossRef CAS; P. R. Elowe, C. McCann, P. G. Pringle, S. K. Spitzmesser and J. E. Bercaw, Organometallics, 2006, 25, 5255 CrossRef CAS and references cited.
- I. Bachert, P. Braunstein and R. Hasselbring, New J. Chem., 1996, 20, 993 Search PubMed; I. Bachert, P. Braunstein, M. K. McCart, F. Fabrizi De Biani, F. Laschi, P. Zanello, G. Kickelbick and U. Schubert, J. Organomet. Chem., 1999, 573, 47 CrossRef CAS; I. Bachert, I. Bartusseck, P. Braunstein, E. Guillon, J. Rosé and G. Kickelbick, J. Organomet. Chem., 1999, 580, 257 CrossRef CAS; I. Bachert, I. Bartusseck, P. Braunstein, E. Guillon, J. Rosé and G. Kickelbick, J. Organomet. Chem., 1999, 588, 143 CAS; P. Braunstein, J. Durand, G. Kickelbick, M. Knorr, X. Morise, R. Pugin, A. Tiripicchio and F. Ugozzoli, J. Chem. Soc., Dalton Trans., 1999, 4175 RSC; I. Bachert, P. Braunstein, E. Guillon, C. Massera, J. Rosé, A. DeCian and J. Fischer, J. Cluster Sci., 1999, 10, 445 CrossRef CAS; P. Braunstein, J. Durand, M. Knorr and C. Strohmann, Chem. Commun., 2001, 211 RSC; F. Balegroune, P. Braunstein, J. Durand, T. Faure, D. Grandjean, M. Knorr, M. Lanfranchi, C. Massera, X. Morise and A. Tiripicchio, Monatsh. Chem., 2001, 132, 885 CrossRef CAS.
- P. Braunstein, J. Durand, X. Morise, A. Tiripicchio and F. Ugozzoli, Organometallics, 2000, 19, 444 CrossRef CAS; P. Braunstein and X. Morise, Chem. Rev., 2000, 100, 3541 CrossRef CAS.
- P. Braunstein, H.-P. Kormann, W. Meyer-Zaika, R. Pugin and G. Schmid, Chem.–Eur. J., 2000, 6, 4637 CrossRef CAS.
- F. Schweyer-Tihay, P. Braunstein, C. Estournès, J. L. Guille, B. Lebeau, J.-L. Paillaud, M. Richard-Plouet and J. Rosé, Chem. Mater., 2003, 15, 57 CrossRef CAS.
- See e.g.: M. Benito, O. Rossell, M. Seco and G. Segalés, J. Organomet. Chem., 2001, 619, 245 Search PubMed; J. Geng, H. Li, D. Zhou, W. T. S. Huck and B. F. G. Johnson, Polyhedron, 2006, 25, 585 CrossRef CAS; J. R. Aranzaes, C. Belin and D. Astruc, Angew. Chem., Int. Ed., 2006, 45, 132 CrossRef CAS; S.-H. Hwang, C. D. Shreiner, C. N. Moorefield and G. R. Newkome, New J. Chem., 2007, 31, 1192 CrossRef CAS; M. Häußler, J. W. Y. Lam, A. Qin, K. K. C. Tse, M. K. S. Li, J. Liu, C. K. W. Jim, P. Gao and B. Z. Tang, Chem. Commun., 2007, 2584 RSC.
- K. G. Gaw, M. B. Smith and J. W. Steed, J. Organomet. Chem., 2002, 664, 294 CrossRef CAS.
- F. Majoumo-Mbe, P. Lönnecke, E. V. Novikova, G. P. Belov and E. Hey-Hawkins, Dalton Trans., 2005, 3326 RSC.
- M. I. Bruce, M. E. Smith, N. N. Zaitseva, B. W. Skelton and A. H. White, J. Organomet. Chem., 2003, 670, 170 CrossRef CAS.
- M. I. Bruce, N. N. Zaitseva, P. J. Low, B. W. Skelton and A. H. White, J. Organomet. Chem., 2006, 691, 4273 CrossRef CAS.
- S. L. Brock, S. C. Perera and K. L. Stamm, Chem.–Eur. J., 2004, 10, 3364 CrossRef CAS; S. T. Oyama, J. Catal., 2003, 216, 343 CrossRef CAS.
- A. Choualeb, P. Braunstein, J. Rosé, S.-E. Bouaoud and R. Welter, Organometallics, 2003, 22, 4405 CrossRef.
- A. Naitabdi, O. Toulemonde, J.-P. Bucher, J. Rosé, P. Braunstein, R. Welter and M. Drillon, Chem.–Eur. J. Search PubMed , in press.
- F. Schweyer, P. Braunstein, J. Rosé, C. Estournès, J. Guille, H. Kessler and J.-L. Paillaud, Chem. Commun., 2000, 1271 RSC; F. Schweyer-Tihay, C. Estournès, P. Braunstein, J. Guille, J.-L. Paillaud, M. Richard-Plouet and J. Rosé, Phys. Chem. Chem. Phys., 2006, 8, 4018 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental data and ORTEP plots for 10 and 11. See DOI: 10.1039/b713540h |
| ‡ Current address: Laboratoire de Chimie de Coordination (UPR 8241 CNRS), 205 route de Narbonne, F-31077 Toulouse Cedex, France. |
| § Dipartimento di Ingegneria delle Acque e di Chimica, Politecnico di Bari, via Orabona 4 (CAMPUS), I-70125 Bari, Italy. |
| ¶ CCDC 659619 and 659620. For crystallographic data in CIF or other electronic format, see DOI: 10.1039/b713540h |
| This journal is © The Royal Society of Chemistry 2008 |