DOI:
10.1039/B713071F
(Paper)
Analyst, 2008,
133, 133-138
Enhanced stability and sensitivity of ionic liquid–carbon paste electrodes at elevated temperatures
Received 24th August 2007, Accepted 25th October 2007
First published on 14th November 2007
Abstract
We describe the operation of ionic liquid–carbon paste electrodes at elevated temperatures and the effect of heating on the electrode performance and response. Using cyclic and square wave voltammetry and amperometry, it is shown that signals can be enhanced and stabilized by increasing the temperature of the operating solution. At low temperature, the electrode was susceptible to electrode fouling and showed poor stability, sensitivity, and linearity. An order of magnitude improvement of response for ascorbic acid was possible by operating the electrode at 60 °C compared to 0 °C. This study represents the first report showing that the analytical response of ionic liquid–carbon paste electrodes can be improved by operating them at elevated temperatures for a number of applications.
Introduction
In recent years ionic liquids (ILs) have emerged as credible replacements for current conventional organic solvents in many areas including electrochemical analysis.1 For example, owing to their high thermal stability and low volatility, ionic liquids allow the design of robust gas sensors under extreme conditions. Room temperature ionic liquids are defined as liquid electrolytes composed entirely of ions which have melting points below 100 °C.2 The basic structure of any ionic liquid is based on an asymmetric heterocyclic cation such as N,N-dialkyl-substituted imidazolium ions, and a small anion such as tetrafluoroborate (BF4–), hexafluorophosphate (PF6–), or bis(trifluoromethylsulfonyl)imide (NTf2).3 The use of PF6– or NTf2 as the counter ion to an N,N-dialkyl-substituted imidazolium cation causes the resulting structure to be water immiscible.4 The PF6–- or NTf2-based ILs have been shown to be electrochemically stable with a wide potential window of over 5 V. They reveal excellent electrical (ionic) conductivity and thermal stability over the temperature range of –25 to 85 °C.3 They also exhibit a high boiling temperature and hence a very low vapor pressure in ambient conditions.5The preparation of carbon paste electrodes for analytical purposes where ILs are used as a binder has been reported in just a few studies.6–9 In one application it was found that the IL–carbon paste electrode exhibits electrocatalytic activity for the reduction of nitrite.6 Recent work by Compton's group showed that the increased capacitive charging current which is a major disadvantage typical of these IL-paste electrodes can be overcome by the use of rotating disk electrodes.10 The increased capacitive currents are basically due to the greater accumulation of ions at the water interface of the liquid salts compared to non-conductive binders such as mineral oil.
In this report we investigate the effect of operating IL–carbon paste electrodes at temperatures above and below room temperature and how this would affect their analytical performance in terms of stability and sensitivity. Owing to their high thermal stability, ILs can be a good candidate for fabricating carbon paste electrodes for operation at high temperatures in extreme environments. Operating analytical devices and sensors at elevated temperatures has always been important for many applications. Among these applications are process control, gas analysis, reactor testing, corrosion analysis, and in the pharmaceutical industry.11–14 One amperometric sensor designed for hydrogen analysis in boiling water proved to be efficient even at 300 °C.11 Besides the need for sensors with good stability in high temperature conditions, heat by itself could have a significant effect in improving the sensitivity and reproducibility of measurement by affecting the properties of the measuring device and the measurement medium. Heated wire electrodes have been shown to be useful for greatly enhancing the voltammetric and chronopotentiometric behavior of important analytes, such as heavy metals.15,16 Heated platinum electrodes were useful in minimizing the fouling of the electrode surface upon NADH oxidation in addition to improving the sensitivity of measurement.17 Heated carbon paste electrodes were also useful in the detection of nucleic acids.18,19 To the best of our knowledge, no one has evaluated the effect of operating IL–carbon paste electrodes at elevated temperatures although it has been shown that the conductivities of ILs increase with temperature.20 Another article describes the better stabilization effect of the membrane in fuel cells operating at high temperature by using the ionic liquid 1-butyl-3-methylimidazolium trifluoromethanesulfonate in place of water along with a Nafion membrane.21 In the following sections we describe the operation of an IL-paste electrode at elevated temperature and the enhancement of sensitivity and stability compared to the unheated paste as well as with a carbon paste using mineral oil as a non-conductive binder.
Experimental
Apparatus
Amperometric, square wave voltammetric, and cyclic voltammetric measurements were performed using a computer-controlled µ-Autolab II potentiostat (Ecochemie, The Netherlands) with a three-electrode configuration. The working electrode, the reference electrode [a saturated calomel electrode (SCE), Radiometer, Copenhagen, Denmark] and the counter electrode (a bright platinum wire) were inserted into the 20 mL glass cell (home-made) through holes in its Teflon cover. A magnetic stirrer provided the convective transport during the amperometric measurements. Square wave (SWV) and cyclic voltammetry (CV) experiments were carried out under quiescent conditions.Chemicals and reagents
All solutions were prepared from double-distilled water. Potassium dihydrogen phosphate, dipotassium hydrogen phosphate, potassium ferrocyanide, potassium ferricyanide, ascorbic acid, mineral oil, and synthetic graphite powder consisting of irregularly shaped microparticles 2–20 µm in diameter were purchased from Aldrich.The room temperature ionic liquids: 1-butyl-3-methylimidazolium hexafluorophosphate (C4mim-PF6), N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide (C4mpyrr-NTf2), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C4mim-NTf2), and 1-octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C8mim-NTf2) were kindly donated by QUILL/Belfast.
The required amount of ionic liquid or mineral oil was mixed for 5 min using a pestle and mortar with the required amount of graphite. The paste was then packed at the end of a pipette tip (diameter 350 µm) to a depth of 3 mm. Electrical contact was achieved via a copper wire.Procedure
Measurements were carried out in a phosphate buffer (0.05 M, pH 7.5) supporting electrolyte medium for amperometric, square wave and cyclic voltammetry measurements. Amperometric detection proceeded under forced convection, whilst quiescent conditions were used for square wave and cyclic voltammetry experiments. In the former the desired working potential was applied, and transient currents were allowed to decay to a steady-state value. Measurements were performed at low temperature by placing the measurement cell into an ice bath. Measurements were initiated when the required temperature was reached. For measurements at elevated temperatures, they were performed by placing the measurement cell into a water bath heated by means of a thermostated hotplate with continuous stirring of the water bath to maintain a homogeneous distribution of temperature. Measurement of solution temperature was made using a mercury thermometer.Results and discussion
Initial experiments compared the effect of temperature on different compositions of IL–carbon pastes and mineral oil–carbon pastes to evaluate the effect of binder amount on the response as a function of temperature. As can be seen in Fig. 1B, all the IL-containing pastes show a linear increase in response as a function of temperature which is evaluated by using the anodic current for 25 mM potassium ferricyanide. The use of this large concentration was required to overcome the large background current. Please note, however, that lower concentrations were possible to study analytically using square wave voltammetry as discussed below. The largest change in response as the temperature was increased from 0 to 50 °C was observed for the 50% IL-paste. This is partly attributed to the increased rate of diffusion in the aqueous phase and the enhanced rate of heterogeneous electron transfer at elevated temperatures. A smaller change in response was observed for the 10 and 30% IL-pastes. On replacing the IL with a non-conductive binder such as the mineral oil, a completely opposite response was observed as can be seen in Fig. 1A when the content of mineral oil was above 10%. At 10% loading similar behavior was observed as compared to the IL-containing paste. The exact reason for the diminishing of the anodic current at higher temperatures using higher loadings of mineral oil is not fully clear but, one probable reason could be the decrease in viscosity of the mineral oil which allows better distribution of the oil and hence increased insulation of the graphite particles. |
| | Fig. 1 Anodic peak currents measured for the second scan using 25 mM potassium ferricyanide at mineral oil/graphite paste (A) and ionic liquid C4mim-NTf2/graphite paste (B) at 0, 25, and 50 °C using a liquid-to-graphite ratio of 10 : 90 (◆), 30 : 70 (▲), and 50 : 50 (■) respectively. Supporting electrolyte, phosphate buffer (0.05 M, pH 7.4); scan rate 50 mV s–1. | |
A plot of background (charging) current versus scan rate in phosphate buffer was used for estimating the double layer capacitance versus temperature as shown in Fig. 2, and the inset of Fig. 2. The corresponding double layer capacitance values were calculated for each temperature and a similar trend was observed as shown in the inset. Although this behavior was typical for C4mim-NTf2/graphite with a 20 : 80 ratio, a similar trend was observed for other ionic liquids with other paste compositions (data not shown). Although the increase in temperature leads to a slight increase in capacitance, as shown in Fig. 2, this did not affect the analytical signal due to the improved mass transport as a result of heating. To evaluate the effect of increasing temperature for some analytical applications, two redox probes were used: ascorbic acid and potassium ferricyanide using cyclic voltammetry as shown in Fig. 3A and 3B respectively. As temperature was increased from 0 to 50 °C for ascorbic acid, a well-noticed enhancement in peak current is observed along with a peak potential shift from 0.08 to –0.04 V as shown in the insets of Fig. 3A. In addition to that, the shape of the voltammogram changed from peak shape at low temperature to steady-state wave shape at high temperature which indicates a hydrodynamic mass transport control as a result of convection arising from heating the solution. As can be observed from the voltammograms for ascorbic acid, the process of its oxidation is chemically irreversible, where peak potentials are only reflective of the electrode kinetics rather than ΔEp. According to Kenten and McCreery,22 the oxidation of ascorbic acid is an inner-sphere reaction with electron-transfer kinetics sensitive to the surface. So the possible enhancement of the oxidation signal upon heating could be attributed to several factors including improved electron-transfer rates due to heating, and improved mass transport due to solution heating. A similar behavior was confirmed with potassium ferricyanide at other temperatures as shown in Fig. 3B. Enhancement in both anodic and cathodic currents was observed as the temperature was increased. The increase in cathodic current was, however, more systematic with a slower increase from 0 to 25 °C, followed by a faster and linear increase from 25 to 65 °C, and a tendency to stabilize thereafter. Both anodic and cathodic peak potentials shifted linearly to more negative values as the temperature was increased as shown in the inset of Fig. 3B. The peak-to-peak separation slightly increased with increasing temperature, where the lowest value was 0.1 V at 0 °C, and the highest was 0.15 V at 75 °C. One possible explanation for this increase is the decrease in the viscosity of the ionic liquid film which tends to make it more mobile and hence to induce a better coating and coverage of the graphite microparticles. This is effectively equivalent to increasing the ratio of the IL-to-graphite. If this is true, then higher electrode resistance would be expected with increasing loading of IL (or heating in this case) and hence slower electron-transfer rates and larger peak-to-peak separations. It is clearly seen that the observed voltammograms for ferricyanide/ferrocyanide redox couple at different temperatures are typical of a quasi-reversible redox process, associated with the composite character of the carbon paste electrodes. This behavior fully agrees with what was reported earlier for carbon paste electrodes using mineral oil,18 and using ionic liquid as a binder.10 Fig. 4 depicts the effect of temperature on three more ionic liquids along with a mineral oil-based carbon paste electrode. The selection of these ionic liquids was made in comparison with C4mim-NTf2, based on the length of the carbon chain within the cation using C8mim-NTf2 (Fig. 4A), with a different anion using C4mim-PF6 (Fig. 4B), a different cation using C4mpyrr-NTf2 (Fig. 4C), and with a different binder using the non-conductive mineral oil binder (Fig. 4D). It is obvious that ILs with different structural features all behaved in a similar fashion when the temperature was increased and showed an increase in response. Using a less hydrophobic IL such as C4mim-PF6, a new peak appeared in the reverse scan of the voltammogram at the highest temperature which reflects the accelerated diffusion of ferrocyanide into the IL interface at higher temperatures. In the case of mineral oil, a strange behavior was observed as the temperature increased as mentioned above. The anodic current was decreasing as the temperature was increasing, while the cathodic current was increasing with a large potential shift. The exact reason behind this is not well understood. One possible explanation could be that the decrease in viscosity of the mineral oil at higher temperatures tends to encapsulate the graphite particles in isolated islands of micron size which would explain the change in the shape of the voltammogram from peak shape to sigmoidal shape at higher temperature, which is typical for microelectrodes. Fig. 5 compares five repetitive cyclic voltammograms using 25 mM ascorbic acid for (A) hot (50 °C) and (B) cold (0 °C) at the C4mim-NTf2/graphite (20 : 80) electrode. The cold electrode shows a fast diminishing of current, with 70 and 60% peak suppressions during the second and fifth scan, respectively. On the contrary, the heated electrode exhibits a very stable response and no change in peak currents during the first five scans. This improved stability can be attributed to an improved removal of oxidation products, thus, decreasing the formation of blocking oxidation products which causes electrode fouling. Furthermore, it is well known that adsorption of oxidation products is reduced at elevated temperature which would explain the improved stability at the heated electrode. Evaluating the reproducibility of response at the IL–carbon paste electrode upon switching between cold and hot conditions is displayed in Fig. 6. Using four alternating cyclic voltammograms at two temperatures, 20 and 50 °C, the response was nearly identical at each temperature. The switching between the two temperatures was made without any need for electrode surface polishing or activation. Extending the number of switching cycles to ten cycles still showed very good reproducibility with a RSD of 5% (data not shown). In order to utilize their analytical applications and to overcome the large background associated with ILs, square wave voltammetry was used to study the effect of temperature on the calibration curves for potassium ferrocyanide as shown in Fig. 7A. A three-fold enhancement in response was observed at the IL–carbon paste electrode at 60 °C compared to that measured at 0 °C with better linear response, ca. R2 0.997 vs. 0.98. Similar to the results observed with cyclic voltammetry, a negative potential shift is also observed with SWV of around 100 mV upon raising the temperature. The dependence of potential on temperature is greatly attributed to the increased kinetics at higher temperature and to a lower extent to the dependence of the reference potential on temperature. In contrast to that, the mineral oil–carbon paste electrode response decreased upon increasing the temperature with an increase in background current and large potential shift in strong agreement with the cyclic voltammetry results. Even the scans at low temperature showed broad peaks at low concentration, with peak broadening increasing as concentration was increasing. In addition to that, the peak potential was shifting gradually as the concentration was increasing. Such behavior was not observed in the case of the IL–carbon paste electrode which shows better stability and reproducibility at the IL–carbon-based electrode. Calibration curves corresponding to the measured peak currents are shown in the inset of Fig. 7B, which lack any degree of linearity either at cold or hot conditions. The dependence of the amperometric response on concentration at two temperatures is shown in Fig. 8 using ascorbic acid. A seven- to eight-fold enhancement was observed under such conditions as the temperature was increased from 20 to 50 °C.
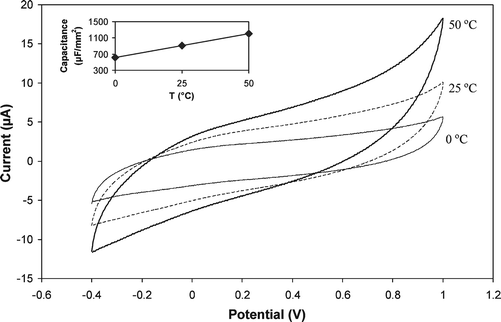 |
| | Fig. 2 Cyclic voltammograms for blank solution recorded at 0, 25, and 50 °C using ionic liquid C4mim-NTf2/graphite (20 : 80). Capacitance values calculated at the above temperatures are shown in the inset. Other conditions as in Fig. 1. | |
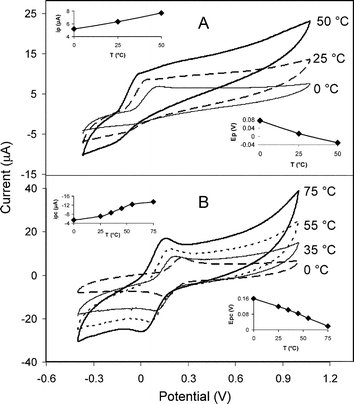 |
| | Fig. 3 Cyclic voltammograms for 25 mM ascorbic acid (A) and potassium ferricyanide (B) using ionic liquid C4mim-NTf2/graphite (20 : 80) at temperatures of 0, 25, and 50 °C (A) and 0, 35, 55, and 75 °C (B). Peak current versus temperature and peak potential versus temperature are shown in the upper left corner inset and lower right corner inset of A and B respectively. Other conditions as in Fig. 1. | |
 |
| | Fig. 5 Cyclic voltammograms for 25 mM ascorbic acid using ionic liquid C4mim-NTf2/graphite (20 : 80) at temperatures of 50 °C (A) and 0 °C (B). Different scans represent first scan ( ), second scan (- - -), and fifth scan (—). Other conditions as in Fig. 1. ), second scan (- - -), and fifth scan (—). Other conditions as in Fig. 1. | |
 |
| | Fig. 6 Alternating cyclic voltammograms for 25 mM potassium ferricyanide using ionic liquid C4mim-PF6/graphite (30 : 70) at 20 °C (a and c) and at 50 °C (b and d). Other conditions as in Fig. 1. | |
 |
| | Fig. 7 Square wave voltammograms for ionic liquid C4mim-NTf2/graphite (30 : 70) (A) and mineral oil/graphite (30 : 70) (B) at cold (0 °C) and hot (60 °C) solutions. Insets show the corresponding calibration curves for each paste at hot and cold conditions. Other conditions as in Fig. 1 | |
 |
| | Fig. 8 Current–time recordings obtained using ionic liquid C4mim-NTf2/graphite (30 : 70) at cold (20 °C) and hot (50 °C) conditions upon increasing the concentration of ascorbic acid in increments of 2 mM. Operating potential –0.1 V. Inset shows the corresponding calibration curves at hot and cold conditions. Other conditions as in Fig. 1. | |
Conclusion
In this report, IL–carbon paste electrodes were evaluated to study the effect of operating temperature on their response. It was shown that signal stabilization and enhancement can be achieved with such heating regimes. Highly reproducible signals along with improvement in linearity were manageable with such controlled heating schemes. It was clear from voltammetric and amperometric studies that the signal can be improved by an order of magnitude at high temperature compared to that at low temperature. Potential shifts were also observed whenever the electrode was heated. One more advantage was the improvement in electrode resistance to fouling and hence improving the electrodes' stability. The greatly improved sensitivity, stability, and linearity, can be further exploited for designing enzyme-based biosensors for several applications that can survive extreme conditions such as high temperature. Other than that, such electrodes can be very helpful for important applications in the oil, energy, and pharmaceutical industries, where measurements at elevated temperature are essential or beneficial.
References
- D. S. Silvester and R. G. Compton, Z. Phys. Chem., 2006, 220, 1247 CrossRef CAS.
- K. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS.
- M. Buzzeo, R. Evans and R. G. Compton, ChemPhysChem, 2004, 5, 1106 CrossRef CAS.
- P. Suarez, S. Einloft, J. Dullius, R. de Souza and J. Dupont, J. Chim. Phys. Phys.-Chim. Biol., 1998, 95, 1626 CAS.
- Y. Lee and T. Chou, Biosens. Bioelectron., 2004, 20, 33 CrossRef CAS.
- H. Liu, P. He, Z. Li, C. Sun, L. Shi, Y. Liu, G. Zhu and J. Li, Electrochem. Commun., 2005, 7, 1357 CrossRef CAS.
- N. Maleki, A. Safavi and F. Tajabadi, Anal. Chem., 2006, 78, 3820 CrossRef CAS.
- A. Safavi, N. Maleki and F. Tajabadi, Analyst, 2007, 132, 54 RSC.
- G. Shul, J. Sirieix-Plenet, L. Gaillon and M. Opallo, Electrochem. Commun., 2006, 8, 1111 CrossRef CAS.
- R. T. Kachoosangi, G. G. Wildgoose and R. G. Compton, Electroanalysis, 2007, 19, 1483 CrossRef CAS.
- L. Kriksunov and D. Macdonald, Sens. Actuators, B, 1996, 32, 57 CrossRef.
- C. Liu and D. Macdonald, J. Supercrit. Fluids, 1995, 8, 263 CrossRef CAS.
- D. Morris, F. Jiang, J. McInerney and D. Lister, J. Appl. Electrochem., 1999, 29, 971 CrossRef CAS.
- W. Gopel1, G. Reinhardt and M. Rosch, Solid State Ionics, 2000, 136–137, 519 CrossRef.
- J. Wang, P. Grundler, G.-U. Flechsig, M. Jasinski, J. Lu, J. Wang, Z. Zhao and B. Tian, Anal. Chim. Acta, 1999, 396, 33 CrossRef CAS.
- G.-U. Flechsig, O. Korbut, S. B. Hocevar, S. Thongngamdee, B. Ogorevc, P. Grundler and J. Wang, Electroanalysis, 2002, 14, 192 CrossRef CAS.
- C. Laua, G.-U. Flechsig, P. Grundler and J. Wang, Anal. Chim. Acta, 2005, 554, 74 CrossRef.
- J. Wang, P. Grundler, G.-U. Flechsig, M. Jasinski, G. Rivas, E. Sahlin and J. L. L. Paz, Anal. Chem., 2000, 72, 3752 CrossRef CAS.
- J. Wang, G.-U. Flechsig, A. Erdem, O. Korbut and P. Grundler, Electroanalysis, 2004, 16, 928 CrossRef CAS.
- J. Widegren, E. Saurer, K. Marsh and J. Magee, J. Chem. Thermodyn., 2005, 37, 569 CrossRef CAS.
- M. Doyle, S. Choi and G. Proulx, J. Electrochem. Soc., 2000, 147, 34 CrossRef CAS.
- K. Kenten and R. McCreery, Anal. Chem., 1992, 64, 2518 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2008 |
Click here to see how this site uses Cookies. View our privacy policy here. 



 ). Other conditions as in Fig. 1.
). Other conditions as in Fig. 1.
 ), second scan (- - -), and fifth scan (—). Other conditions as in Fig. 1.
), second scan (- - -), and fifth scan (—). Other conditions as in Fig. 1.


