Diamond microelectrodes for in vitro electroanalytical measurements: current status and remaining challenges
Jinwoo
Park
a,
Veronika
Quaiserová-Mocko
a,
Bhavik Anil
Patel
d,
Martin
Novotný
a,
Aihua
Liu
a,
Xiaochun
Bian
b,
James J.
Galligan
bc and
Greg M.
Swain
*ac
aDepartment of Chemistry, Michigan State University, East Lansing, MI 48824, USA. E-mail: swain@chemistry.msu.edu
bDepartment of Pharmacology and Toxicology, Michigan State University, East Lansing, MI 48824, USA
cThe Neuroscience Program, Michigan State University, East Lansing, MI 48824, USA
dDepartment of Bioengineering, Imperial College London, London, UK SW7 2AZ
First published on 28th August 2007
Abstract
An emerging research field in electrochemistry today is the preparation, characterization and application of diamond microelectrodes for electroanalytical measurements in biological media. Interest in this new electrode material stems from its outstanding properties: (i) hardness, (ii) low, stable and pH-independent background current, (iii) morphological and microstructural stability over a wide range of potentials, (iv) good electrochemical responsiveness for multiple redox analytes without any conventional pre-treatment and (v) weak molecular adsorption of polar molecules that leads to a high level of resistance to response deactivation and electrode fouling. Diamond electrodes have advanced in recent years from being simply a scientific curiosity into a viable material for electroanalysis. In this article, we highlight the current state of progress by our laboratory and others on the preparation, study of the basic electrochemical properties, and application of this new type of microelectrode for in vitro electroanalytical measurements, and discuss some of the remaining challenges.
Introduction
Ideally, an electrochemical electrode for use in biological environments should possess biocompatibility, good sensitivity for the target analyte, rapid response time, a stable background current that is unaffected by changes in the surrounding solution environment (e.g. pH), and resistance to molecular adsorption, either by the redox molecule of interest and its reaction products or by other constituents in the sample. In the worst case, the molecular adsorption inhibits electron transfer leading to electrode deactivation (i.e. fouling). Carbon fiber, which has been routinely used for in vivo and in vitro electrochemical measurements since the 1970s,1–5 possesses an sp2-bonded carbon microstructure with an extended π-electron system and a surface chemistry that normally consists of various carbon–oxygen functional groups. Therefore, the surface is polar and hydrophilic; properties that generally promote strong adsorption of polar molecules viahydrogen bonding , dipole–dipole and or ion–dipole interactions. A problem that electrochemists have always faced when using sp2-bonded carbon electrodes is the fact that the microstructure and surface chemistry can be quite variable from fiber type-to-type and even from fiber-to-fiber of the same type. Additionally, the multitude of pre-treatment methods routinely used to activate the electrodes further exacerbates this problem of response variability because of the differential manner in which the electrode microstructure and surface chemistry are affected by each. As a consequence, measurements with carbon fiber can be plagued by poor response precision and irreversible molecular adsorption. Furthermore, the oxygen-rich surface chemistry makes the carbon fiber background response highly sensitive to the solution composition and pH. For biological measurements, it is desirable to have an electrode that does not require pre-treatment for activation and one that possesses a surface chemistry rendering it less prone to the molecular adsorption that leads to electrode fouling.Some 15 years ago now, researchers embarked on an effort to develop electrically-conducting diamond thin film for use as an electrode for electroanalytical measurements.6–10 Most of the work over the years has utilized thin films of boron-doped diamond supported on conducting planar substrates, like highly doped silicon. Only more recently has there been an evolution to microelectrode architectures. Originally, it was our hypothesis that the diamond electrode would exhibit superior response reproducibility and stability in electroanalytical measurements, as compared to sp2-bonded carbon electrodes (e.g. glassy carbon). Furthermore, it was hypothesized that the microstructure and surface chemistry would be more stable than that of sp2 carbon electrodes during the application of the anodic and cathodic potentials used for the amperometric detection of biological molecules of interest. Such behavior would lead to improved signal-to-background and signal-to-noise ratios. It turns out that both hypotheses were correct.10 Conductive diamond exhibits improved electrochemical performance because of its stable sp3-bonded carbon microstructure, and its relatively non-polar surface chemistry due to the absence of an extended π-electron system and carbon–oxygen functional groups (i.e a largely hydrogen surface termination at least after growth). Characteristic features of good quality diamond include a low and stable voltammetric background current, excellent response reproducibility, and resistance to deactivation and fouling via molecular adsorption. These properties make the diamond microelectrode attractive for measurements in complex biological environments.
Diamond deposition and microelectrode preparation
Electrically-conducting diamond thin film can be deposited on several substrates (e.g. quartz, platinum, tungsten, molybdenum, titanium, silicon and carbon) by either hot filament or microwave plasma -assisted chemical vapor deposition (CVD ) through well understood growth mechanisms.11–14 In our laboratory, boron-doped diamond thin film is deposited on a metal wire substrate using microwave plasma CVD (1.5 kW, 2.54 GHz, ASTeX, Woburn, MA).15–17 Platinum is used by our group for reasons given below. Both 76 µm and 40 µm diameter wires are routinely employed. In some cases, 25 and 10 µm diameter wires are used but coating such small wires is a more challenging task. Details of the growth have been reported elsewhere.15–18 Briefly, the preparation of diamond microelectrode involves three steps: shaping, cleaning and seeding the substrate wire; growth of boron-doped diamond from a CH4/H2/B2H6 source gas mixture; and post-growth annealing in an H2plasma . Poor nucleation on non-diamond substrates is the general rule so the cleaning and seeding process is essential for enhancing the initial nucleation density (i.e. the achievement of a continuous film in a short growth period). Post-growth annealing in an H2plasma is a key step to cool the sample in the presence of atomic hydrogen, thus maintaining an sp3-bonded carbon microstructure and ensuring full hydrogen surface termination.After deposition, the diamond-coated wire is attached to a longer copper wire with conductive silver epoxy for ease of electrical connection. The microelectrode is then insulated in some manner but it turns out that the process of insulating diamond to form a well-defined architecture is more complicated than for carbon fiber. There are a couple of reasons for this. First, it is difficult to seal diamond in a pulled glass capillary without the glass cracking due to the difference in thermal expansion coefficients for the two materials as well as the surface roughness. Second, diamond cannot be cut or polished to form microcylinder or disc geometry after insulation. In our laboratory, a procedure has been adopted that involves insulating the diamond with polypropylene to form a conically-shaped microelectrode (see refs 15–18 and also 19,20). This process involves inserting a diamond-coated platinum wire into a polypropylene pipet tip such that the diamond protrudes from the tip end by several hundred microns. A heat gun is used to first melt the polypropylene at the top of the microelectrode assembly, thereby securing the electrode in the polymer. The end of the pipet tip nearest the diamond electrode is then carefully heated in the filament of a glass capillary puller. This softens the polymer and causes it to form a thin, conformal layer around the morphologically-rough diamond.
Fig. 1 shows three different diamond microelectrode architectures that can be produced: bevelled, cylindrical and conical. All electrodes were formed using 76 µm diameter wire. The conically-shaped electrode, shown in the lowest image, is the architecture most often used by our group.15–18
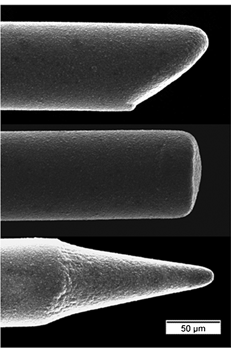 | ||
| Fig. 1 SEM images of different diamond microelectrode architectures that can be produced: bevelled, cylindrical and conical. The substrate was a platinum wire overcoated with a polycrystalline boron-doped diamond film. | ||
Fig. 2a shows SEM images of sharpened 76, 25 and 10 µm diameter platinum wires (labelled 1, 2 and 3, respectively) prior to diamond deposition. The wires were electrochemically etched to a sharp point with a radius of curvature of 100 nm, or so. Fig. 2b shows SEM images of the same wires after coating with a thin film of boron-doped diamond. Clearly, the diamond film possesses a rough and faceted polycrystalline morphology. The base diameters of some of the larger crystallites are on the order of 0.5–1 µm. Importantly, the images reveal that a continuous film can be formed over the entire substrate. As mentioned above, platinum is used as a substrate in our group for a couple of reasons. First, the metal is easily coated with well-adhering diamond and, second, it affords a very distinctive electrochemical signature, particularly on the cathodic end, if any of the underlying metal is exposed to the solution due to incomplete diamond coverage. Fig. 2c shows a schematic drawing of a conically-shaped diamond microelectrode after insulation.
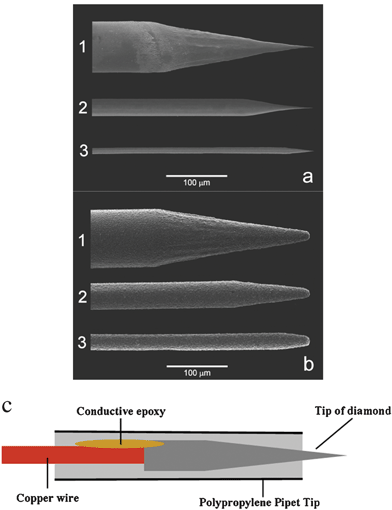 | ||
| Fig. 2 SEM images of (a) electrochemically sharpened platinum wires and (b) platinum wires covered with a polycrystalline diamond film. The wires were (1) 76 µm, (2) 25 µm and (3) 10 µm in diameter (magnification of 300×). The SEM images were reproduced from ref. 15 (copyright 2003, American Chemical Society). (c) Schematic of an insulated diamond microelectrode. | ||
Other groups have also reported methods for preparing diamond microelectrodes. For instance, Cooper et al., the first to report on the preparation of a diamond microelectrode, produced two types by hot filament CVD : deposition of an isolated crystal of conducting diamond on the tip of a sharpened tungsten wire, and formation of a conducting film over a conically-shaped substrate wire.21 Deposition of a micron-sized poly-crystal of diamond was accomplished by inserting a properly prepared tungsten wire into a stainless steel shaft such that only the wire tip is exposed to the reactants in the plasma . In the case of the continuous film, the entire end of the conically-shaped wire was coated. Both electrode types were insulated by sealing in a glass capillary. Sarada et al. used a similar approach to produce diamond tip electrodes by microwave plasma CVD .22 The end of a sharpened 50 µm diameter tungsten wire was coated with a few crystals of conducting diamond. The electrode was then inserted into a pulled glass capillary and back-filled with low viscosity epoxy. This insulated the electrode but also completely covered the diamond. Exposure of the diamond tip was then accomplished by lightly polishing the end to remove the polymer. Xie et al. selectively deposited boron-doped diamond onto the end of a sharpened tungsten wire using hot filament CVD .23 A 25 µm diameter tungsten wire was first pre-sealed into a quartz capillary and then polished to expose the end of the wire for diamond growth. After appropriate pre-treatment, boron-doped diamond growth occurred on the exposed end of the wire to produce a disk-shaped microelectrode that was about 25 µm in diameter. Basu et al. reported on the preparation of a conically-shaped diamond microelectrode by microwave plasma CVD .24 In their approach, the 130 µm diameter tungsten wire was first partially coated with a layer of insulating diamond. An uncoated end of the wire was then electrochemically etched into a conical shape. The etching had no effect on the portion of the wire insulated by the diamond. A boron-doped diamond layer was then deposited over the etched tip by microwave plasma CVD . A layer of negative photoresist was then applied to the entire diamond-coated wire for insulation, similar to the procedure described by Lambie et al.25 The electrode tip was exposed by curing the photoresist with UV light followed by chemical dissolution. Finally, Holt et al. described the fabrication of disc- and hemispherically-shaped boron-doped diamond ultramicroelectrodes 1–25 µm in diameter.26 Electrically-conducting diamond was deposited on sharpened and pre-treated tungsten wires by hot filament CVD . The microelectrodes were insulated with nail varnish to produce the hemispherical architecture. To produce the disc shape, the microelectrode was completely coated with epoxy followed by a light polish to expose the diamond tip. The authors used the ultramicroelectrodes in scanning electrochemical microscopy measurements to image the respiratory activity of immobilized Escherichia coli bacteria. A recent review by Herlambang et al.27 discusses the preparation of diamond microelectrodes and cites other electroanalytical applications (e.g. electrochemical detection coupled with separation science for environmental analysis) not covered in the present article.
Slow and fast scan cyclic voltammetric behavior of diamond microelectrodes
Investigation of the basic electrochemical properties of boron-doped diamond electrodes has been an active area of research over the years.6–10,28–30 Most of this work has focused on thin films of diamond deposited on planar substrates, like silicon; however, these same material properties are characteristic of the microelectrode architecture, as well. In electroanalytical measurements, diamond often provides significant improvements over conventional sp2 carbon electrodes (e.g. glassy carbon) in terms of linear dynamic range, limit of detection, response precision, and response stability. Diamond electrodes have advanced beyond a scientific curiosity as there are now commercial suppliers worldwide.9 As important as the commercial availability is the relatively low cost of the material.The basic voltammetric properties of diamond at slow scan rates (<1 V s−1) have been reported on in several recent papers.6–10,15–18,28–30 Hydrogen-terminated diamond microelectrodes exhibit lower and more featureless background voltammetric currents than do carbon fibers. The lower background current is due to a smaller double layer capacitance, ca. 10 µF cm−2, and the absence of redox-active and or ionizable surface carbon–oxygen functionalities that give rise to the pseudo-capacitance often seen with disordered sp2 carbon electrodes. This leads to improved signal-to-background ratios in electroanalytical measurements. Furthermore, the background voltammetric current is relatively insensitive to changes in solution pH at constant ionic strength.18
The basic voltammetric behavior of diamond toward several redox systems has been investigated.8–10,15–18 In general, for the complicated inner-sphere redox system, Fe(CN)6–3/–4, and the less complicated outer-sphere redox system, Ru(NH3)6+3/+2, the E1/2 or Ep/2 values are similar for diamond and carbon fiber microelectrodes.15 Typical E1/2 values of 283 ± 2 and –186 ± 2 mV vs. Ag/AgCl are seen for these two compounds, respectively, at the diamond microelectrode. For other redox systems, however, there are significant differences in the response of the two microelectrode types. For example, Table 1 shows a comparison of nominal oxidation E1/2 values for several catechols and catecholamines at the two microelectrode types. Clearly, the diamond microelectrode yields a reproducible response for all the redox analytes in terms of the peak position (ca. 1–4% RSD), but E1/2 values for all are some 200 mV more positive for diamond than for carbon fiber.8,15–17 This is a common observation for catecholamine electrochemistry at diamond. The more positive E1/2 is due to more sluggish electrode reaction kinetics and not ohmic resistance effects. While not fully understood, we have attributed the sluggish kinetics to weak molecular adsorption on the diamond surface. This is based on the fact that McCreery and DuVall have shown that catecholamines adsorb strongly on glassy carbon (an electrode with a disordered microstructure like that of many types of carbon fibers) and they have correlated the adsorption with more rapid electrode reaction kinetics.31 Thus far, we have detected no adsorption of these or similarly structured molecules on the diamond surface.32 Importantly, from an electroanalytical view, the more positive detection potential for these compounds at diamond does not appear to be a major drawback as the background current remains low and the physiochemical properties remain unchanged.
| Redox system | E 1/2 (diamond)/mV | E 1/2 (carbon fiber)/mV | Scan rate/mV s−1 |
|---|---|---|---|
a The solutions were 0.1 mM of the redox system in 0.1 M phosphate buffer (pH 7.2). Data are presented for the oxidation of each redox system. These data have been reproduced from ref. 18. Results are presented as mean ± std. dev. (n ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 3).
b
DOPAC = dihydroxyphenylacetic acid. 3).
b
DOPAC = dihydroxyphenylacetic acid.
|
|||
| Fe(CN)6–3/–4 | 286 ± 8 | 262 ± 4 | 25 |
| Ru(NH3)6+3/+2 | –196 ± 5 | –179 ± 3 | 25 |
| Dopamine | 421 ± 17 | 188 ± 3 | 20 |
| Norepinephrine | 456 ± 12 | 236 ± 10 | 20 |
| Epinephrine | 472 ± 18 | 266 ± 14 | 20 |
| Catechol | 543 ± 8 | 291 ± 12 | 20 |
| 4-Methylcatechol | 495 ± 6 | 241 ± 8 | 20 |
| DOPAC b | 614 ± 19 | 352 ± 11 | 20 |
Investigation of the diamond microelectrode response at high scan rate has also been performed. For example, Fig. 3a shows cyclic voltammetric i–E curves for 50 µM 4-methylcatechol in 0.1 M phosphate buffer (pH 7.4) (dashed line) and 0.1 M phosphate buffer only (solid line) at a potential sweep rate of 250 V s−1. The diamond was a conically-shaped electrode 40 µm in diameter. It can be seen that the faradaic current for the redox system is not easily distinguishable from the background current at this high scan rate. Fig. 3b shows the background-subtracted curve in which the faradaic peak currents are well resolved. The Epa is 712 mV and Epc is –292 mV, resulting in a ΔEp of 1004 mV. Other redox systems have been studied and the results will be reported in a future manuscript. Table 2 presents a comparison of Epa values for other redox-active bioanalytes at diamond and carbon fiber microelectrodes. These preliminary data indicate that the diamond microelectrode can be used for electroanalytical measurements at both low and high scan rates.
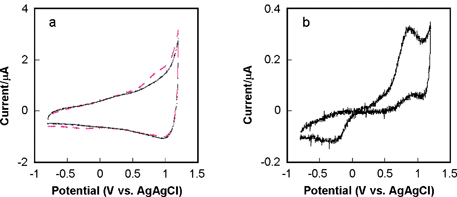 | ||
| Fig. 3 Fast scan cyclic voltammetric i–E curves for a 40 µm diameter diamond microelectrode at 250 V s−1. (a) Curves for 0.1 M phosphate buffer (pH 7.4) (solid line) and 50 µM 4-methylcatechol in 0.1 M phosphate buffer (pH 7.4) (dashed line). (b) Background-subtracted curve. | ||
| Redox system | E p a (diamond)/mV | E p a (carbon fiber)/mV | Scan rate/V s−1 |
|---|---|---|---|
a All solutions were 0.01 mM of the redox system, except for ascorbic acid and DOPAC (0.2 mM), in 0.1 M phosphate buffer (pH 7.4). Data are presented for the oxidation of each redox system. Results are presented as mean ± std. dev. (n ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 3).
b
DOPAC = dihydroxyphenylacetic acid. 3).
b
DOPAC = dihydroxyphenylacetic acid.
|
|||
| Dopamine | 829 ± 50 | 370 ± 20 | 250 |
| Norepinephrine | 902 ± 34 | 420 ± 20 | 250 |
| Serotonin | 635 ± 11 | 440 ± 10 | 250 |
| DOPAC b | 1076 ± 55 | 580 ± 150 | 250 |
| Ascorbic acid | 1012 ± 53 | 585 ± 80 | 250 |
In vitro electroanalytical measurements with the diamond microelectrode
Detection of norepinephrine (NE) release from sympathetic nerves innervating rat mesenteric arteries
A few reports have recently appeared in the literature describing the use of the diamond microelectrode for in vitro electroanalytical measurements.16,17,23,33–36 In a series of papers, our group has described the use of diamond to measure norepinephrine (NE) release from sympathetic nerves innervating smooth muscle cells in rat mesenteric arteries.16,18,33NE is a vasoconstrictor neurotransmitter released from sympathetic nerves that acts on the α1-adrenergicreceptors of smooth muscle cells to elicit a contractile response. Gaining a better understanding of the neurogenic control mechanisms of arterial and venous tone, and how these control mechanisms are altered in hypertension is the goal of this work. Two of the key findings from these initial studies are that the bare diamond microelectrode exhibited (i) a pH-independent voltammetric background current and (ii) superb resistance to deactivation and fouling during electrically-evoked NE release measurements.16,18 In contrast, a bare carbon fiber microelectrode exhibited (i) a pH-dependent background voltammetric current response with evidence for electroactive surface carbon–oxygen functional groups and (ii) deactivated irreversibly during exposure to tissue. For example, in continuous amperometric measurements of the electrically-evoked release of NE over a four-hour period, with the adipose and connective tissues removed from the blood vessel, the diamond microelectrode exhibited only a 7% NE oxidation current response attenuation while a carbon fiber lost 30% of the original signal.18 Most of the original signal for diamond could be regained by a simple soak in distilled isopropanol, while the original response for the carbon fiber could not be restored, which is consistent with much stronger molecular adsorption on the sp2 carbon surface. These results demonstrate that this new microelectrode is useful for sensitive, reproducible and stable in vitro electroanalytical measurements in complex biological environments.This work was followed up by a more extensive study that used continuous amperometry with a diamond microelectrode and video microscopy to investigate in vitro endogenous NE release simultaneously with the evoked contractile response of a rat mesenteric artery.33 Using these two techniques along with several drugs, the NE released at sympathetic neuroeffector junctions in the vicinity of the microelectrode was recorded as an oxidation current. Actually, the oxidation current for NE measured reflects a balance between the kinetics of release and clearance. Key to the amperometric measurement was the use of a diamond microelectrode because of the response sensitivity, reproducibility and stability it provided. NE release was elicited by electrical stimulation (40–70 V, 60 pulses, 0.3 ms pulse width) at frequencies between 1 and 60 Hz, with the maximum oxidation current seen at 20 Hz. Detection was accomplished at 0.80 V vs. Ag/AgCl. While continuous amperometry provides excellent temporal resolution of the current, the technique provides no qualitative information about the redox species giving rise to the current. Confirmation that the oxidation current was, in fact, associated with endogenous NE came through the use of several neuropharmacological agents. Tetrodotoxin (TTX, 0.3 µM), a voltage-dependent sodium channel antagonist that blocks nerve conduction, abolished both the oxidation current and the arterial constriction. The α2-adrenergic autoreceptor antagonist, yohimbine (1 µM), caused an increase in the oxidation current and the corresponding constriction. The addition of cocaine (10 µM), an antagonist that inhibits
neuronal
NE reuptake, caused both the oxidation current and the contractile response to increase. These results, combined with the fact that the hydrodynamic voltammetric E1/2 for endogenous NE was identical to that for a standard solution, confirmed that the oxidation current was due to NE and that this compound caused, at least in part, the contractile response. The results demonstrate that continuous amperometry monitoring of NE with a diamond microelectrode and video imaging of vascular tone allow real time local measurement of the temporal relationship between nerve-stimulated NE release and arterial constriction.
In vitro detection of adenosine in the central nervous system
Martin and co-workers recently demonstrated that adenosine, an important neuromodulator in the central nervous system, can be monitored in vitro in brain tissue using a diamond microelectrode.23 The authors fabricated their microelectrodes by a different procedure from the one described above. Tungsten wire, pre-sealed in quartz, was the substrate. Only the end of the wire was coated with a thin layer of conducting diamond, producing a disc-shaped architecture with a 25 µm diameter. Cyclic voltammetric measurements indicated a primary oxidation peak for a 10 nM solution of adenosine in artificial cerebrospinal fluid at 1.1 V vs. Ag/AgCl. The ability to detect this concentration, which is below the basal level in brain tissue, with a signal-to-noise ratio greater than three was a major finding from this work. The authors used fast scan voltammetry and 3-D false color plots of the resulting oxidation current as a function of potential and time to map adenosine concentrations in the PreBötzinger Complex of the rat brain stem. This site is rich in adenosine-releasing neurons, which control respiration. The results demonstrate that the diamond microelectrode can be used to stably map adenosine concentrations and provide evidence suggesting that the molecule modulates respiration.Serotonin (5-HT) monitoring in Aplysia californica
Halpern et al. reported preliminary results on neurodynamic studies of neurons from the marine mollusc, Aplysia californica.34 The authors used a 30 µm diameter diamond microdisc electrode to study feeding patterns in the animal model based on extracellular measurements of 5-hydroxytryptamine (5-HT, serotonin). Serotonin is a neuromodulator that regulates feeding patterns. Stable oxidation currents were recorded with the diamond microelectrode for electrically-evoked release of 5-HT from metacerebral cells. The key finding from this work was the fact that the diamond microelectrode can be used for both stimulation and recording of neurotransmitter release.Detection of serotonin (5-HT) released from enterochromaffin cells in the intestinal mucosa of the guinea pig
The diamond microelectrode has also successfully employed in in vitro measurement of 5-HT overflow from enterochromaffin (EC) cells in the intestinal mucosal layer of the guinea pig.35,365-HT is an important neurotransmitter and paracrine signalling molecule in the enteric nervous system, which is involved in the regulation of gastrointestinal (GI) tract function. There are two sources of 5-HT in the gut wall. First, 5-HT is contained in a population of neurons in the myenteric plexus, which is a collection of neurons and support cells that resides between the longitudinal and circular muscle layers of the GI tract. The myenteric plexus is the division of the enteric nervous system responsible for control of gastrointestinal motility. About 2% of myenteric neurons contain 5-HT. The second store of gastrointestinal 5-HT is contained in the EC cells that reside in the intestinal mucosal layer. More than 80% of whole body 5-HT content is contained in these cells. EC cells function as sensory transducers that respond to mechanical or chemical stimulation of the mucosa by releasing 5-HT. Released 5-HT then acts on mucosal endings of enteric primary afferent neurons and extrinsic primary afferent neurons to initiate motorreflexes and intestinal sensation.36 and refs cited therein5-HT released from EC cells is cleared by the serotonintransporter (SERT), which is expressed by enterocytes in the gastrointestinal mucosa. Alterations in 5-HT handling in the mucosa are likely associated with gastrointestinal motility disorders.In the measurements, continuous amperometry with the diamond microelectrode poised at a detection potential of 0.70 V vs. Ag/AgCl was used to measure 5-HT release as an oxidation current. 5-HT release from multiple EC cells in the vicinity of the microelectrode was elicited by both mechanical and electrical stimulation. Some minor electrode fouling, a common problem with the oxidative detection of 5-HT, was seen for diamond but the response stabilized rapidly. Both 5-HT and the paracrine hormone, melatonin, were detected in the extracellular solution. The 5-HT oxidation current increased in the presence of the serotonintransporter (SERT) inhibitor, fluoxetine (1 µM), providing evidence that the oxidation current was associated with 5-HT. Electrochemical monitoring of 5-HTin vitro or in vivo is often hindered by the tendency of oxidation products to form an insulating film on the carbon fiber surface causing electrode fouling and signal loss.37,38 The key finding from this work was that the diamond microelectrode is resistant to fouling and can be used for sensitive and stable measurement of 5-HT in the intestinal mucosa in vitro .35 Fig. 4 shows continuous amperometric i–t curves for 5-HT released from enterochromaffin cells. The currents were monitored with a 76 µm diameter, conically-shaped diamond microelectrode. Moving the diamond microelectrode to the tissue surface (a few hundreds of microns away) produces an increase in current from the background level of ca. 0.5 nA to ca. 1.5 nA. This is referred to as the over tissue current. The flowing bath causes 5-HT to be released from enterochromaffin cells at the mucosa surface. This leads to an increase in the extracellular5-HT concentration that is recorded with the microelectrode. Pressing the tissue near the recorded microelectrode elicits an increase in the current to ca. 2 nA. This is referred to as the touching tissue current. Ceasing the mechanical stimulation with the capillary causes the current to decrease to the over tissue level, and removing the recording microelectrode from the surface of the tissue causes the current to decrease back to the original baseline level. If the same measurements are performed in the presence of the SERT blocker fluoxetine, then both the over tissue and touching tissue currents increase by a factor of 2.5. With SERT blocked, there is less clearance of the extracellular5-HT and, therefore, a greater extracellular concentration during the course of the measurements.
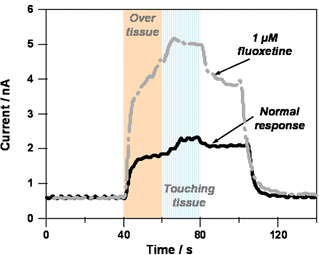 | ||
| Fig. 4 Continuous amperometric i–t curves for 5-HT release from enterochromaffin cells of the guinea pig intestine. The measurements were made with a diamond microelectrode poised at 700 mV vs. Ag/AgCl. Krebs' buffer, pH 7.4, was flowing through the bath at 2 mL min−1. | ||
The above study was followed up by a more comprehensive investigation of 5-HT release from EC cells in the intestinal mucosa of neonate and adult guinea pigs.36Serotonin oxidation current measurements with the diamond microelectrode in vitro were a key component of a comprehensive effort to understand physiological differences in 5-HT handling in the two age groups. Specifically, it was found that functional SERT expression is not yet developed and therefore, there is no mechanism for clearance of 5-HT released by EC cells in the neonatal intestine. The absence of functional SERT expression causes enhanced basal and mechanically-stimulated overflow of 5-HT from the mucosa. As 5-HT released from EC cells is an important stimulant of motorreflexes elicited by mucosal stimulation, it might be expected that these reflexes would not be fully-developed in the neonatal intestine where SERT expression is low and 5-HT availability is high. The findings suggest that maturation of the 5-HTsignalling system in the gut might be required for post-natal maturation of motility patterns as the neonate transitions to oral intake of nutrients.
Conclusions and remaining challenges
Work reported to date clearly demonstrates that diamond microelectrodes are useful for in vitro electroanalytical measurements, providing improved detection figures of merit over the commonly used carbon fiber. Specific material advantages include low, stable and pH-independent background current; excellent response sensitivity and reproducibility; and superb response stability (i.e. resistance to deactivation and fouling). At least for the measurements reported so far, the diamond microelectrode can be used bare, that is without any protective polymer coating, for measurements of electroactive neurotransmitters in tissue and extracellular fluid. Noteworthy, is the excellent response stability exhibited for serotonin; a notorious electrode fouler! The microelectrodes are hard and lubricious, properties that make them attractive for in vivo measurements.There are, however, some remaining challenges that must be overcome before this new electrode will find widespread use in neurophysiological and neuropharmacological studies. First, the electrodes must become commercially available to the general research community. At present, there are at least six companies worldwide that sell boron-doped diamond electrodes but these are offered as planar macro-sized electrodes, not in a microelectrode geometry. Right now, the microelectrodes are available on a limited basis from only those groups that can grow the diamond in-house.
Second, the size of the microelectrode must be reduced. Progress in this direction has already been made by Martin and co-workers23,34 and Holt et al.26 Much of our work to date has involved the use of conically-shaped electrodes that are large in diameter by microelectrode standards, ca. 76 µm. This has been simply a matter of convenience as larger diameter wires are easier to prepare and handle in our deposition scheme. Additional challenges arise with coating wires by microwave plasma CVD as their diameter gets smaller. We have also made some progress on this front though, as we now are regularly coating and using conically-shaped diamond microelectrodes that are ca. 40 µm in diameter. As revealed in the SEM images (see Fig. 1 and Fig. 2), microelectrodes of this diameter can be either bevelled, cylindrical or conical in shape. Our ultimate goal is to routinely prepare and use microelectrodes that are 10–20 µm in diameter. The materials science community, at large, could be helpful on this front by refining the methods used to prepare and coat small diameter wire substrates with electrically-conducting diamond (<20 µm diameter). Microarray-type sensors are also needed for the detection of analytes over a region of tissue in order to gain spatial information on how a network of cells is behaving. A recent paper by Lawrence et al. describes the preparation and electroanalytical application of diamond microelectrode arrays.39
Third, better methods for insulating the microelectrodes need to be developed. At present, we use polypropylene for this purpose. The microelectrodes can in fact be insulated with this polymer, but controlling the exposed electrode length is not possible with any great precision (±100–200 µm). Furthermore, this approach is laborious and produces conically-shaped microcylinder electrodes that are prohibitively large for any kind of in vivo measurement (overall millimeters in diameter). Basic research is needed to develop alternate methods for insulating these morphologically-rough microelectrodes in terms of the insulation material, its prolonged use in aqueous environments, and the ease/speed of application. Sealing these microelectrodes in glass, as is commonly done with carbon fiber, is not practical because of difference in thermal expansion coefficients for the two materials. A recent article by Lindsay and O'Hare reviewed the different materials and procedures used to insulate gold microelectrodes,40 and certainly these methods could be tried with diamond. Based on this review, progress by other groups, and work performed in our laboratory, insulation material candidates include (i) electrophoretic paints, (ii) Teflon-like polymers, (iii) allylphenol–phenol copolymer and (iv) cured photoresist . Additionally, one could use the selective deposition a layer of undoped diamond for insulating a doped diamond microelectrode.
Fourth, while diamond electrodes exhibit relatively fast electron transfer for some redox systems without any conventional pre-treatment, one class of redox molecules for which the electrode response is sluggish is catecholamines . Molecules, like dopamine, norepinephrine and serotonin, undergo relatively slow electrochemical reaction kinetics at hydrogen-terminated diamond surfaces. We have suggested that this is due to a lack of molecular adsorption on the diamond surface, which can lower the activation barrier for electron transfer and lead to enhanced rates. The weak adsorption is beneficial because it renders the electrode resistant to fouling. It is problematic because the electrode kinetics are sluggish for these redox molecules that undergo electron transfer mediated by some specific surface interaction. Research should be conducted to learn how to appropriately modify the surface chemistry of diamond in order to promote stronger adsorption of catecholamines as this may be a way to enhance the electron-transfer kinetics. For example, the electrochemically-assisted modification of diamond by substituted aryl diazonium salt molecules would be one strategy that could be followed.41
Acknowledgements
The work of the authors was made possible by generous support from the National Institutes of Health [R01 HL084258 (G. M. S.), P01 HL70687 (J. J. G.) and R01 DK57039 (J. J. G.)]. Contributions toward the preparation and characterization of diamond microelectrodes were also made by Hua Dong, Shihua Wang and Tom Loegel.References
- R. N. Adams, Anal. Chem., 1976, 48, 1128A.
- R. M. Wightman, Science, 1988, 40, 415 CrossRef.
- J. A. Stamford, J. Neurosci. Met., 1986, 17, 1 Search PubMed.
- K. T. Kawagoe, J. B. Zimmerman and R. M. Wightman, J. Neurosci. Met., 1993, 48, 225 Search PubMed.
- K. P. Troyer, M. Heien, B. J. Venton and R. M. Wightman, Curr. Opin. Chem. Biol., 2002, 6, 696 CrossRef CAS.
- Y. Pleskov, A. Sakharova, M. Krotova, L. Bouilov and B. Spitsyn, J. Electroanal. Chem. Interfacial Electrochem., 1987, 228, 19 CrossRef CAS.
- G. M. Swain and R. Ramesham, Anal. Chem., 1993, 65, 345 CrossRef CAS.
- M. C. Granger, M. Witek, J. Xu, J. Wang, M. Hupert, A. Hanks, M. D. Koppang, J. E. Butler, G. Lucazeau, M. Mermoux, J. W. Strojek and G. M. Swain, Anal. Chem., 2000, 72, 3793 CrossRef CAS.
- A. E. Fischer, Y. Show and G. M. Swain, Anal. Chem., 2004, 76, 2553 CrossRef CAS.
- G. M. Swain, ‘Electrically Conducting Diamond Thin Films’, in Electroanalytical Chemistry, ed. A. J. Bard and I. Rubinstein, Marcel Dekker, New York, 2004, vol. 22, p. 181 Search PubMed.
- J. C. Angus and C. C. Hayman, Science, 1988, 241, 913 CAS.
- P. E. Pehrsson, F. G. Celii and J. E. Butler, ‘Chemical Mechanisms of Diamond CVD’, in Diamond Films and Coatings, ed. R. F. Davis, Noyes Publications, Park Ridge, NJ, 1993, pp. 68–146 Search PubMed.
- J. E. Butler and R. L. Woodin, Phil. Trans. R. Soc. London, Ser. A, 1993, 342, 209 CrossRef CAS.
- Diamond Films Handbook, ed. J. Asmussen and D. K. Reinhard, Marcel Dekker, Inc., New York, 2002 Search PubMed.
- J. Cvačka, V. Quaiserová, J. Park, Y. Show, A. Muck, Jr. and G. M. Swain, Anal. Chem., 2003, 75, 2678 CrossRef CAS.
- J. Park, V. Quaiserová-Mocko, K. Pecková, J. J. Galligan, G. D. Fink and G. M. Swain, J. Electroanal. Chem., 2005, 583, 56 CrossRef CAS.
- G. W. Muna, V. Quaiserová-Mocko and G. M. Swain, Anal. Chem., 2005, 77, 6542 CrossRef CAS.
- J. Park, V. Quaiserová-Mocko, K. Pecková, J. J. Galligan, G. D. Fink and G. M. Swain, Diamond Relat. Mater., 2006, 15, 761 CrossRef CAS.
- Z. Zhou and S. Misler, J. Biol. Chem., 1996, 271, 270 CrossRef CAS.
- R. H. Chow, L. von Ruden and E. Neher, Nature, 1992, 356, 60 CrossRef CAS.
- J. B. Cooper, S. Pang, S. Albin, J. Zheng and R. M. Johnson, Anal. Chem., 1998, 70, 464 CrossRef CAS.
- B. V. Sarada, T. N. Rao, D. A. Tryk and A. Fujishima, J. Electrochem. Soc., 1999, 146, 1469 CrossRef CAS.
- S. Xie, G. Shafer, C. G. Wilson and H. B. Martin, Diamond Relat. Mater., 2006, 15, 225 CrossRef CAS.
- S. Basu, W. P. Kang, J. L. Davidson, B. K. Choi, A. B. Bonds and D. E. Cliffel, Diamond Relat. Mater., 2006, 15, 269 CrossRef CAS.
- B. A. Lambie, O. Orwar and S. G. Weber, Anal. Chem., 2006, 78, 5165 CrossRef CAS.
- K. B. Holt, J. Hu and J. S. Foord, Anal. Chem., 2007, 79, 2556 CrossRef CAS.
- O. Herlambang, B. V. Sarada, T. N. Rao and A. Fujishima, ‘Diamond Microelectrodes’, in Diamond Electrochemistry, ed. A. Fujishima, Elsevier, Amsterdam, Netherlands, 2005, pp. 396–413 Search PubMed.
- H. Martin, A. Argoitia, U. Landeau, A. B. Anderson and J. C. Angus, J. Electrochem. Soc., 1996, 143, L133 CAS.
- Yu. V. Pleskov, Russ. Chem. Rev., 1999, 68, 381 RSC.
- T. N. Rao, T. A. Ivandini, C. Terashima, B. V. Sarada and A. Fujishima, New Diamond Frontier Technol., 2003, 13, 79 Search PubMed.
- S. H. DuVall and R. L. McCreery, J. Am. Chem. Soc., 2000, 122, 6759 CrossRef CAS.
- J. Xu, Q. Chen and G. M. Swain, Anal. Chem., 1998, 70, 3146 CrossRef CAS.
- J. Park, J. J. Galligan, G. D. Fink and G. M. Swain, Anal. Chem., 2006, 78, 6756 CrossRef CAS.
- J. M. Halpern, S. Xie, G. P. Sutton, B. T. Higashikubo, C. A. Chestek, H. Liu, H. J. Chiel and H. B. Martin, Diamond Relat. Mater., 2006, 15, 183 CrossRef CAS.
- B. A. Patel, X. Bian, V. Quaiserová-Mocko, J. J. Galligan and G. M. Swain, Analyst, 2007, 132, 41 RSC.
- X. Bian, B. A. Patel, X. Dai, J. J. Galligan and G. M. Swain, Gastroenterology, 2007, 132, 2438 CrossRef CAS.
- J. E. Baur, E. W. Kristensen, L. J. May, D. J. Wiedemann and R. M. Wightman, Anal. Chem., 1988, 60, 1268 CrossRef CAS.
- B. P. Jackson, S. M. Dietz and R. M. Wightman, Anal. Chem., 1995, 67, 1115 CrossRef CAS.
- N. S. Lawrence, M. Pagels, A. Meredith, T. G. J. Jones, C. E. Hall, C. S. J. Pickles, H. P. Godfried, C. E. Banks, R. C. Compton and L. Jiang, Talanta, 2006, 69, 829 CrossRef CAS.
- A. E. Lindsay and D. O'Hare, Electrochim. Acta, 2006, 51, 6572 CrossRef CAS.
- A. Hermans, A. T. Seipel, C. E. Miller and R. M. Wightman, Langmuir, 2006, 22, 1964 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
