Synthesis of bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) derivatives functionalised with two, four or eight hydroxyl groups
Abstract
Short synthetic routes to a range of BEDT-TTF derivatives functionalised with two, four or eight hydroxyl groups are reported, of interest because of their potential for introducing hydrogen bonding between donor and anion into their radical cation salts. The cycloaddition of 1,3-dithiole-2,4,5-trithione with
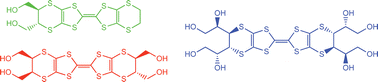
- This article is part of the themed collection: In memory of Peter Day

 Please wait while we load your content...
Please wait while we load your content...