Robust and stable pyrogallol[4]arene molecular capsules facilitated via an octanuclear zinc coordination belt†
Nicholas P.
Power
,
Scott J.
Dalgarno
and
Jerry L.
Atwood
*
Department of Chemistry, University of Missouri-Columbia, Columbia, MO 65211, USA. E-mail: Atwoodj@missouri.edu; Fax: (+1) 573-884-9606; Tel: (+1) 573-882-8374
First published on 6th December 2006
Abstract
The first metal coordinated pyrogallol[4]arene encapsulating dimer has been synthesised and shows high nuclearity with respect to ZnII, arranged as a polar coordination belt.
Molecular capsules, self-assembled from calix[4]arenes, resorcin[4]arenes and pyrogallol[4]arenes, have received increasing interest in recent years. These capsules tend to be either dimeric or hexameric in their assembly. Many of the dimers are comprised of monomers seamed together, either directly or with hydrogen bond donor/acceptor solvent molecules.1 The hexameric pyrogallol[4]arene spheroidal structures are held together by hydrogen bonding between monomers,2 while the hexameric resorcinol[4]arenes exhibit both direct and solvent bridged hydrogen bonding.3 Capsules based on metal coordinate covalent bonding have been encountered only with regard to CuII and GaIII hexameric spheres,4 and to PtII, PdII, CoII and AgI in covalently modified calix[4]arene and resorcin[4]arene dimers.5
Whilst ZnII has been used in complexing and dimerizing calix[4]arenes and resorcin[4]arenes, neither have previously been reported to form capsules in any successful manner.6 Herein, we report the synthesis and structure of [Zn8(C-propylpyrogallol[4]arene)2(pyridine)8 ⊂ pyridine] (1), a crystal structure that is complemented by MALDI-TOF MS analysis of the first metal coordinated pyrogallol[4]arene dimer capsule, which has high nuclearity with respect to ZnII and also encapsulates a pyridine molecule.
Of particular interest to this work is Rebek and co-workers’1aasymmetric [(C-ethylpyrogallol[4]arene)2·Cl–·7MeOH·H2O] capsule (2) (see Fig. 1a), which has a charged quinuclidine species as a guest, where the dimer is seamed by a complex polar belt of hydrogen bonds, and which is bridged via one water and seven methanol molecules. Of the 12 hydroxy groups from the respective pyrogallol[4]arenes, four from each ring are directly involved in intramolecular hydrogen bonding and the remaining eight connect with the opposing ring via hydrogen bonds through the bridging solvent molecules. On closer examination of this chiral conformation of the dimer, we envisaged the potential of the system to form a metal-seamed dimer complex. Given that the O–O (O–solvate–O) bond distance between opposing pyrogallolarenes is in the order of 3.44 Å, ZnII should comfortably sit in this environment.
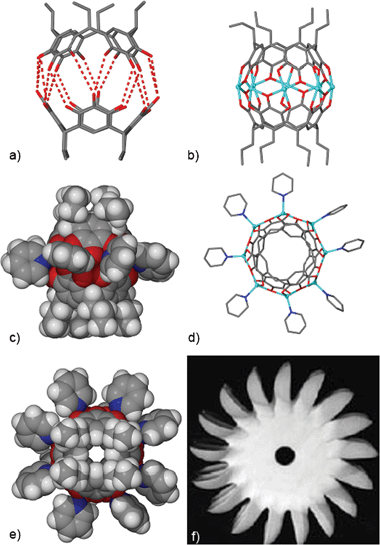 | ||
| Fig. 1 (a) Rebek’s capsule, showing the dimer’s asymmetry and the direct bond distances (in red) between the opposing phenoxy groups, and (b) its conversion to the zinc-seamed capsule (axial pyridines and hydrogens omitted for clarity). (c) A side-on view of the space-filling model of the octanuclear zinc dimer. (d) A top-down view of the X-ray structure of dimer 1, with alkyl chains and hydrogens omitted for clarity, and (e) a top-down view of the space-filling model of the octanuclear zinc dimer alongside (f) a Pelton wheel. | ||
Synthesis of the metal dimeric capsule was readily achieved by the combination of a zinc complex precursor, [ZnII(NO3)2Pyridine4·6H2O], and C-propylpyrogallol[4]arene in MeOH, with 5–10 min sonication affording a yellow powder. However, for the single crystal growth of 1, pyridine as a solvent was an essential requirement (see the experimental section), as other solvents or combinations thereof did not adequately yield crystals suitable for X-ray crystallography. The crystal structure‡ reveals the formation of a dimer, where the phenoxy groups are seamed by eight ZnII centres, which are axially coordinated by eight pyridines. The dimer was found to encapsulate a single pyridine molecule.
The MALDI-TOF MS analysis (see Fig. 2) shows two major peaks: 2027 Da, which represents an octanuclear zinc dimer encapsulating a pyridine molecule, but which is stripped of its axial pyridine ligands in the gas phase, and the smaller peak of 1951 Da, for an unoccupied dimer, which is also stripped of its pyridine ligands in the gas phase. Notably, this is not unusual for complexes which are weakly ligated. The isotopic mass distribution for the unoccupied dimer is offset by 3 mass units from the calculated distribution of 1948 Da, but for the occupied dimer, experimental and calculated values are in good agreement.
 | ||
| Fig. 2 MALDI-TOF MS spectra of the octanuclear zinc dimer minus the eight axial pyridines. The peaks at 2027 and 1951 Da represent the occupied and unoccupied dimers, respectively. Calculated isotopic distributions of the relative capsules are shown as an inset. | ||
Interestingly, there seems to be a significant population of the “empty” zinc dimer observed by MS analysis. It is feasible that the empty capsule is a consequence of the mechanism of zinc dimer formation rather than gas phase fragmentation and rearrangement. However, this does not appear to be supported by 1H NMR analysis of the zinc dimer, where the peaks for the encapsulated pyridine experience a significant upfield shift (Δδ = −3.55 (Hb), −2.86 (Hc) and −3.54 (Ha)) (see Fig. 3a) from that expected for pyridine in DMSO. Relative integration of the peaks of the weakly-ligated pyridines with respect to the encapsulated pyridine shows a clear 8 : 1 ratio, as one would expect for full population occupancy. Thus, the differences in the mass value of 3 units can best be explained by protonation in the gas phase during fragmentation and rearrangement. With respect to the 1H NMR values for the encapsulated pyridine, such large shift changes are not unusual for molecules when encapsulated. This is often a consequence of the orientation and movement restrictions enforced by the host on the entrapped guest, along with the proximity of the guest to the arene moiety of the host, reflecting possible C–H-π interactions.7
 | ||
| Fig. 3 (a) Pyridine1H NMR nomenclature. (b) Visual depiction of the chemical space available for a guest within the octanuclear zinc dimer 1 (axial pyridines omitted for clarity). | ||
The octanuclear ZnII complex is unique in that each of the zinc are pentacoordinated by an outer axial pyridine, and by four equatorial phenoxy groups, giving a distorted square pyramidal configuration, leading to an asymmetric conformation for the dimer, which is similar to the Rebek dimer. The zincs are singularly linked to each other via the central bridging oxygen from the pyrogallol sub-unit of the opposing pyrogallol[4]arenes to form a ring of eight zinc and eight oxygens (Fig. 1b). The two remaining coordination sites of each ZnII are filled by the ortho-phenoxy groups (relative to the central oxygen atom) of the opposing pyrogallol sub-units. The overall assembly is neutral, thus leaving four hydroxy groups from each pyrogallol[4]arene, which are directly involved in intramolecular hydrogen bonding with the ortho-phenoxide anion of the neighbouring pyrogallol sub-unit. The connectivity of the zinc with oxygen affords an octagonal perimetry to the shape of the molecule, and along with the axial coordination of the pyridines gives a “Pelton Wheel”8-like appearance to the molecule (see top-down view of the space-filling model in Fig. 1e). For the coordination of ZnII, there are few reports in the literature of such high nuclearity , and none where the metals are organized as a polar belt.9
The average O–O (O–Zn–O) distance for 1 is 2.92 Å, compared to the mean O–O (O–solvate–O) distance for 2 of 3.44 Å. This reflects the volume restrictions on the encapsulated pyridine. Space availability is further emphasised by the calculated volumes of 141 Å3 and 193 Å3 for the octanuclear zinc dimer 1 (see Fig. 3b for a visual depiction of the void space using a Connolly surface plot)10 and Rebek’s dimer 2, respectively.11Pyridine is estimated to occupy 75.1 Å3, thus, there are more severe limitations on the potential guests for the octanuclear zinc dimer in contrast to Rebek’s capsule, which has been noted to have contained such guests as the charged species quinuclidine, tropylium ion , tetramethylammonium and tetramethylphosphonium cations.1a
The crystallographic packing arrangement is clearly determined by interdigitation of the alkyl chains (Fig. 4). However, there is a C–H-π interaction (2.8 Å) between the pyridine and the aromatic region of neighbouring capsules. The head-to-tail arrangement for the neighbouring interdigitating ligated pyridines may also allow one to speculate a tentative π–π interaction between them.
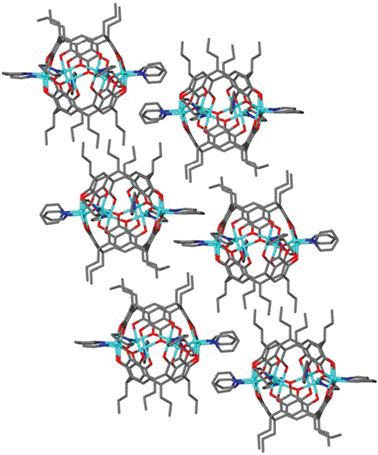 | ||
| Fig. 4 Packing arrangement in the crystal structure of 1 showing alkyl chain interdigitation. Hydrogen atoms are removed for clarity. | ||
In conclusion, this high nuclearity zinc pyrogallol[4]arene dimer capsuleis a successful follow-through on a model concept. This offers a spectrum of new and unusual opportunities for further investigations of metal chelation with molecular encapsulation, and where one can envisage chemistry on the sphere with respect to potential ligand exchange, ligand chemistry manipulation and coordination network construction in situ.
Experimental
Instrumentation
NMR spectra were acquired on a Bruker AXR500 (500 MHz). MALDI-TOF MS were acquired on a Voyager DE-PRO instrument.C-propylpyrogallol[4]arene was prepared by a known procedure.12 Synthesis of [Zn8(C-propylpyrogallol[4]arene)2(pyridine)8 ⊂ pyridine]: [ZnII(NO3)2Pyridine4·6H2O] (0.328 g, 0.555 mmol) was placed in flask along with C-propylpyrogallol[4]arene (0.10 g, 0.139 mmol) and MeOH (4 ml). The mixture was sonicated for 5–10 min to afford a yellow powder. The yellow solid was filtered and dried under vacuum. Yield: 0.160 g, 90%. 1H NMR (500 MHz, DMSO-d6, shifts relative to DMSO): δ = 8.58 (16 H, m), 7.87 (8 H, m), 7.47 (16 H, m), 6.50 (8 H, s), 4.99 (2 H, m, encapsulated pyridine), 4.49 (2 H, m, encapsulated pyridine), 4.21 (8 H, t, overlaying 1 H encapsulated pyridine), 2.20 (16 H, m), 1.36 (16 H, m) and 0.98 (24 H, m); 13C NMR: δ = 149.62, 145.0 (encapsulated pyridine), 137.7 (encapsulated pyridine), 137.58, 124.76, 124.39 (encapsulated pyridine), 107.36, 35.53, 32.64, 21.49 and 14.55.
For crystal growth, a solution of C-propylpyrogallol[4]arene (0.25 g, 0.35 mmol) in pyridine (2 ml) was prepared by sonication until a dark colour was achieved, reflecting the deprotonation of the pyrogallol[4]arene. To this was added a pre-sonicated solution of Zn(NO3)2·6H2O (0.416 g, 1.4 mmol) in pyridine (5–7 ml). The mixture was further sonicated for 1–2 h, and was then left to air-evaporate for several weeks in a fume hood.
Data for 1 was collected on a Bruker SMART 1000 CCD diffractometer.‡
For MALDI-TOF MS analysis, a crystal was dissolved and co-crystallized in a dithranol matrix (10 mg ml–1 in CHCl3). All analyses were conducted on a stainless steel MALDI plate. The Voyager DE-PRO instrument was operated in a reflector positive ion mode (20 kV acceleration, 150 ns delayed extraction, 600–4000 mass range). Spectra were acquired at a laser intensity of 1200 with 500 spectra per acquisition, and were accumulated to a total of 2000 spectra by moving the laser around the spot.
Acknowledgements
This work was supported by the National Science Foundation. Thanks go to Dr. Brian Mooney for MALDI-TOF MS analysis, performed at the Charles W. Gehrke Proteomics Center at UMC.References
- (a) A. Shivanyuk, J. C. Friese, S. Doring and J. Rebek, Jr, J. Org. Chem., 2003, 68, 6489 CrossRef; (b) A. Shivanyuk and J. Rebek, Jr, Chem. Commun., 2001, 2374 RSC; (c) A. Shivanyuk, K. Rissanen and E. Kolehmainen, Chem. Commun., 2001, 1107 Search PubMed; (d) K. Murayama and K. Aoki, Chem. Commun., 1998, 607 RSC; (e) K. N. Rose, L. J. Barbour, G. W. Orr and J. L. Atwood, Chem. Commun., 1998, 407 RSC.
- (a) J. L. Atwood, L. J. Barbour and A. Jerga, Chem. Commun., 2001, 2376 RSC; (b) J. L. Atwood, L. J. Barbour and A. Jerga, J. Supramol. Chem., 2001, 1, 131 CrossRef CAS; (c) T. Gerkensmeier, W. Iwanek, C. Agena, R. Frohlich, S. Kotila, C. Nather and J. Mattay, Eur. J. Org. Chem., 1999, 2257 CrossRef CAS.
- (a) O. Ugono and K. T. Holman, Chem. Commun., 2006, 2144 RSC; (b) J. L. Atwood, L. J. Barbour and A. Jerga, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4837 CrossRef CAS; (c) L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS.
- (a) R. M. McKinlay, P. K. Thallapally, G. W. V. Cave and J. L. Atwood, Angew. Chem., Int. Ed., 2005, 44, 5733 CrossRef CAS; (b) R. M. McKinlay, G. W. V. Cave and J. L. Atwood, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5944 CrossRef CAS.
- (a) P. Jacopozzi and E. Dalcanale, Angew. Chem., Int. Ed. Engl., 1997, 36, 613 CrossRef CAS; (b) O. D. Fox, N. K. Dalley and R. G. Harrison, J. Am. Chem. Soc., 1998, 120, 7111 CrossRef CAS; (c) O. D. Fox, N. K. Dalley and R. G. Harrison, Inorg. Chem., 1999, 38, 5860 CrossRef CAS; (d) O. D. Fox, J. F.-Y. Leung, N. K. Dalley and R. G. Harrison, Inorg. Chem., 2000, 39, 783 CrossRef CAS; (e) S. J. Park and J.-I. Hong, Chem. Commun., 2001, 1554 RSC; (f) Z. Zhong, A. Ikeda, M. Ayabe, S. Shinkai, S. Sakamoto and K. Yamaguchi, J. Org. Chem., 2001, 66, 1002 CrossRef CAS; (g) F. Fochi, P. Jacopozzi, E. Wegelius, K. Rissanen, P. Cozzini, E. Marastoni, E. Fisicaro, P. Manini, R. Fokkens and E. Dalcanale, J. Am. Chem. Soc., 2001, 123, 7539 CrossRef CAS; (h) F. A. Cotton, P. Lei, C. Lin, C. A. Murillo, X. Wang, S.-Y. Yu and Z.-X. Zhang, J. Am. Chem. Soc., 2004, 126, 1518 CrossRef CAS; (i) R. Pinalli, V. Cristini, V. Scottili, S. Geremia, M. Campagnolo, A. Caneschi and E. Dalcanale, J. Am. Chem. Soc., 2004, 126, 6516 CrossRef CAS; (j) K. Kobayashi, Y. Yamada, M. Yamanaka, Y. Sei and K. Yamaguchi, J. Am. Chem. Soc., 2004, 126, 13896 CrossRef CAS.
- (a) J. L. Atwood, P. C. Junk, S. M. Lawrence and C. L. Raston, Supramol. Chem., 1996, 7, 15 CrossRef CAS; (b) M. G. Gardiner, S. M. Lawrence, C. L. Raston, B. W. Skelton and A. H. White, Chem. Commun., 1996, 2491 RSC; (c) E. Bukhaltsev, I. Goldberg and A. Vigalok, Organometallics, 2005, 24, 5732 CrossRef CAS; (d) N. Kotzen, I. Goldberg and A. Vigalok, Inorg. Chem. Commun., 2005, 8, 1028 CrossRef CAS.
- R. G. Chapman and J. C. Sherman, J. Org. Chem., 2000, 65, 513 CrossRef CAS and references cited therein.
- A Pelton wheel is an efficient type of water turbine designed for power generation by the use of tangential water flow.
- (a) M. Tesmer, B. Muller and H. Vahrenkamp, Chem. Commun., 1997, 721 RSC; (b) M. Mikuriya, N. Tsuru, S. Ikemi and S. Ikenoue, Chem. Lett., 1998, 879 CrossRef CAS; (c) M. Mikuriya and K. Minowa, Inorg. Chem. Commun., 2000, 3, 227 CrossRef CAS; (d) J. Sanmartin, M. R. Bermejo, A. M. Garcia-Deibe and A. L. Llamas-Saiz, Chem. Commun., 2000, 795 RSC; (e) J. Sanmartin, M. R. Bermejo, A. M. Garcia-Deibe, I. M. Rivas and A. R. Fernandez, J. Chem. Soc., Dalton Trans., 2000, 4174 RSC.
- M. L. Connolly, Science, 1983, 221, 709 CrossRef CAS.
- L. J. Barbour, MCAVITY: Program for calculating the molecular volume of closed capsules, University of Missouri-Columbia, Columbia, MO, USA, 2003, http://www.x-seed.net/cavity.html. The maximum free volume within the cavities was calculated in the absence of guest Search PubMed.
- F. Weinelt and H.-J. Schneider, J. Org. Chem., 1991, 56, 5527 CrossRef CAS.
Footnotes |
| † The HTML version of this article has been enhanced with further colour images. |
‡ Crystal data: C145H147.50N13.50O26.50Zn8, Mr = 3026.23, 0.41 × 0.35 × 0.27 mm, monoclinic, space group P21/n, a = 16.449(2), b = 28.272(4), c = 29.144(4) Å, β = 90.614(3)°, V = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553(3) Å3, Z = 4, ρcalc = 1.483, λ(Mo-Kα) = 0.70930 Å, T = 173(2) K, µ = 1.469 mm, –196 553(3) Å3, Z = 4, ρcalc = 1.483, λ(Mo-Kα) = 0.70930 Å, T = 173(2) K, µ = 1.469 mm, –196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 reflections, 29 293 reflections, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 783 unique (19 783 unique (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 observed, Rint = 0.0354), R1 = 0.0567, wR2(all data) = 0.1872 for 1917 parameters and 21 restraints. CCDC 628358. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b615947h 564 observed, Rint = 0.0354), R1 = 0.0567, wR2(all data) = 0.1872 for 1917 parameters and 21 restraints. CCDC 628358. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b615947h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2007 |
