Complexation of C-methyl pyrogallarene with small quaternary and tertiary alkyl ammonium cations†‡
Antti
Åhman
,
Minna
Luostarinen
,
Kari
Rissanen
and
Maija
Nissinen
*
Nanoscience Center, Department of Chemistry, University of Jyväskylä, P.O. Box 35, FIN-40014 JYU, Finland. E-mail: majoni@cc.jyu.fi; Fax: +358 14 260 4756; Tel: +358 14 260 4242
First published on 27th October 2006
Abstract
Complexation properties of pyrogallarene 1 towards small quaternary and tertiary alkyl ammonium cations were studied in gas phase, solution and in solid state. In gas phase both dimeric capsules and monomeric 1 : 1 complexes of all cations 2a+–d+ are detected but only in the case of 2a+ is the abundance of the capsule form higher than the monomeric 1 : 1 complex. A similar trend is observed in NMR experiments, which reveal a favourable dimeric complex for 2a+ and a weaker dimeric complex for 2b+ but only monomeric complexes for 2c+ and 2d+. Also in solid state, 2a+ and 2b+ form capsules when crystallized from MeOH while 2c+ and 2d+ form dimeric 1 : 1 complexes. As a reference, hetero-conformational dimeric capsule EtOH@12 obtained from the crystallization without any guest, is reported. The capsule consists of two directly hydrogen-bonded pyrogallarenes 1, one in boat conformation and the other in crown conformation, and encloses a molecule of ethanol.
Introduction
Hydrogen-bonded capsules1–3 as well as open inclusion complexes2c,4 of artificial receptors have enhanced our current understanding of chemical and biological recognition and self-assembly. For example, size and stereoselective inclusion5 of guest molecules and catalysis of chemical reactions6 within a capsule give valuable information of the function of the receptor molecules and biocatalysis.One family among these capsular and open inclusion complexes is based on resorcinarenes and pyrogallarenes 1, which are versatile and readily available host compounds.7 Pyrogallarenes and resorcinarenes are usually all-cis configured (r-ccc), bowl-shaped cyclic tetramers, which are, due to their favorable shape, π-basic cavity and ability to form multiple hydrogen bonds, suitable for the complexation both in dimeric and open 1 : 1 fashion2,3,8,9 as well as for the formation of larger complexation assemblies, such as hexameric capsules10,11 or nanotubes.12,13
Previous studies in solid state reveal that two halves of dimeric resorcinarene and pyrogallarene capsules are without exception hydrogen bonded to each other via mediating solvent molecules or in some cases also via spherical halide anions (Cl− or Br−).2,3,8 CH⋯π and cation–π interactions act as the driving force for the capsule formation since several weak interactions between the aromatic parts of the host and the carbon atoms of the guest are detected. Previous studies emphasize the significance of the cation size for encapsulation, while the effect of the counter anion and solvent is evident for the solid state assembly in general.2c,d
In order to compare the complexation properties of pyrogallarenes 1 with earlier results of resorcinarenes2,12 we studied the complexation of small alkyl ammonium cations 2a+–d+ with pyrogallarene 1 in solid state, solution and in gas phase (Scheme 1).
 | ||
| Scheme 1 Structural formula of C-methyl pyrogallarene 1 and alkyl ammonium cations 2a+–d+. | ||
Results and discussion
Single crystal X-ray diffraction analysis
Slow crystallization of pyrogallarene 1 with tetramethyl ammoniun chloride (2aCl) from methanol solution in 2 : 1 ratio gives single crystals of a dimeric capsule enclosing the cation with the total composition of 2a+@1·1−0.5·0.5Cl−·8MeOH (Fig. 1). The composition of the crystal is somewhat unusual, since only a half of the chloride anion is found in the asymmetric unit and, additionally, one of the hydroxyls of the other pyrogallarene host is deprotonated with a disorder between protonated OH and deprotonated O−. The deprotonation is proved by the shorter hydrogen bonding distance of the deprotonated hydroxyl group (2.63 Å vs. average of 2.70 Å) and the lack of electron density indicating hydrogen in that particular hydroxyl group. This result is in line with the earlier observation with C-ethyl pyrogallarene·2a+ capsule, in which one of the pyrogallarene hydroxyl groups was similarly deprotonated.3 | ||
| Fig. 1 X-ray crystal structure of the dimeric capsule of pyrogallarene 1 with 2a+ presented as VDW/stick presentation. The capsule halves are linked by hydrogen bonds via four methanol molecules. Methanol molecules and the anion, which are not linking pyrogallarenes, are omitted for clarity. | ||
Both capsule-forming molecules of 1 adopt fairly symmetrical crown conformation stabilized by four intramolecular hydrogen bonds in each host molecule. The cation, which is located at the centre of the capsule, interacts with the host molecules via CH⋯π interactions, the shortest distances between the cation carbons (CH3) and centers of the closest aromatic rings of 1 being 3.70–3.88 Å (Table 1). Capsule halves are in staggered orientation and hydrogen bonded via four mediating methanol molecules. Additionally, and differing from resorcinarene capsules2,8 there is also one direct hydrogen bond of length 3.02(1) Å between the pyrogallarenes owing to the additional hydroxyl group in the 2-position of pyrogallol ring which is in suitable spatial position for direct hydrogen bonding. Despite the direct hydrogen bond the dimensions of the capsule do not differ significantly from the respective values of the resorcinarene capsules,2 for example, the distance between the planes of the methine bridges of the opposing hosts is 8.67 Å in pyrogallarene capsule and varies from 8.26 to 8.99 Å in resorcinarene capsules.
| Structure/Distance [Å] | 2a +@1·1−0.5·0.5 Cl− | 1·2c+Cl− | 1·2d+Cl− | EtOH@12 |
|---|---|---|---|---|
| a Distances given as O⋯O or O⋯X− distances. b Defined as a distance between the planes formed by the methine bridges of 1. c Distance between the centroids of the opposite aromatic rings of 1. | ||||
| Intramolecular O–H⋯Oa | 2.63(1)–2.75(2) | 2.624(4)–2.709(4) | 2.658(5)–2.748(6) | 2.732(5)–2.761(5) |
| Intermolecular O–H⋯Oa | 3.02(1) | 2.964(5) | 2.858(5) | 2.954(5)–3.102(5) |
| Intermolecular O–H⋯O to solventa | 2.67(1)–2.75(1) | 2.822(4) | — | — |
| Intermolecular O–H⋯X− to aniona | — | 3.057(3)–3.072(3) | 3.080(4)–3.272(7) | — |
| N–H⋯O (cation to 1) | — | 2.780(5)–3.059(5) | 2.846(5)–2.986(6) | — |
| CH⋯π (the closest cation C to the closest aromatic ring centroid of 1) | 3.70 | 3.34 | 3.60 | 3.72 |
| Capsule heightb | 8.67 | — | — | 8.77 |
| Capsule/molecule widthc | 6.70/6.97 for molecule A; 6.72/6.84 for molecule B | 6.72/6.91 | 6.72/6.92 | 5.13 (boat) 6.83/6.88 (crown) |
| Dihedral angles between the adjacent pyrogallol rings/° | 67.0/79.5 for molecule A; 65.8/73.5 for molecule B | 65.8/74.0 | 67.9/75.5 | 6.6/187.1 (boat) 70.4/77.9 (crown) |
The packing of the capsules of 2a+@1·1−0.5·0.5Cl− is shown in Fig. 2. The capsules form ribbons, in which the lower rim methyl groups of the pyrogallarene are facing the methyl groups of the adjacent pyrogallarene and the ribbons form crossing layers which are at 65° angle to each other.
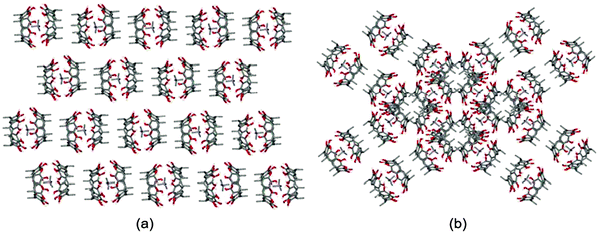 | ||
| Fig. 2 Crystal packing of capsules of 1 with 2a+ showing (a) layers formed by sequential pyrogallarene capsules (b) crossing layers at 65° angle to each other. Solvent molecules, anions and hydrogen atoms attached to carbons are omitted for clarity. | ||
Several attempts to obtain solid state structure of pyrogallarene 1 with 2b+Cl− from methanol solution in 2 : 1 ratio resulted in bad quality crystals and hence the quality of the data was also poor and the results of a low quality structure can only be considered preliminary.14 However, the low quality structure clearly reveals that also in this case pyrogallarene 1 forms a dimeric complex where cation 2b+ is located inside the capsule. This result is in line with previously reported results of resorcinarene capsules with 2b+.8a
Crystallization of 1 with trimethyl ammonium chloride, 2cCl, from methanol and triethyl ammonium chloride, 2dCl, from methanol–ethanol solution in 2 : 1 molar ratio afforded remarkably similar structures of 1 : 1 inclusion complex, 1·2c+Cl−·2MeOH and 1·2d+Cl−·MeOH·EtOH, respectively (Fig. 3). The position of the cation inside the cavity is similar in both cases. One of the alkyl arms of the cation is situated deeply in the cavity of 1 interacting via CH⋯π interactions with the aromatic rings of the host. The difference between the two structures arises from the interactions of the two remaining alkyl chains. With 2c+ also the other two methyl arms interact with the aromatic walls of the pyrogallarene, but with 2d+ two other ethyl chains show no interactions with the host.
 | ||
| Fig. 3 X-ray crystal structures of dimeric assemblies of (a) 2(1·2c+·Cl−) and (b) 2(1·2d+·Cl−) connected via hydrogen bonds. Solvent molecules, which are not linking host molecules, are omitted for clarity. | ||
The inspection of the crystal packing of 1·2c+ and 1·2d+ reveals that two 1 : 1 complexes are connected to a dimeric assembly by hydrogen bonds from cation N–H to two hydroxyl groups of the adjacent pyrogallarene, two direct hydrogen bonds between pyrogallarenes and hydrogen bonds mediated by chloride (Fig. 3). In addition, the dimer of 1·2c+ shows altogether four methanol mediated hydrogen bonds, while no solvent mediated bonds are observed in 1·2d+.
The probable reason for the formation of this shifted-type of capsule-like dimer instead of typical directly facing capsule is the non-spherical shape and hydrogen-bonding ability of the cation, which clearly directs the orientation of host molecules in respect to each other.
The major difference between the two complexes arises from the crystal packing of dimeric assemblies (Fig. 4). 2(1·2c+·Cl−) dimers form layers in which they are hydrogen bonded to each other via chloride anions, while 2(1·2d+·Cl−) dimers are placed next to each other with the methyl groups of pyrogallarenes pointing toward each other and forming dimer ribbons running in two directions throughout the crystal.
 | ||
| Fig. 4 Crystal packing of (a) 2(1·2c+·Cl−) and (b) 2(1·2d+·Cl−). Solvent molecules and hydrogen atoms attached to carbons are omitted for clarity. | ||
To get a reference how the host 1 alone behaves in similar crystallization conditions, we crystallized 1 from small polar alcohols and alcohol–water mixtures. To our great surprise the crystallization of 1 from ethanol–water mixture gave unique, directly hydrogen-bonded hetero-conformational capsule where one pyrogallarene 1 is in crown and the other in boat conformation. The diminished cavity is occupied by a disordered ethanol molecule, which is hydrogen bonded to one of the pyrogallarene hydroxyls of the molecule in boat conformation (Fig. 5). To our knowledge this is the very first example of the simultaneous crystallization of two different conformations of resorcinarene-type molecules in the same crystal, as well as the first example of hetero-conformational capsule, which, in addition, is the first truly directly hydrogen-bonded resorcinarene-type dimeric capsule without any mediating solvent or anionic species.
 | ||
| Fig. 5 X-ray crystal structure of directly hydrogen-bonded heterodimeric capsule EtOH@12 shown as WDV/stick presentation, two views. Solvent molecules, except for ethanol inclusion, are omitted for clarity. | ||
The formation of this type of novel capsule relates to the optimal hydrogen bonding and effective crystal packing. The change of the conformation of one of the hosts is needed to fulfill these requirements and therefore all four intramolecular hydrogen bonds of other pyrogallarene are replaced by intermolecular hydrogen bonds and the conformation changes from crown to boat. Similar types of guest induced conformational change caused by the replacement of intramolecular hydrogen bonds by intermolecular interactions to guests have earlier been detected in open inclusion complexes.15
The molecule in boat conformation is described by the two parallel pyrogallol rings (6.5° dihedral angle and 5.13 Å centroid-to-centroid distance) while the other two opposing rings are nearly coplanar and bent towards the lower rim of the pyrogallarene core. The other capsule forming pyrogallarene molecule remains in crown conformation and is, as usual, stabilized by four intramolecular hydrogen bonds of length 2.73–2.76 Å. Its cone shaped cavity also efficiently nests the molecule of ethanol.
Investigation of the crystal packing reveals that pyrogallarenes in boat conformation are connected to each other via multiple hydrogen bonds and π⋯π interactions (closest C to C distance 3.47 Å) so that capsules form very tight continuous chains emphasizing the significance of the closest packing and stabilising effect of π-stacking interactions in addition to hydrogen bonding (Fig. 6). The chains are further interloced to a zipper-like packing motif.
 | ||
| Fig. 6 Crystal packing of EtOH@12 showing hydrogen bonded and π-stacked continuous chains, which further form zipper-like motifs, two views. Solvent molecules and hydrogen atoms of C are omitted for clarity. | ||
The other important reason for the unusual formation of a hetero-conformational capsule can be reasoned by comparing the sizes and shapes of the guests. Roughly spherical and symmetrical guest 2a+ fits perfectly into the crown-shaped cavity of host 1 and being symmetrical, it is able to withdraw yet another host for dimer formation. More ellipsoidal shape and hydrogen bonding ability of 2c+ and 2d+, on the other hand, seems to induce the formation of a paired assembly. In hetero-conformational capsule EtOH@12 the shape of the guest is roughly linear and the size is smaller than the size of the studied cations, so a true directly hydrogen-bonded capsule can form and no mediating hydrogen bonds are needed at all. It is also noteworthy to realize that ethanol as a neutral molecule is still a good enough guest for encapsulation and no reinforcing cationic interactions are required in this case.
NMR spectroscopic studies
At 213 K exchange of components became slow on the NMR time scale and the integration of the signals at different host–guest ratios clearly shows that cation 2a+ is complexed by 1 in 1 : 2 ratio (Fig. 7). This observation can either mean the formation dimeric (2 : 1 host–guest ratio) or hexameric (6 : 3 host–guest ratio) capsule. However, hexameric capsules in this case are very unlikely, since methanol solution have been reported to be too polar solvent for hexameric pyrogallarene capsules.10d Additionally, small alkyl ammonium cations are known to form 1 : 1 and 1 : 2 complexes with high affinity,2,3,8,9 and the formation of hexameric 6 : 3 would, as a matter of fact, mean three positively charged cations packed in a small volume with high charge density.
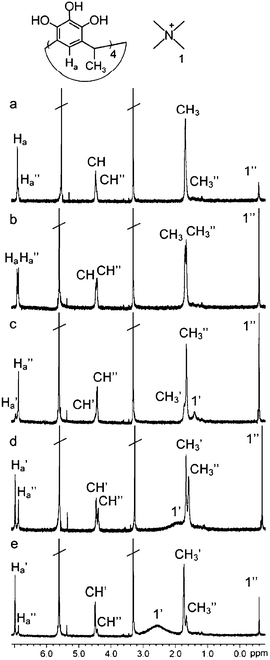 | ||
| Fig. 7 1H NMR spectra of 2aCl and 1 in methanol-d4 at 213 K. No apostrophe = free host or guest, ′ = host or guest in 1 : 1 complex and ″ = host or guest in capsule. 2aCl: 1; (a) 1 : 8, (b) 1 : 4, (c) 1 : 2, (d) 1 : 1, (e) 2 : 1. | ||
In the presence of 4 equiv. of 1 per 1 equiv. of 2a+, the signals of both free and complexed pyrogallarene are found in a 1 : 1 ratio (Fig. 7b) and a strong up-field shift of the protons of 2a+ (Δδ = −3.79 ppm) indicates dimeric capsular structure. Addition of salt (2a+ : 1 > 1 : 2) leads to the appearance of an open inclusion 1 : 1 complex, whose components are in fast exchange, and to the reduction of the amount of the dimeric capsule (Fig. 7c–e), which indicates that the 1 : 1 complex is favored when an excess of the guest is used.
As expected on the basis of solid state and gas phase studies pyrogallarene 1 can form dimeric capsules also with 2b+ in methanol solution, but not as willingly as 2a+ and hence 1 : 1 stoichiometry is favored. The addition of 2bCl to the solution of 1 in methanol-d4 at 303 K did not change 1H NMR spectrum of pyrogallarene 1 and weaker induced shifts for the resonances of the methylene and methyl protons of 2b+ (Δδ = −0.92 ppm and −0.78 ppm, respectively) are observed than with 2a+. At 213 K the exchange of components is still fast and hence the dilution experiments were made at 195 K, where the exchange becomes slow at NMR time scale.
In the presence of 4 equiv. of 1 per 1 equiv. of 2b+, the shoulder of the signal of the methine bridge and broadening of the aromatic proton signal indicate the presence of free and complexed pyrogallarene. Strong upfield shift of the protons of 2b+ (Δδ = −3.69 and −1.75 ppm), which are comparable to the shifts of 2a+, indicate the formation of a dimeric capsule also in this case (Fig. 8a). When the amount of 1 is increased in respect to the amount of 2b+ (Fig. 8b; 2 : 1 ratio) two singlets for methine bridge, a slight broadening of the signal of the aromatic proton and appearance of guest peaks at 0.22 and 1.73 ppm (Δδ = −1.52 and −0.98 ppm) indicate also the presence of 1 : 1 complex. The ratio of 1 : 1 complex and capsule is 2 : 1 which indicates that capsule formation is weaker than in the case of 2a+ which gave only capsule form in the same host-to-guest ratio. Further addition of the salt (2a+ : 1 > 1 : 1) led to the appearance of the signal of the free salt and disappearance of signal of dimeric capsule (Fig. 8c–e). This difference in NMR behavior between 2a+ and 2b+ hints that 2b+ is indeed already too large to be efficiently encapsulated inside two pyrogallarene hosts.
 | ||
| Fig. 8 1H NMR spectra (500 MHz) of 2bCl and 1 in methanol-d4 at 195 K. * = complexed host, ′ = guest in 1 : 1 complex and ″ = guest inside capsule. 2bCl : 1; (a) 1 : 4, (b) 1 : 2, (c) 1 : 1, (d) 2 : 1, (e) 4 : 1. | ||
Trimethyl ammonium 2c+ and triethyl ammonium 2d+ cations did not show any signal for dimeric capsules with 1 in methanol-d4 and only the formation of 1 : 1 complex was observed. At 213 K the spectrum of a methanol-d4 solution containing 1 and 2c+ in a 4 : 1 molar ratio reveals upfield shift for the methyl (Δδ = −2.12 ppm which is approximately half of the corresponding shift of the 2a+) protons of the cation while there was no change in the spectrum of 1. In the same conditions up-field shifts for the methyl and methylene protons of the 2d+ cation are −1.20 ppm and −1.63 ppm, respectively. This indicates the formation of an open monomeric complex that is in fast exchange with its components.
Mass spectrometric studies
Mass spectra of pyrogallarene–alkyl ammonium cation mixtures in 1 : 1 ratio in methanol gave peaks representing both 1 : 1 and 2 : 1 pyrogallarene–ammonium ion complexes, which is in line with the mass spectrometric studies of resorcinarene complexes.2b When the ratio of pyrogallarene to ammonium ion was increased to 2 : 1 the abundance of capsule in respect to monomeric complex was increased as well, which together with NMR titration results and former investigation with resorcinarenes in gas phase16 indicates that the ratio of the guest and host controls the formation of dimeric capsules. Fig. 9a presents the mass spectrum of 2 equiv. of 1 mixed with 1 equiv. of 2aCl. The peak at m/z 682 corresponds to the monomeric complex [1·2a]+ and m/z 1290 to the dimeric capsule [2a@12]+. Only in the case of 2a+ was the amount of the capsule form higher than that of the monomeric complex, which is in good agreement with X-ray structural and NMR results. Respectively, other spectra of 2 : 1 pyrogallarene–ammonium ion ratio are shown in Fig. 9b–d.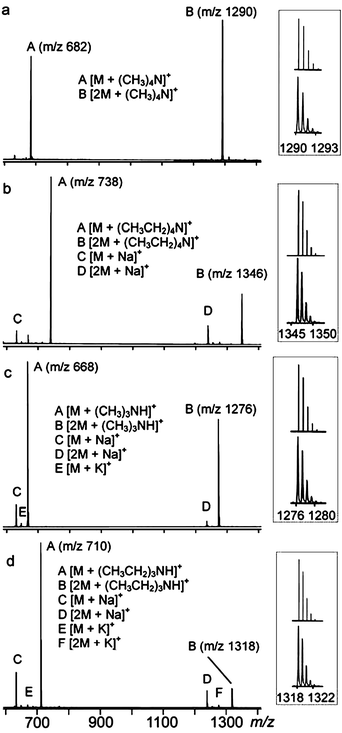 | ||
| Fig. 9 ESI spectra of pyrogallarene 1 with alkyl ammonium ion 2a+–d+ in 2 : 1 ratio. The inserts show the experimental isotope patterns (curves) of dimeric capsules (B) and those calculated on the basis of the natural abundance (line spectra), which agree well with each other. | ||
The competition studies between the equimolar amounts of cations 2a+–d+ and host 1 are shown in Fig. 10a. Monomeric complexes form with all cations (1·2a+ > 1·2b+ ≈ 1·2c+ > 1·2d+) and dimeric capsules were detected with cations 2a+, 2b+ and 2c+, but not for 2d+ (2a+@12 ≫ 2b+@12 ≈ 2c+@12). The competition experiment reveals that host 1 has the highest affinity for cation 2a+ both as a monomeric complex and in capsule form. The lowest affinity for monomeric complex was detected with 2d+ and in these conditions it did not form capsules at all. The abundances of 1 : 1 complexes and capsules of 2b+ and 2c+ were almost equal which corresponds to the results of 1H NMR experiments in methanol solutions at 303 K where upfield shifts of monomeric complexes of 2b+ and 2c+ with 1 were same order of magnitude. Also the competition studies support the earlier results with resorcinarenes.2b
 | ||
| Fig. 10 ESI spectrum of pyrogallarene 1 with alkyl ammonium ions 2a+–d+ (a) and relative abundance of dimeric capsules in soft (b) and harder (c) ionization conditions. | ||
Relative stability of the capsules was also studied in methanol solution with equimolar amounts of cations 2a+–d+ and host 1. Different voltages of the sample cone were used to induce ion source fragmentation. Increasing sample cone from 15 V to 35 V led to the reduction of the 2b+@12 relative to 2a+@12 while the relative abundancies of 2c+@12 and 2a+@12 stayed almost unchanged (Fig. 10b and c). This is line with NMR results which show that dimeric capsule 2a+@12 is more stable than 2b+@12 in methanol-d4 solution due to the more suitable size of the guest.
Conclusions
In solid state pyrogallarene 1 forms dimeric capsules with tetraalkyl ammonium cations 2a+ and 2b+ which is in accordance with the earlier reported capsules of closely related resorcinarenes.2,3,8a,b Capsule-like dimeric assemblies of 2c+ and 2d+ are more open than tetraalkyl ammonium capsules and their formation is also governed by hydrogen bonding ability of the guest.NMR titration experiments show that both 2a+ and 2b+ form dimeric capsules also in methanol-d4 solution, but capsule formation of 2b+ is considerably weaker than that of 2a+, which emphasizes the sensitivity of the guest size for the efficient encapsulation. The titration experiments of 2c+ and 2d+ showed only the formation of 1 : 1 complexes.
In gas phase both capsule and 1 : 1 complex were detected for all cations 2a+–d+ but only in the case of 2a+ was the abundance of capsule form higher than that of the monomeric 1 : 1 complex. The competition studies between the equimolar amounts of cations 2a+–d+ in gas phase show that host 1 has the highest affinity for cation 2a+ both as a monomeric complex and especially in capsule form.
In general it can be concluded on the basis of NMR studies that the affinity of the pyrogallarene 1 towards the capsule formation especially with 2a+ is greater than that of earlier much studied resorcinarenes.2 Otherwise solid state and gas phase behavior of pyrogallarene 1 is comparable to the behavior of the resorcinarenes emphasizing the significance of the cation size for the type of the complex formed. These results are complemented by the novel hetero-conformational capsule, which underlines the versatility of pyrogallarene 1 as a building block for crystal engineering studies and brings out the unusual effect of the neutral, roughly linear guest as a template for encapsultion.
Experimental
Single crystal X-ray diffraction
Data were recorded on a Nonius Kappa CCD (2a+@1·1−0.5, 1·2c+ and 1·2d+) or Nonius Kappa Apex II (EtOH@12) diffractometer using graphite monochromatized MoKα radiation [λ(MoKα) = 0.710 73 Å] and temperature of 173.0 ± 0.1 K. The data were processed with Denzo-SMN v0.97.638.17 The structures were solved by direct methods (SHELXS-9718) and refinements based on F2, were made by full-matrix least-squares techniques (SHELXL-9719). The hydrogen atoms were calculated to their idealized positions with isotropic temperature factors (1.2 or 1.5 times the C temperature factor) and refined as riding atoms.In the structure 2a+@1·1−0.5·0.5Cl− only a half of the chloride anion was found in the asymmetric unit and it was disordered with a methanol molecule (occupancies 0.5 : 0.5). Additionally, one of the hydroxyls of the other host was partially deprotonated with a disorder between protonated OH and deprotonated O−. Five methanol molecules were refined isotropically and four of them were disordered as follows: MeOH 1: occupancy factor 0.75 and carbon disordered over two positions with site occupancy factors 0.35 : 0.40; MeOH 2: disordered over two positions with occupancies of 0.4 : 0.6; MeOH 3: carbon disordered over two positions with occupancies of 0.4 : 0.6; MeOH 4: oxygen disordered over two positions with occupancies of 0.25 : 0.75. The fifth methanol was refined anisotropically with occupancy factor 0.75.
In the structure 1·2d+Cl− chloride was disordered over two positions with site occupancy factors 0.5 : 0.5. Ethanol was refined isotropically and its oxygen was disordered over two positions (0.75 : 0.25). The methanol molecule was disordered over two positions (0.5 : 0.5). Residual electron density of 1.19 and 1.11 was detected near disordered Cl−.
In the structure EtOH@12 ethanol molecule was disordered over two positions with site occupancy factors 0.5 : 0.5 and its oxygen was refined isotropically.
The crystal data and collection parameters are compiled in Table 2.§
| 2a + @1·1−0.5 | 1·2c+ | 1·2d+ | EtOH@12 | |
|---|---|---|---|---|
| a For I > 2 σI. | ||||
| Formula | C32H32O12·C32H31.5O12·(CH3)4N+·0.5·Cl−·8CH3OH | C32H32O12·(CH3)3NH+Cl−·2CH3OH | C32H32O12·(CH3CH2)3NH+Cl−·CH3OH·CH3CH2OH | 2C32H32O12·3CH3CH2OH |
| Formula weight | 1564.85 | 768.2 | 824.3 | 1355.4 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n (No. 14) | P21/c (No. 14) | C2/c (No. 15) | C2/c (No. 15) |
| a/Å | 15.2741(8) | 13.9176(7) | 21.228(1) | 28.6719(6) |
| b/Å | 23.811(1) | 15.1093(9) | 25.343(1) | 14.5214(5) |
| c/Å | 21.702(1) | 18.653(1) | 17.9561(5) | 20.4197(6) |
| β/° | 91.297(2) | 109.008(2) | 122.883(2) | 131.685(2) |
| V/Å3 | 7891.0(8) | 3708.5(4) | 8112.1(6) | 6349.3(3) |
| Z | 2 | 4 | 8 | 4 |
| μ(MoKα)/mm−1 | 0.119 | 0.174 | 0.164 | 0.109 |
| No. of refl. measured | 48118 | 23613 | 27871 | 9463 |
| No. of independent refl. | 12515 | 7254 | 7814 | 5779 |
| R int | 0.2204 | 0.1278 | 0.088 | 0.064 |
| R/Rw/%a | 14.89/27.42 | 8.37/15.49 | 10.21/22.96 | 9.42/21.98 |
| GOF | 1.036 | 1.095 | 1.111 | 1.111 |
NMR experiments
NMR experiments were performed in methanol-d4 by adding a 60 mM solution of corresponding alkyl ammonium chloride salt (2a+–d+) to a 5 mM solution (0.5 ml) of the pyrogallarene 1. The 1H spectra were measured with Bruker Avance 400 and Bruker Avance DRX 500 (400 and 500 MHz for 1H) equipped with temperature regulation.ESI-TOF mass spectra
The mass spectrometric studies were performed with a Micromass LCT ESI-TOF instrument equipped with a Z geometry electrospray ion source. The samples were introduced into the ion source as methanol solutions of 1 (50 μM) and the salts of cations 2a+–d+ (50 or 25 μM) using 1 : 1 and 2 : 1 pyrogallarene–alkyl ammonium ion molar ratio at flow rates of 10–20 μL min−1.The highest intensities were achieved with a capillary voltage of 4500 V at a source temperature of 80 °C and a desolvation temperature of 120 °C. Other selected source parameters were as follows: Sample cone voltage: 10–33 V, extraction cone voltage: 6 V, flow of gone gas: 10 L h−1, flow of desolvation gas: 150 L h−1.
Acknowledgements
Financial support of Academy of Finland (proj. no. 211240; MN and AÅ) and TEKES (proj. no. 40476/01; KR and ML) are gratefully acknowledged. We thank Mr Reijo Kauppinen for his help in NMR experiments and Prof. C. A. Schalley for his helpful comments.References
- (a) M. M. Conn and J. Rebek, Jr, Chem. Rev., 1997, 97, 1647 CrossRef CAS; (b) J. Rebek, Jr, Acc. Chem. Res., 1999, 32, 278 CrossRef; (c) C. A. Schalley, Adv. Mater., 1999, 11, 1535 CrossRef CAS.
- (a) H. Mansikkamäki, M. Nissinen and K. Rissanen, Chem. Commun., 2002, 1902 RSC; (b) H. Mansikkamäki, M. Nissinen, C. A. Schalley and K. Rissanen, New J. Chem., 2003, 27, 88 RSC; (c) H. Mansikkamäki, M. Nissinen and K. Rissanen, CrystEngComm, 2005, 7, 519 RSC; (d) H. Mansikkamäki, C. A. Schalley, M. Nissinen and K. Rissanen, New J. Chem., 2005, 29, 116 RSC.
- M. Luostarinen, A. Åhman, M. Nissinen and K. Rissanen, Supramol. Chem., 2004, 16, 505 CrossRef CAS.
- E. A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210 CrossRef CAS.
- J. M. Rivera, T. Martín and J. Rebek, Jr, Science, 1998, 279, 1021 CrossRef CAS.
- J. Kang and J. Rebek, Jr, Nature, 1997, 385, 50 CrossRef CAS.
- (a) L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A. Bryant, J. C. Sherman, R. C. Helgeson, C. B. Knobler and D. J. Cram, J. Org. Chem., 1989, 54, 1305 CrossRef CAS; (b) T. Gerkensmeier, C. Agena, W. Iwanek, R. Frölich, S. Kotila, C. Näther and J. Mattay, Z. Naturforsch., B: Chem. Sci., 2001, 56, 1063 CAS.
- (a) K. Murayama and K. Aoki, Chem. Commun., 1998, 607 RSC; (b) A. Shivanyuk, K. Rissanen and E. Kolehmainen, Chem. Commun., 2000, 1107 RSC.
- (a) A. Shivanyuk and J. Rebek, Jr, Chem. Commun., 2001, 2374 RSC; (b) A. Shivanyuk, J. C. Friese, S. Döring and J. Rebek, Jr, J. Org. Chem., 2003, 68, 6489 CrossRef.
- (a) L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS; (b) T. Gerkensmeier, W. Iwanek, C. Agena, R. Fröhlich, S. Kotila, C. Näther and J. Mattay, Eur. J. Org. Chem., 1999, 2257 CrossRef CAS; (c) J. L. Atwood, L. J. Barbour and A. Jerga, Chem. Commun., 2001, 2376 RSC; (d) L. Avram and Y. Cohen, Org. Lett., 2003, 5, 3329 CrossRef CAS.
- (a) A. Shivanyuk and J. Rebek, Jr, Chem. Commun., 2001, 2424 RSC; (b) A. Shivanuyk and J. Rebek, Jr, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 7662 CrossRef CAS; (c) L. Avram and Y. Cohen, J. Am. Chem. Soc., 2003, 125, 16180 CrossRef CAS.
- (a) H. Mansikkamäki, M. Nissinen and K. Rissanen, Angew. Chem., Int. Ed., 2004, 43, 1243 CrossRef; (b) H. Mansikkamäki, S. Busi, M. Nissinen, A. Åhman and K. Rissanen, Chem.–Eur. J., 2006, 12, 4289 CrossRef CAS.
- S. J. Dalgarno, G. W. V. Cave and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 570 CrossRef CAS.
- X-ray structure data of 2b+Cl−: a = 16.4671(7), c = 19.2680(6) Å, space group I4/m, R1 = 14.94%.
- (a) M. Munakata, L. P. Wu, T. Kuroda-Sowa, M. Maekawa, Y. Suenaga, K. Sugimoto and I. Ino, J. Chem. Soc., Dalton Trans., 1999, 373 RSC; (b) M. Nissinen, E. Wegelius, D. Falábu and K. Rissanen, CrystEngComm, 2000, 28, 151 RSC; (c) A. Åhman and M. Nissinen, Chem. Commun., 2006, 1209 RSC.
- M. Mäkinen, P. Vainiotalo, M. Nissinen and K. Rissanen, J. Am. Soc. Mass Spectrom., 2003, 14, 143 CrossRef CAS.
- Z. Otwinowski, W. Minor, Processing of X-ray Diffraction Data Collected in Oscillation Mode, in Methods in Enzymology, Macromolecular Crystallography Part A, ed. C. W. Carter, Jr and R. M. Sweet, Academic Press, New York, 1997, vol. 276, p. 307 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXL-97, Program for refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: crystallographic data in CIF format. See DOI: 10.1039/b609117b |
| ‡ The HTML version of this article has been enhanced with colour images. |
| § CCDC reference numbers 611014–611017†. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2007 |
