Quantitative on-chip determination of taurine in energy and sports drinks†
Sebastian
Götz
,
Tobias
Revermann‡
and
Uwe
Karst‡
*
University of Twente, Chemical Analysis Group and MESA+ Institute for Nanotechnology, P.O. Box 217, 7500 AE Enschede, The Netherlands
First published on 6th October 2006
Abstract
A new method for the quantitative determination of taurine in beverages by microchip electrophoresis was developed. A rapid and simple sample preparation procedure, only including two dilution steps and the addition of the fluorogenic labeling reagent NBD-Cl (4-chloro-7-nitrobenzofurazan), is applied. Using a home-built wavelength-resolved fluorescence detector, the separation and determination of the taurine derivative could be achieved in only 12 s, while the additional spectral information was utilized to ensure peak purity. Spanning from 0.1 to 50 mmol L−1, the linear dynamic range of the applied method was adapted to the apparent contents in common taurine containing beverages. The smallest detectable amount of the taurine derivative actually injected into the separation channel was as low as 60 amol. The method was successfully validated by an independent liquid chromatographic method.
Introduction
Since first appearances of lab-on-a-chip applications1 and the proposal of micro total analysis systems,2 many researchers were fascinated by the apparent advantages of miniaturized separation and detection systems. Ultra short separation times on low-cost disposable devices combined with a strongly reduced amount of sample and organic solvents needed, encouraged a large number of research groups to work in this field. Because of the fact that no pumps or pressure-tight connections to the macro world were needed, electrophoretic separations could most easily be adapted to the dimensions of microchips3 and are still mainly used today. Combined with fluorescence detection, electrophoresis offers very favorable selectivity and sensitivity, rapid separations and very high separation efficiencies. In the following years, many impressive results have been reported, ranging from ultra-trace detection4 and on-chip derivatization5 to single cell lysis experiments6 and ultra fast chiral separations.7 Despite all the advances made in this area, most of the remarkable work published is of qualitative or semi-quantitative nature. On-chip electrophoresis still suffers from bad reproducibility compared to the results of desktop instruments; analytical figures of merit are often extrapolated or calculated from signal-to-noise ratios of single measurements. Only few reports of truly quantitative on-chip determinations with real samples can be found in literature. Inorganic ions, such as nitrite in water,8 calcium in urine9 and lithium in blood10 as well as a few more complex analytes e.g. levoglucosan in aerosols,11 thiols in nerve agent degradation products12 and homocysteine in plasma13 have been determined. Many problems associated with miniaturized separation and detection systems, like pH-changes due to electrolysis,14–16 analyte surface interactions17 and temperature effects,18 have already been addressed and often solved, but mostly in a way not suited for routine use.Taurine (2-aminoethanesulfonic acid) is a semi-essential amino acid, which is abundant in high concentrations in many tissues and body fluids. Although it is not incorporated into proteins, taurine in its free form is associated with a vast variety of physiological functions, such as antioxidation activity,19 neuromodulation, membrane stabilization20 and modulation of intracellular calcium levels.21 While in a healthy state, intercellular taurine levels are strictly controlled, altered concentrations in plasma and urine have been associated with a variety of diseases including epilepsy,22 myocardial infarction23 and cancer.24 With regard to the importance of taurine in retinal development, reproduction and development, it has been added to infant formula as well as to parental solutions.25 In recent years, with the propagation of energy and sports drinks, the normal daily uptake of taurine could easily be exceeded by a factor of 100. Although animal studies have not indicated toxic effects of taurine, the need for a rapid and easy method of taurine determination in food and beverages for quality assurance and product control purposes has become more important.
While taurine can be detected directly by means of pulsed amperometric detection,26 the most frequently used method for taurine determination is the HPLC separation with subsequent UV/vis or fluorescence detection.27 The required derivatization reagents include o-phthalaldehyde,28 2,4-dinitrofluorobenzene29 and fluorescamine.30,31 In recent years, alternative separation methods like ion-exchange chromatography32 and particularly capillary electrophoresis33 became more prominent.
In this paper, we present the quantitative determination of taurine in sports drinks and other taurine containing beverages by means of a very rapid on-chip CE separation and wavelength-resolved fluorescence detection. The applied detector system consisting of a fluorescence microscope, a spectrograph and an intensified CCD-camera delivers information-rich 3-D electropherograms comparable to diode-array detection in UV/vis spectroscopy. This set-up is the first wavelength-resolved detection system specially adapted to the requirements of rapid on-chip separations. More fundamental information about wavelength-resolved detection systems for non-microchip CE applications can be found in the comprehensive review by Sweedler et al.34 This paper describes a fast and easy way of receiving reliable data with reproducibility comparable to other established methods.
Experimental
Taurine standard solutions were derived by dilution from a freshly prepared 100 mM stock solution of taurine in demineralized water. Real samples were diluted 3- to 10-fold, depending on their concentration of acidity regulators to ensure the proper pH during derivatization and yield final taurine concentrations in the linear range of the detector.Taurine standard or real sample solutions (30 µL) were mixed with 30 µL of buffer (700 mM aqueous borate buffer, pH 9.3) spiked with a 30 mM concentration of 6-aminohexanoic acid as internal standard. After addition of 60 µL of a 200 mM solution of 4-chloro-7-nitrobenzofurazan (NBD-Cl) in acetonitrile, the mixture was shaken and incubated at 45 °C for 30 min. Subsequently, the reaction solution was diluted 10-fold with running buffer and then applied to the reservoir of the microchip and injected and separated in triplicate.
Separations were performed on glass microchips (model T3550) from Micronit (Enschede, Netherlands) with an orthogonal channel design (10 × 40 mm) and a channel cross section of 20 × 50 µm. The chips incorporate a double-T crossing with 100 µm offset.
The high voltage power supply (model ECH-135L) from Micronit (Enschede, The Netherlands) has 8 programmable outputs (0-3000 V) and was additionally equipped with a custom-made trigger output (TTL) to provide a starting signal to the camera.
The chip was flushed once every three runs with running buffer (50 mM borate buffer, pH 9.3) and refilled. The voltages applied during analysis are shown in Fig. 1.
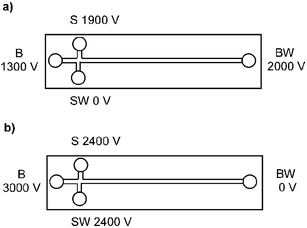 | ||
| Fig. 1 Voltage program for pinched injection (buffer reservoir (BR), buffer waste (BW), sample reservoir (SR) and sample waste (SW)) for injection (5 s) and separation (20 s). | ||
Separations were observed with a wavelength-resolved fluorescence detector consisting of a fluorescence microscope, a spectrograph and a CCD-camera.35 An inverse fluorescence microscope (IX-71S1F) from Olympus (Hamburg, Germany) equipped with a xenon lamp (U-LH75XEAPO) from Olympus was used. A filter cube with components from Chroma (Rockingham, VT, USA) was employed (exciter: HQ470/40x; dichroic: 500DCLP; emitter: HQ510LP) for wavelength selection.
The triple grating turret in the spectrograph SpectraPro 308i (Acton Research, Acton, MA, USA) was provided with a 150 grooves per mm (gr mm−1) grating for standard measurements, a 600 gr mm−1 grating for high resolution spectra and a mirror for imaging.
The light-intensified CCD-camera PI-Max 512RB from Princeton Instruments (Trenton, NJ, USA) was combined with a ST133 controller ver.5 (Princeton Instruments). The data was recorded and evaluated with WinSpec/32 software ver. 2.5.12.0 (Princeton Instruments).
For validation purposes, the analysis was also performed on an HPLC system with fluorescence detection. Liquid chromatographic separations and the detection were performed on the following system (all components from Shimadzu, Duisburg, Germany): two LC-10AS pumps, degasser GT-154, RF-10AXL fluorescence detector, SIL-10A autosampler, software Class LC-10 version 1.6 and CBM-10A controller unit. The injection volume was 10 µL. A Prontosil 120-3-C18 column (Bischoff Chromatography, Leonberg, Germany) was used; particle size 3 µm, pore size 120 Å; column dimensions 150 × 4.6 mm.
To ensure a sufficient retention of the highly polar analytes, a gradient of an acidic buffer (10 mM acetate, pH 5) and acetonitrile with a flow of 1 ml min−1 has been used (Table 1). Sample preparation was performed analogous to the on-chip separation, except for the fact that the reaction solution was diluted by a factor of 100 with water prior to injection.
| 0 min | 10 min | 15 min | 19 min | 23 min | 25 min | |
|---|---|---|---|---|---|---|
| A | 90% | 80% | 60% | 10% | 90% | stop |
| B | 10% | 20% | 40% | 90% | 10% | stop |
Results and discussion
The goal of this work is the quantitative determination of taurine in energy drinks and other taurine containing beverages by means of a microchip capillary electrophoretic separation with wavelength-resolved fluorescence detection. As taurine itself is neither fluorescent nor UV-active, it has to be labeled. In this work, we use 4-chloro-7-nitro-1,2,3-benzofurazan (NBD-Cl) as a reagent. The non-fluorescent NBD-Cl binds to amine functions at elevated pH-values and forms the respective NBD-derivatives, which show strong fluorescence. To improve the reproducibility of the quantitative measurements, all standard solutions and samples were spiked with 6-aminohexanoic acid as an internal standard. The use of the internal standard helps to compensate for fluctuations in the amount of injected sample, in reaction speed during derivatization, in the optical alignment of the detector and in slight changes of migration speed.After dilution, the reaction mixture is directly filled into the sample reservoir of the microchip and injected into the separation channel. The electropherogram of a derivatized taurine standard is shown in Fig. 2 (top left). The electropherogram shows three major peaks with peak (a) being the internal standard and (b) being the taurine derivative. Peak (c) is a hydrolysis product of the NBD-Cl reagent, where the chloride function was substituted by a hydroxy ion. Two very small peaks caused by yet unidentified side products of the derivatization in front of peak (a) can also be seen (*). They are apparent in all separations, but do not interfere with the quantification. The complete separation is accomplished in 12 s.
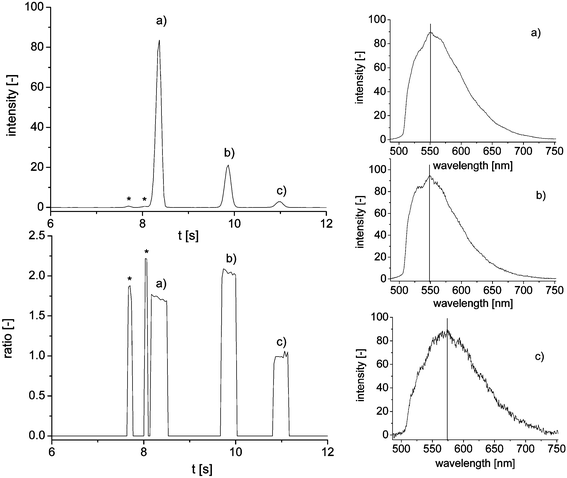 | ||
| Fig. 2 Magnification of the electropherogram of a taurine standard solution (10 mM) with corresponding peak purity plot and extracted emission spectra; (a) internal standard, (b) taurine derivative and (c) hydrolysis product; * mark unidentified peaks. | ||
The applied wavelength-resolved fluorescence detector set-up consists of a fluorescence microscope, a spectrograph and a CCD-camera and yields information-rich electropherograms comparable to a diode-array detector in UV/vis-spectroscopy. Fig. 2 shows the extracted emission spectra of the three major peaks. Compared to the spectrum of the internal standard (a) with an emission maximum of 551 nm, the taurine derivative (b) shows a slight shift of the maximum to 548 nm and a more expressed shoulder on the left side of the curve. Even though the fluorescence in both cases is generated by the same NBD-backbone and the two molecules are very similar, subtle differences in their fluorescent properties can be detected. The third spectrum of the hydroxy derivative shows an even stronger shift of the emission maximum to 573 nm. Using the additional spectral dimension of the recorded data, possible coelutions during the separation can easily be detected by means of a peak purity plot. For this purpose, two wavelengths apparent in all observed spectra are selected (545 and 605 nm). If both measured intensities exceed a previously defined threshold value, the ratio between them is calculated and plotted (Fig. 2 bottom left). A clean peak is then observed as a concentration independent box-shaped peak at the point of time of the original peak and the height of the calculated signal ratio. A coelution would show up as a strong change of ratio during one peak,35 but was not observed in these separations.
A high emphasis was set on a very simple and fast sample preparation. Standards and real samples only had to be mixed with labeling reagent and diluted twice to ensure final taurine concentrations in the linear range of the detector system. With taurine standards covering the expected concentration range of 0.1 to 50 mmol L−1 (before reaction and dilution), the dynamic range of the detector was found to exceed 2.5 concentration decades, yielding a straight calibration line with an R2 value of 0.9992. Each concentration was determined in triplicate, yielding an average standard deviation of 3.4%.
While the derivatization procedure (including an overall 40-fold dilution of the analyte for sample preparation) was not optimized for low limits of detection, the instrumental limit of quantification for the actual taurine derivative was 3 × 10−6 mol L−1 (S/N = 10). Considering the injection volume of approximately 60 pL, the smallest detectable amount of the taurine derivative (S/N = 3) actually injected into the separation channel was as low as 60 amol, corresponding to a concentration of 1 × 10−6 mol L−1.
Eleven energy drinks and other taurine containing beverages were purchased (Table 2) and analyzed analogous to the taurine standard solutions. Whereas most of the energy drinks exploit the legal limit of 0.4% (31.96 mmol L−1) taurine content, mixed beverages based on tap water, fruit juice and also beer were found in the lower concentration range from 0.8 to 3 mmol L−1. No taurine containing beverages with a medium concentration around 10–20 mmol L−1 could be found and used for this analysis.
| Beverage | c taurine (suppl.)/mmol L−1 | c taurine (on-chip CE)/mmol L−1 | c taurine (HPLC)/mmol L−1 |
|---|---|---|---|
| Red Bull | 31.96 | 31.92 | 30.27 |
| Red Bull sugar free | 31.96 | 31.52 | 31.24 |
| Mr. Energy | 31.96 | 32.02 | 30.85 |
| Caps energy | n/a | 3.19 | 2.68 |
| Effect | 31.96 | 31.89 | 31.29 |
| S1 | 31.96 | 33.49 | 32.19 |
| Veltins V+ energy beer | n/a | 1.85 | 1.45 |
| Kick off (bottle) | 31.96 | 33.78 | 32.81 |
| Kick off (can) | 2.40 | 3.26 | 2.68 |
| Xi energy water | 0.80 | 1.01 | 0.87 |
| Xi climax | 31.96 | 32.02 | 31.27 |
Fig. 3 shows a typical electropherogram of a derivatized energy drink sample along with its peak purity plot. Comparable to the taurine standard solutions, the data shows no sign of coelution. All results generated by the on-chip analysis were compared with the suppliers' information and found to be in good accordance (Table 2). The average standard deviation for all real sample measurements was 3.0%.
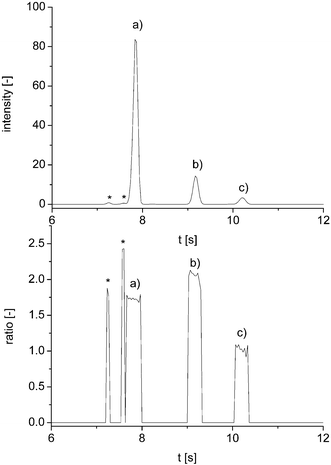 | ||
| Fig. 3 Electropherogram of a taurine real sample (Red Bull Sugar Free) with corresponding peak purity plot; (a) internal standard, (b) taurine derivative and (c) hydrolysis product; * mark unidentified peaks. | ||
To validate the results of the microchip separation, the analysis was also performed on a commercially available HPLC system with fluorescence detection. Using a column with C18 material, the aqueous part of the acetonitrile/water gradient had to be acidified (10 mM acetate, pH 5) to ensure enough retention of the highly polar NBD-derivatives. Fig. 4 shows the liquid chromatogram of a derivatized energy drink sample. In contrast to the electrophoresis, the chromatographic separation with its different retention mechanism results in a changed elution order of the three major peaks. The analytes are separated after 15 min; one complete run takes 23 min. Threefold injection of the diluted reaction mixtures yielded average standard deviations of 1.8%. The resulting taurine concentrations determined by means of HPLC are in good agreement with the on-chip measurements and the suppliers' information (Table 2). Both detection methods delivered very similar results, which is obvious from the correlation plot (Fig. 5).
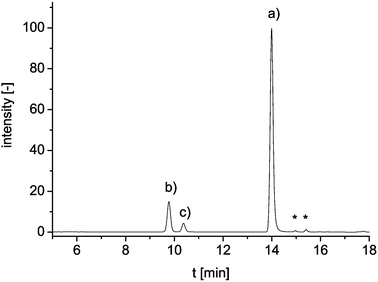 | ||
| Fig. 4 HPLC chromatogram of taurine standard solution (10 mM); (a) internal standard, (b) taurine derivative and (c) hydrolysis product; * mark unidentified peaks. | ||
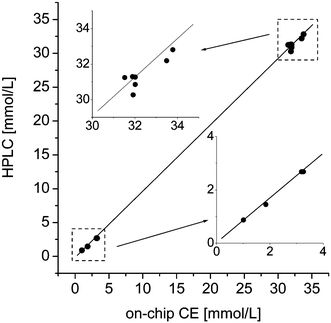 | ||
| Fig. 5 Correlation plot of HPLC and on-chip CE results of real sample measurements. Lower and higher concentration ranges have been enlarged in the inserts. | ||
Conclusion
This work demonstrates the development of a method for the quantitative on-chip determination of taurine in energy drinks and other taurine containing beverages. After derivatization with NBD-Cl, the sample is filled into the reservoir of a glass microchip and separated by capillary electrophoresis. Three major peaks, the internal standard, the taurine derivative and the hydrolysis product of the reagent are separated in only 12 s. The separation is observed with a wavelength-resolved fluorescence detector consisting of a fluorescence microscope, a spectrograph and an intensified CCD-camera. The yielded information-rich electropherogram shows even subtle differences in the fluorescent properties of the observed analytes. Furthermore, it enables peak purity calculations, ensuring no hidden coelution is taking place. Including only dilution and addition of the labeling reagent, a very fast and straightforward sample preparation method was applied, which was adapted to the anticipated taurine concentrations in real samples. A calibration curve with standards covering the expected concentration range from 0.1 to 50 mmol L−1 was recorded. The smallest detectable amount (S/N = 3) of taurine derivative actually injected into the separation channel was 60 amol, corresponding to a concentration of 1 × 10−6 mol L−1. The method was successfully validated by liquid chromatography with fluorescence detection.Acknowledgements
Financial support of the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO, Den Haag, The Netherlands) and the Fonds der Chemischen Industrie (Frankfurt, Germany) is gratefully acknowledged.References
- S. C. Terry, IEEE Trans. Electron Devices, 1997, 26, 1880–1886.
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244–248 CrossRef.
- D. J. Harrison, A. Manz, Z. Fan, H. Lüdi and H. M. Widmer, Anal. Chem., 1992, 64, 1926–1932 CrossRef.
- A. Ros, W. Hellmich, T. Duong and D. Anselmetti, J. Biotechnol., 2004, 112, 65–72 CrossRef CAS.
- S. C. Jacobsen, L. B. Koutny, R. Hergenröder, A. W. Moore and J. M. Ramsey, Anal. Chem., 1994, 66, 3472–3476 CrossRef CAS.
- W. Hellmich, C. Pelargus, K. Leffhalm, A. Ros and D. Anselmetti, Electrophoresis, 2005, 26, 3689–3696 CrossRef CAS.
- N. Piehl, M. Ludwig and D. Belder, Electrophoresis, 2004, 25, 3848–3852 CrossRef CAS.
- G. M. Greenway, S. J. Haswell and P. H. Petsul, Anal. Chim. Acta, 1999, 387, 1–10 CrossRef CAS.
- N. Malcik, J. P. Ferrance, J. P. Landers and P. Caglar, Sens. Actuators, B, 2005, 107, 24–31 CrossRef.
- E. X. Vrouwe, R. Luttge, W. Olthuis and A. van den Berg, Electrophoresis, 2005, 26, 3032–3042 CrossRef CAS.
- C. D. Garcia, G. Engling, P. Herckes, J. L. Collett and C. S. Henry, Environ. Sci. Technol., 2005, 39, 618–623 CrossRef CAS.
- J. Wang, J. Zima, N. S. Lawrence, M. P. Chatrathi, A. Mulchandani and G. E. Collins, Anal. Chem., 2004, 76, 4721–4726 CrossRef CAS.
- S. A. Pasas, N. A. Lacher, M. I. Davies and S. M. Lunte, Electrophoresis, 2002, 23, 759–766 CrossRef CAS.
- M. Macka, P. Andersson and P. R. Haddad, Anal. Chem., 1998, 70, 743–749 CrossRef CAS.
- A. Oki, Y. Takamura, Y. Ito and Y. Horiike, Electrophoresis, 2002, 23, 2860–2864 CrossRef CAS.
- I. Rodriguez and N. Chandrasekhar, Electrophoresis, 2005, 26, 1114–1121 CrossRef CAS.
- D. Belder and M. Ludwig, Electrophoresis, 2003, 24, 3595–3606 CrossRef CAS.
- N. J. Petersen, R. P. H. Nikolajsen, K. B. Mogensen and J. P. Kutter, Electrophoresis, 2004, 25, 253–269 CrossRef CAS.
- J. Milei, R. Ferreira, S. Llesuy, P. Forcada, J. Covarrubias and A. Boveris, Am. Heart J., 1992, 123, 339–345 CrossRef CAS.
- R. J. Huxtable and L. A. Sebring, Trends Pharmacol. Sci., 1986, 7, 481–485 CrossRef CAS.
- R. J. Huxtable, Physiol. Rev., 1992, 72, 101–163 Search PubMed.
- S. G. Hartley, H. O. Goodman and Z. Shihabi, Neurochem. Res., 1989, 14, 149–152 CrossRef CAS.
- S. K. Bhatnagar, J. D. Welty and A. R. Al Yussuf, Int. J. Cardiol., 1990, 27, 361–366 CrossRef CAS.
- G. E. Gray, A. M. Landel and M. M. Meguid, Nutrition, 1990, 10, 11–15.
- G. B. Schuller-Levis and E. Park, FEMS Microbiol. Lett., 2003, 226, 195–202 CrossRef CAS.
- T. R. I. Cataldi, G. Telesca, G. Bianco and D. Nardiello, Talanta, 2004, 64, 626–630 CrossRef CAS.
- S. Muo, X. Ding and Y. Liu, J. Chromatogr., B: Biomed. Appl., 2002, 781, 251–267 CrossRef CAS.
- C. J. Waterfield, J. Chromatogr., B: Biomed. Appl., 1994, 657, 37–45 CrossRef CAS.
- Z. Chen, G. Xu, K. Specht, R. Yang and S. She, Anal. Chim. Acta, 1994, 296, 249–253 CrossRef CAS.
- T. Sakai and T. Nagasawa, J. Chromatogr., 1992, 576, 155–157 CAS.
- G. P. McMahon, R. O'Kennedy and M. T. Kelly, J. Pharm. Biomed. Anal., 1996, 14, 1287–1294 CrossRef CAS.
- F. Qu, Z. Qi, K. Liu and S. Mou, J. Chromatogr., B: Biomed. Appl., 1999, 730, 161–166 CrossRef CAS.
- M. T. Kelly, H. Fabre and D. Perett, Electrophoresis, 2000, 21, 699–705 CrossRef CAS.
- X. Zhang, J. N. Stuart and J. V. Sweedler, Anal. Bioanal. Chem., 2002, 373, 323–343 CrossRef.
- S. Götz and U. Karst, Sens. Actuators, B DOI:10.1016/j.snb.2006.08.027.
Footnotes |
| † Electronic supplementary information (ESI) available: Derivatization of taurine with NBD-Cl under basic conditions (Fig. S1); electropherogram of taurine standard solution (Fig. S2). See DOI: 10.1039/b609739a |
| ‡ Present address: University of Münster, Institute of Inorganic and Analytical Chemistry, Corrensstr. 30, 48149 Münster, Germany. E-mail: uk@uni-muenster.de; Fax: +49-251-8336013 |
| This journal is © The Royal Society of Chemistry 2007 |
