Containerless reaction monitoring in ionic liquids by means of Raman microspectroscopy†
Mercedes
López-Pastor
ab,
Ana
Domínguez-Vidal
b,
María José
Ayora-Cañada
c,
Thomas
Laurell
d,
Miguel
Valcárcel
a and
Bernhard
Lendl
*b
aDepartment of Analytical Chemistry, University of Cordoba, Campus de Rabanales, Marie Curie Annex Building, E-14071, Córdoba, Spain
bInstitute of Chemical Technologies and Analytics, Vienna University of Technology, Getreidemarkt 9/164AC, A-1060, Wien, Austria
cDepartment of Physical and Analytical Chemistry, University of Jaen, Paraje Las Lagunillas S/N, E-23071, Jaén, Spain
dDepartment of Electrical Measurements, Lund University, PO Box 118, 221 00 Lund, Sweden
First published on 13th October 2006
Abstract
Reaction monitoring by Raman microspectroscopy in levitated room temperature ionic liquid (RTIL) droplets is reported. Due to their non-volatility, RTIL droplets are well-suited to act as wall-less microreactors. The droplets were produced by a piezoelectric flow-through microdispenser connected to an automated flow injection system and were levitated by an acoustic trap. Taking advantage of the flow system versatility, the sequence of reagents was easily changed to study a model organic reaction: the Knoevenagel condensation. The reaction was followed by Raman microspectrometry and the obtained spectra were analysed using multivariate curve resolution to retrieve the concentration profiles and pure spectra of reactants, intermediates and products involved in the reaction. In addition, information about solvation interactions was obtained by monitoring the desolvation process taking place when a volatile co-solvent evaporated from the droplet.
Introduction
Monitoring reactions by means of spectroscopic techniques is a general trend in synthetic chemistry, because it can provide structural information of reagents and products, as well as information about reaction mechanisms.1–4 Furthermore, modern chemistry has been developed toward miniaturization, with the aim of saving time and cost. In this way, recent advances in the fields of miniaturized reaction and separation systems, including the construction of fully integrated lab-on-a-chip systems have been developed.5 However, common problems found in such miniaturized systems are the risk of analyte adsorption to walls and interfaces,6–9 and the optical interference at the walls of the container hampering detection8 when spectroscopic techniques are used. These main problems can be avoided by means of levitation of small sample volumes. Levitation can be achieved by several manners: aerodynamically, electrostatically, diamagnetically, optically, and acoustically. Aerodynamic levitation has found much use in industrial applications and for levitating solid spheres10 where an upward stream of an inert gas maintains a solid sample stationary at a point where the gas velocity relative to the sample equals the gravitational force. In electrostatic levitation, charged samples are levitated in a electric field.11,12 Another means to achieve levitation is the application of a strong magnetic field.13 Optical levitation allows the manipulation of small particles with laser light, and it has found many applications in biology, chemistry, and physics.14 Finally, acoustic levitation is the most generally applicable levitation technique. In this kind of levitation, developed for the first time by Bücks and Müller,15 small solid or liquid particles are suspended in the nodal points of a standing ultrasonic wave formed by means of multiple reflections between an ultrasonic radiator and a concave solid reflector.16 Moreover, containerless processing by using acoustic field positioning was first developed by Whymark et al.17 The range of size which can be levitated is very wide. The largest drop that can be suspended at the standard frequency (58 kHz) of the most frequently employed bench-top levitators without any significant drop deformation measures about 2.5 mm in diameter; while the smallest drop measures only around 15 µm.18Raman spectroscopy in levitated drops offers a possibility to study in situ chemical processes, providing detailed molecular and structural information. It has already been employed for a wide range of applications including the study of crystallization processes, which take place as a consequence of solvent evaporation.19,20 Moreover, Santesson et al.20 and Leopold et al.21 have measured surface-enhanced Raman scattering (SERS) in levitating droplets. For robust monitoring of chemical reactions in levitated droplets, stable levitation of samples of a given size must be achieved. This can be difficult, especially considering droplets of fast evaporating solvents and under laser illumination. Recently, Wood et al.22 reported the acquisition of Raman spectra of levitating living algal cells. They had to add water during their experiments to compensate for solvent evaporation during the 150 s of measuring time. This necessity, together with continuous solvent fill-up, can be avoided when using levitated droplets of non-volatile substances such as room temperature ionic liquids (RTILs).
RTILs are salts which are liquid at room temperature. Their unique properties, such as negligible vapor pressure even at elevated temperature, non-flammability, and high thermal stability, make RTILs ideal solvents to perform chemical reactions, separations and analytical procedures.23 Furthermore, the interest in using those compounds as clean solvents in green chemistry has recently risen exponentially.24–26
In this work, we propose a flexible and robust approach in which a levitated RTIL droplet is used as a reaction medium into which reagents are dispensed by a flow-through microdispenser and employing Raman microspectroscopy to monitor the drop. With the use of an automated flow system, the dispensing sequence of reagents, as well as the reagent volumes, can be easily changed and adapted to given requirements of the reaction under study. This makes the system suitable for combinatorial chemistry or to identify optimal reaction conditions. To demonstrate the applicability of the proposed setup, a Knoevenagel condensation was selected as a model reaction. This base catalyzed-condensation is known as a special case of an aldol reaction, defined as the reaction of a carbonyl (ketone or aldehyde) with an active methylene compound. This reaction has been studied in 1-alkyl-3-methylimidazolium based ionic liquids,27–29 where it could be seen that this condensation could be performed more efficiently in ionic liquids than in traditional solvents. With this new contribution, we demonstrate the feasibility of handling chemical reactions in ultrasonically levitated drops of ionic liquid and monitoring them with Raman microspectroscopy.
Experimental section
Chemicals
1-ethyl-3-methylimidazolium tetrafluoroborate (emimBF4) was purchased from Solvent Innovation GmbH (Köln, Germany) and used as received without any pre-treatment. The rest of the chemicals (malononitrile, cinnamic aldehyde, and 3,4-dihydroxybenzaldehyde) were of reagent grade and they were purchased from Fluka (Steinheim, Germany).Setup and instruments
Fig. 1 shows the experimental setup for on-line reaction monitoring in levitated nano-droplets by means of Raman spectroscopy. Four main parts can be considered in the setup: flow system, flow-through microdispenser, levitator, and detector unit. The flow system was a sequential injection analysis system (SIA) composed of a Cavro (Sunnyvale, CA, USA) XP 3000 pump with a 100 µl syringe, a 200 µl holding coil, and a 10 port Valco (Houston, TX, USA) selection valve equipped with an electronic microactuator. All PTFE tubings (0.5 mm i.d.) and fittings used were obtained from Global FIA (Gig Harbour, WA, USA). The flow system was connected to a flow-through microdispenser30 to dose the droplets into the trap. It consisted of two microstructured silicon plates that were joined to form a channel with a pyramidal nozzle in the middle. A piezoelectric element was connected to the channel wall opposing the nozzle, in such a way that when a voltage pulse was applied, the droplets were ejected. To implement the pulse, a computer-controlled arbitrary waveform generator (Agilent 33120 A, Agilent Technologies, Palo Alto, CA, USA) was used. The flow and dispensing systems were computer controlled by custom software (Sagitarius V3) allowing sequential introduction of the different reagents into the microdispenser.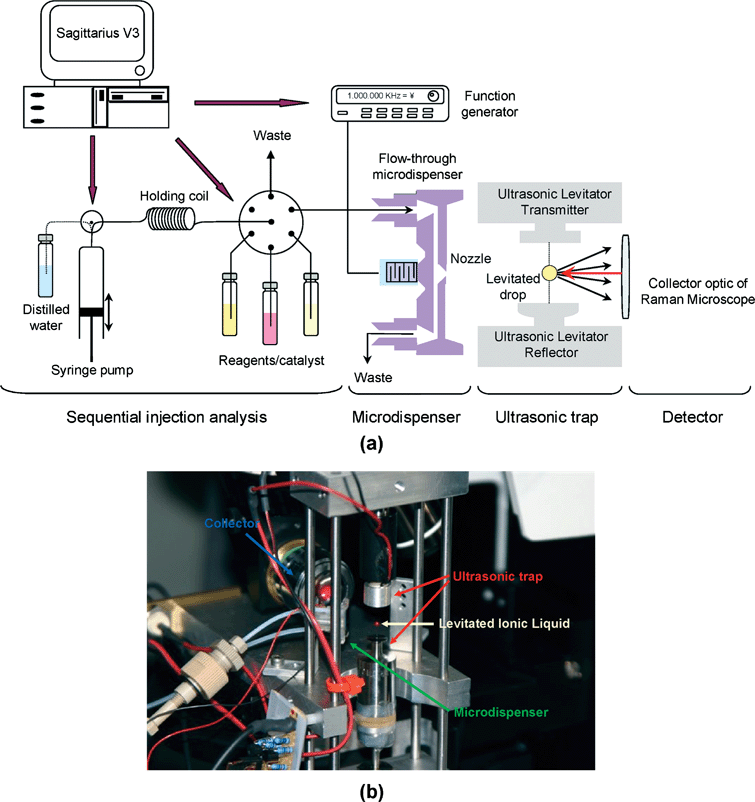 | ||
| Fig. 1 (a) Experimental setup consisting of flow system for liquid handling, microdispenser, ultrasonic levitator and Raman microscope. (b) Image of the ultrasonic levitator (attached to the Raman microspectrometer) holding a droplet in a node that is aligned with the collection optics of a Raman microscope. | ||
The microdispenser dosed pico-droplets into the node of an acoustic trap. The used trap was based on a piezoelectric vibrator and a concave reflector to generate a standing ultrasonic wave with equally spaced nodes and antinodes by means of multiple reflections between them. The acoustic wave had several nodes into which liquid or solid samples could be placed without contact and levitated. The ultrasonic levitator used (tec5 AG, Dantec/invent Measurement Technology, Erlangen, Germany) operated with a frequency of 58 kHz. Finally, the detection unit was a Raman Confocal Microspectrometer LabRam HR800 (Jobin Ybon GmbH, Bensheim, Germany) equipped with a coupled charge detector (CCD). Fig. 1b shows how a droplet located in one of the nodes was illuminated by a He–Ne laser emitting at 632.817 nm with power set to 14.5 mW. The microscope objective of the spectrometer was arranged at 180° to the laser beam axis and 90° to the levitator axis by means of a modified lens configuration (20 magnifications). The working distance was 20.5 mm. Each spectrum was gathered over 14 s.
Reaction and procedure
Two aldehydes were selected to carry out the condensation together with malononitrile (2) (which acted as the methylene compound) and KOH (which acted as the catalyst). These two aldehydes were 3,4-dihydroxybenzaldehyde (1), which produced (3,4-dihydroxybenzylidene)malononitrile (3), (see Scheme 1) and cinnamic aldehyde (4), which produced cinnamylidenemalononitrile (5) (see Scheme 2). 1-Ethyl-3-methylimidazolium tetrafluoroborate RTIL was selected as the reaction medium.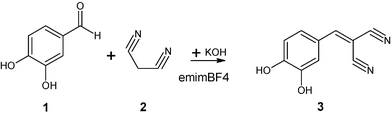 | ||
| Scheme 1 | ||
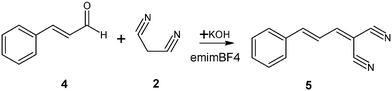 | ||
| Scheme 2 | ||
Malononitrile, as well as 3,4-dihydroxybenzaldehyde, was prepared in a mixture of emimBF4 and distilled water (1 : 1 v/v), while cinnamic aldehyde was prepared in a mixture of emimBF4 with acetonitrile (1 : 1 v/v), due to its non-solubility in water. All of them were freshly prepared at a concentration of 1 M before monitoring the reaction. Potassium hydroxide, KOH (0.2 M), which acted as catalyst in the Knoevenagel condensation, was prepared in distilled water. Each solution was filtered prior to introduction into the microdispenser using filters with 2 µm pore size (Upchurch Science Inc., Oak Harbour, WA, USA). To monitor the reaction, several pico-droplets were dosed into the node by dispensing with a frequency of 1000 Hz. Since the droplet ejection from the microdispenser depends on liquid properties, emimBF4 was mixed with water (1 : 1, v/v) to reduce its viscosity and surface tension in order to facilitate its dispensing. The experiments were carried out in the following sequence: aldehyde, followed by malononitrile, were dispensed first for 7 s each. Finally the catalyst KOH was added for 3 s. At the beginning of each working day, the set-up was aligned for maximum intensity in the recorded Raman spectra using a drop of ionic liquid produced by dispensing it for 17 s at 1000 Hz. For reaction monitoring, spectra acquisition was started immediately after addition of the catalyst and continued until no spectral changes were recorded, indicating the end of the reaction. The volume of the levitated droplets was determined gravimetrically. Considering the IL density, the volume of the droplets was ca. 250 nL, corresponding to 300 µm in diameter.
The raw spectra were baseline corrected and smoothed using a 13-point Savitzy Golay filter and then they were analysed by multivariate curve resolution-alternating least squares (MCR-ALS) using the freely available program (Matlab code) by A. de Juan and R. Tauler.31
Results and discussion
The Knoevenagel condensation was firstly studied in conventional solvents, namely, water and acetonitrile, using the proposed setup. The use of these solvents for this reaction has been described previously.32,33 When carrying out the reaction under laser illumination, the solvent evaporation was very fast. Due to the small size of the droplets, the continuous addition of solvent was necessary. This continued feed of droplets added to slight instabilities19 in the position of the levitated drop. Due to sampling with the objective of a microscope, these fluctuations were reflected in intensity shifts in the recorded Raman spectra. However, significantly more stable measurement conditions, were obtained when using the ionic liquid as solvent. Spectra of 250 nL drop of levitated emimBF4 were recorded for 30 min in order to check the stability of the droplet. Since the ionic liquid was dispensed in a 50% (v/v) water solution, at the beginning of the experiment (ca. 3 min) water evaporated. During this time interval, the intensity of the emimBF4 spectrum increased and a slight red-shift in the band centered around 760 cm−1 was observed (Fig. 2). This band corresponds to the symmetric stretching of BF4−,34 which has been reported to be mainly responsible for the interaction of the ionic liquid with water.35 After water evaporation, the intensity remained constant, demonstrating the stability of emimBF4 in a levitated droplet under laser illumination during a prolonged time. It thus may be concluded that emimBF4 is suitable as reaction medium in airborne reaction monitoring.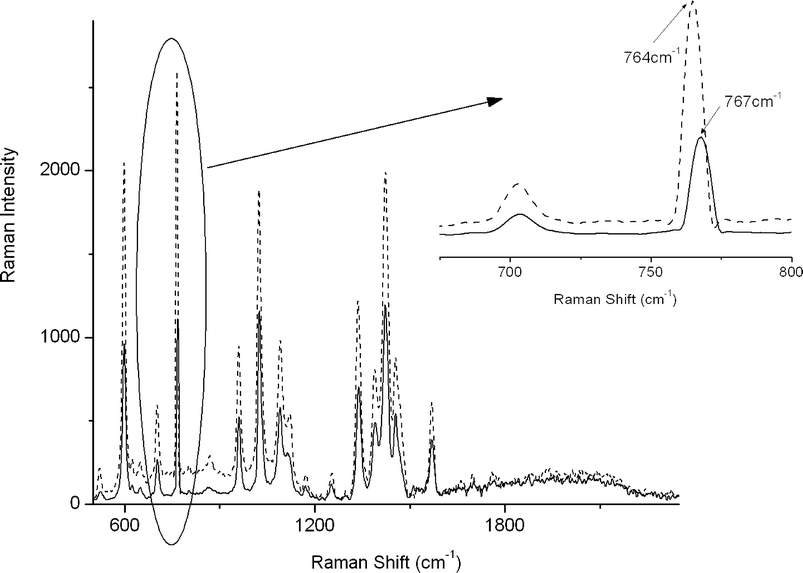 | ||
| Fig. 2 Raman spectra of levitated emimBF4, wet (solid line) and dry (dotted line). Inset: shift of the band at 760 cm−1 due to the evaporation process. | ||
The stability of the reagents under the measurement conditions was also tested. Aldehyde and malononitrile were levitated separately for 20 min while recording Raman spectra. Complete evaporation of the co-solvent took place in less than two and three minutes in the case of acetonitrile and water, respectively. A slight shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands of the aldehydes was observed. As an example, the co-solvent evaporation effect for cinnamic aldehyde is shown in Fig. 3, in which it can be observed that the C
O stretching bands of the aldehydes was observed. As an example, the co-solvent evaporation effect for cinnamic aldehyde is shown in Fig. 3, in which it can be observed that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band located at 1689 cm−1 was shifted to higher wavenumbers. The bands related to double bonds and the aromatic ring were not affected. That is, solvation of cinnamic aldehyde with acetonitrile took place through the carbonyl group. After 20 minutes of levitation, no other spectral changes were observed.
O stretching band located at 1689 cm−1 was shifted to higher wavenumbers. The bands related to double bonds and the aromatic ring were not affected. That is, solvation of cinnamic aldehyde with acetonitrile took place through the carbonyl group. After 20 minutes of levitation, no other spectral changes were observed.
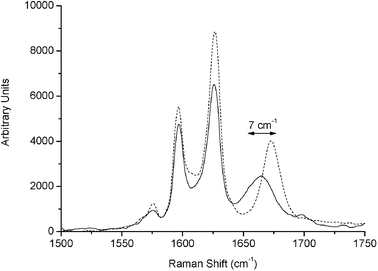 | ||
| Fig. 3 Comparison of the spectra at the beginning and at the end of the co-solvent evaporation process of cinnamic aldehyde, when it was levitated in a mixture of emimBF4 : acetonitrile 1 : 1 v/v. The solid line corresponds to levitated aldehyde in a mixture of ACN : emimBF4, while the dotted line corresponds to levitated aldehyde in emimBF4. The shifted band is shown by an arrow. | ||
After the stability of the ionic liquid and reagents under the experimental conditions had been assured, the reactions were monitored in the region between 1400–2350 cm−1. Raw spectra were normalized to eliminate the influence of concentration in the first two minutes due to co-solvent evaporation and the possible fluctuations of the droplet. Normalization of data obtained from levitated droplets has been also used by other authors. For example, Wood et al.22 used a characteristic feature of their spectra to compensate for the fluctuations in the Raman intensities due to slight movements of the drop in the levitator. Weis et al.36 introduced an internal standard to compensate for the effect of solvent evaporation in the study of enzyme kinetics in supercooled water droplets. In the present work, the ionic liquid used as reaction medium acted as an internal standard itself, thus avoiding the need of an additional substance for normalization. For this, the RTIL band located at 1423 cm−1 was selected since reagents and product show no Raman bands here. This band is related to the symmetric bending of the methyl group in the cation of emimBF4.34
In Fig. 4a the Knoevenagel reaction carried out with 3,4-dihydroxybenzaldehyde is shown. In the region 1500–1750 cm−1 the carbonyl stretching band decreased and one band arose at 1580 cm−1, which can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching. Due to conjugation with the aromatic ring and the nitrile groups, this band appears at lower frequency and has a strong Raman intensity. More complex spectra were obtained when the reaction was carried out with cinnamic aldehyde (Fig. 4b), where bands at 1565, 1580, and 1610 cm−1 appeared. During the reaction, the C
C stretching. Due to conjugation with the aromatic ring and the nitrile groups, this band appears at lower frequency and has a strong Raman intensity. More complex spectra were obtained when the reaction was carried out with cinnamic aldehyde (Fig. 4b), where bands at 1565, 1580, and 1610 cm−1 appeared. During the reaction, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bands of cinnamic aldehyde decreased. Moreover, in the region from 2200 to 2350 cm−1, where nitrile groups can be observed (data not shown), the initial nitrile stretching band of malononitrile located at 2273 cm−1 was shifted towards lower wavenumbers (ca. 2231 cm−1) and suffered a strong intensity increase due to the conjugation with double bonds present in both products.
C bands of cinnamic aldehyde decreased. Moreover, in the region from 2200 to 2350 cm−1, where nitrile groups can be observed (data not shown), the initial nitrile stretching band of malononitrile located at 2273 cm−1 was shifted towards lower wavenumbers (ca. 2231 cm−1) and suffered a strong intensity increase due to the conjugation with double bonds present in both products.
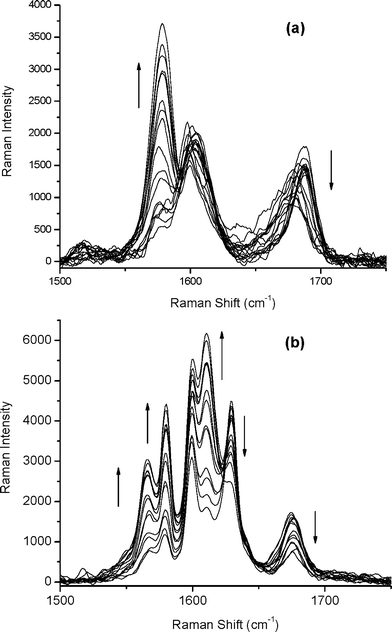 | ||
| Fig. 4 Monitoring of the Knoevenagel condensation by Raman microscopy when the reaction was carried out with (a) 3,4-dihydroxybenzaldehyde or (b) with cinnamic aldehyde. | ||
With the proposed setup, the sequence in which the reagents are dispensed into the node could be easily permutated. In this way, different experiments were executed. The reaction was also carried out dispensing firstly malononitrile, then the corresponding aldehyde, and finally the catalyst. The results agreed with those above. Similar results were obtained for the sequence aldehyde/catalyst/malononitrile. Nevertheless, the reaction hardly progressed if malononitrile and KOH were dispensed before the aldehyde. The reason for this is seen in the dimerization of malononitrile in the presence of the base.37 In that case, the carbanion, which is formed by deprotonation of malononitrile, as well as the formation of the dimer could be detected spectroscopically (data not shown).
The ability of the proposed system to rapidly explore reaction conditions not only offers opportunities for optimizing yield and selectivity, but also provides sufficient data to investigate reaction paths and chemical kinetics. In this sense, the multivariate curve resolution (MCR) technique can be used to extract information about the temporal evolution of the concentrations of reactants and products and to recover the pure spectra of the involved compounds. Experimental data from the reaction between cinnamic aldehyde and malononitrile were analyzed as example. The first step was to establish the number of components in the system using principal component analysis. Two principal components explained 99.93% of the accumulative variance for the reaction. It is clear that the rank of the data matrix was lower than the number of chemical components involved. The two reactants were considered as only one component since their concentration changed at the same rate during the experiment. Furthermore, since data have been normalized to a Raman band of the ionic liquid and due to the fact that it never appeared alone, the ionic liquid could not be detected as an independent contribution thus adding rank-deficiency.38 Resolution of a single rank-deficient matrix is not feasible. Therefore, rank augmentation of the rank deficient data matrix was carried out by column-wise matrix augmentation39 with a set of Raman spectra of levitated pure emimBF4. In this way the rank of the data matrix could be augmented to three.
Initial estimates of concentration profiles were built by taking traces at three wavenumbers corresponding to characteristic bands of emimBF4 (1569 cm−1), aldehyde (1629 cm−1) and product (1610 cm−1). Non-negativity for spectra and concentration profiles and unimodality for concentration profiles were the constraints applied during ALS iteration. Results are shown in Fig. 5, where concentration profiles and spectra of pure components for the condensation have been plotted. First of all, the spectrum of the ionic liquid, emimBF4, was recovered and the concentration profile showed that it was constant during the reaction, as expected. As the acquisition of Raman spectra started with the addition of the catalyst, some product was already present in the first recorded spectrum. This is reflected in the obtained concentration profiles. The pure spectra of the reagent and product were also obtained. As can be seen in Fig. 5b, the reagent presented bands at 1689, 1629, and 1600 cm−1, which corresponded to the carbonyl group, conjugated double bond to the carbonyl group and aromatic ring, respectively, as assigned above. On the other hand, 5 has bands at 1610, 1600, 1580, and 1565 cm−1. The band centered at 1600 cm−1 corresponds with aromatic ring that is present in both, reagent and product, whereas, the band centered at 1580 cm−1 is assigned to the double bond conjugated with the nitrile groups. Finally, bands centered at 1610 and 1565 cm−1 are characteristic for the symmetric and asymmetric stretching observed in the conjugated dienes (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) without centre of symmetry.40
C–) without centre of symmetry.40
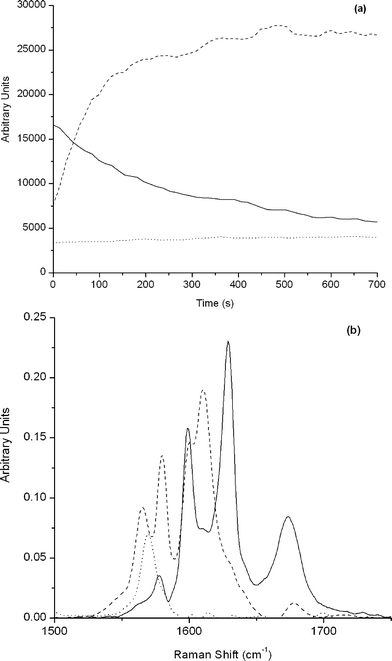 | ||
| Fig. 5 Multivariate curve resolution analysis for the reaction between cinnamic aldehyde and malononitrile. (a) Concentration profiles and (b) spectra of pure components. Solid line, dashed and dotted line correspond to cinnamic aldehyde, cinnamylidenemalononitrile and emimBF4, respectively. | ||
Conclusions and outlook
A versatile system comprised of a microdispenser to dose picolitre-sized droplets, and an ultrasonic trap able to catch them, has been developed for levitating a nano-litre droplet of a room temperature ionic liquid. This drop acted as a miniaturized wall-free reactor located in the focus of a confocal Raman microscope to monitor the reaction. The versatility of the system has also been demonstrated by changing the dispensing sequence of reagents. In this way, information about chemical interactions, such as solvation processes as well as occurring chemical reactions has been obtained. Further developments in this field could be envisioned. For example, as the ultrasonic field can create several nodes at once, in which droplets can be levitated, it would be possible to place an array of levitated droplets to in situ monitor different reactions or the same reaction, but under different conditions. Similarly to chip based approaches, low reagent consumption can be achieved with the important advantage of avoiding channel blockage problems. Thus, it could be possible to perform heterogeneous reactions. Finally, special properties of room temperature ionic liquids could also be exploited; for example by using chiral RTILs as the reaction medium, the enantiomeric ratio of products could be determined.41Acknowledgements
M.L.P., A.D.V., and M.J.A.C. thank the Spanish Ministry of Education and Science for pre-, postdoctoral and mobility grants, respectively.References
- A. R. de Carvalho, M. N. Sanchez, J. Wattoom and R. G. Brereton, Talanta, 2006, 68, 1190–1200 CrossRef CAS.
- E. Venardou, E. Garcia-Verdugo, S. J. Barlow, Y. E. Gorbaty and M. Poliakoff, Vib. Spectrosc., 2004, 35, 103–109 CrossRef CAS.
- E. Furusjo and L. G. Danielsson, Anal. Chim. Acta, 1998, 373, 83–94 CrossRef CAS.
- A. Dominguez-Vidal, M. P. Saenz-Navajas, M. J. Ayora-Cañada and B. Lendl, Anal. Chem., 2006, 78, 3257–3264 CrossRef CAS.
- M. Krishnan, V. Namasivayam, R. S. Lin, R. Pal and M. A. Burns, Curr. Opin. Biotechnol., 2001, 12, 92–98 CrossRef CAS.
- M. Petersson, J. Nilsson, L. Wallman, T. Laurell, J. Johansson and S. Nilsson, J. Chromatogr., B: Biomed. Appl., 1998, 714, 39–46 CrossRef CAS.
- O. Rohling, C. Weitkamp and B. Neidhart, Fresenius’ J. Anal. Chem., 2000, 368, 125–129 CrossRef CAS.
- S. Santesson, M. Andersson, E. Degerman, T. Johansson, J. Nilsson and S. Nilsson, Anal. Chem., 2000, 72, 3412–3418 CrossRef CAS.
- E. Welter and B. Neidhart, Fresenius’ J. Anal. Chem., 1997, 357, 345–350 CrossRef CAS.
- E. G. Lierke, Forsch. Ingenieurwes., 1995, 61, 201–215 Search PubMed.
- S. K. Chung and E. H. Trinh, J. Cryst. Growth, 1998, 194, 384–397 CrossRef CAS.
- K. F. Kelton, G. W. Lee, A. K. Gangopadhyay, R. W. Hyers, T. J. Rathz, J. R. Rogers, M. B. Robinson and D. S. Robinson, Phys. Rev. Lett., 2003, 90, 195504-1–195504-4.
- M. D. Simon and A. K. Geim, J. Appl. Phys., 2000, 87, 6200–6204 CrossRef CAS.
- J. Huisken and E. H. Stelzer, Opt. Lett., 2002, 27, 1223–1225 Search PubMed.
- K. Bücks and H. Müller, Z. Phys., 1933, 84, 75–86.
- E. G. Lierke, Forsch. Ingenieurwes., 1996, 62, 21–31 Search PubMed.
- R. R. Whymark, Ultrasonics, 1975, 13, 251–261 CrossRef CAS.
- C. Esen, T. Weigel, W. Sprynchak and G. Schweiger, J. Quant. Spectrosc. Radiat. Transfer, 2004, 89, 79–85 CrossRef CAS.
- A. Biswas, J. Cryst. Growth, 1995, 147, 155–164 CrossRef CAS.
- S. Santesson, J. Johansson, L. S. Taylor, I. Levander, S. Fox, M. Sepaniak and S. Nilsson, Anal. Chem., 2003, 75, 2177–2180 CrossRef CAS.
- N. Leopold, M. Haberkorn, T. Laurell, J. Nilsson, J. R. Baena, J. Frank and B. Lendl, Anal. Chem., 2003, 75, 2166–2171 CrossRef CAS.
- B. R. Wood, P. Heraud, S. Stojkovic, D. Morrison, J. Beardall and D. McNaughton, Anal. Chem., 2005, 77, 4955–4961 CrossRef CAS.
- J. L. Anderson, D. W. Armstrong and G. T. Wei, Anal. Chem., 2006, 78, 2892–2902 CrossRef.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297 CrossRef CAS.
- U. Kragl, M. Echstein and N. Kaftzik, Curr. Opin. Biotechnol., 2002, 13, 565–571 CrossRef CAS.
- H. Olivier-Bourbigou and L. Magna, J. Mol. Catal. A: Chem., 2002, 182–183, 419–437 CrossRef CAS.
- P. Formentín, H. García and A. Leyva, J. Mol. Catal. A: Chem., 2004, 214, 137–142 CrossRef CAS.
- F. A. Khan, J. Dash, R. Stapathy and S. K. Upadhyay, Tetrahedron Lett., 2004, 45, 3055–3058 CrossRef CAS.
- D. W. Morrison, D. C. Forbes and J. H. Davis, Tetrahedron Lett., 2001, 42, 6053–6055 CrossRef CAS.
- T. Laurell, L. Wallman and J. Nilsson, J. Micromech. Microeng., 1999, 9, 369–376 CrossRef.
- R. Tauler and A. de Juan, Multivariate Curve Resolution – Alternating Least Squares, MATLAB code, University of Barcelona, 1999 Search PubMed.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and G. Sartori, Green Chem., 2000, 2, 101–103 RSC.
- K. Yamashita, T. Tanaka and M. Hayashi, Tetrahedron, 2005, 61, 7981–7985 CrossRef CAS.
- S. A. Katsyuba, P. J. Dyson, E. E. Vandyukova, A. V. Chernova and A. Vidis, Helv. Chim. Acta, 2004, 87, 2556–2565 CrossRef CAS.
- M. López-Pastor, M. J. Ayora-Cañada, M. Valcárcel and B. Lendl, J. Phys. Chem. B, 2006, 110, 10896–10902 CrossRef CAS.
- D. D. Weis and J. D. Nardozzi, Anal. Chem., 2005, 77, 2558–2563 CrossRef CAS.
- M. López-Pastor, A. Dominguez-Vidal, M. J. Ayora-Cañada, M. Valcárcel and B. Lendl, J. Mol. Struct., 2006 DOI:10.1016/j.molstruc.2006.03.004 , in press.
- J. Diewok, A. de Juan, R. Tauler and B. Lendl, Appl. Spectrosc., 2002, 56, 40–50 CrossRef CAS.
- J. Saurina, S. Hernandez-Cassou, R. Tauler and A. Izquierdo-Ridorsa, J. Chemom., 1998, 12, 183–203 CrossRef CAS.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies, Wiley, 2001 Search PubMed.
- C. D. Tran, D. Oliveira and S. Yu, Anal. Chem., 2006, 78, 1349–1356 CrossRef CAS.
Footnote |
| † The HTML version of this article has been enhanced with colour images. |
| This journal is © The Royal Society of Chemistry 2007 |
