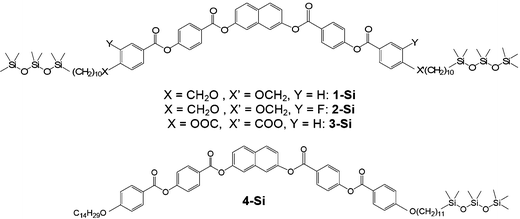
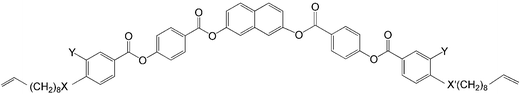
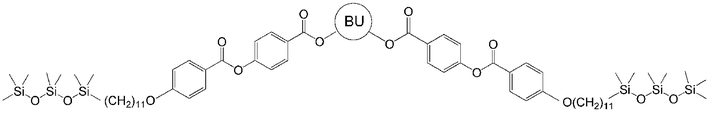
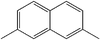
Influence of the core structure on the development of polar order and superstructural chirality in liquid crystalline phases formed by silylated bent-core molecules: naphthalene derivatives†‡
R. Amaranatha Reddy§a, U. Baumeisterb, C. Keitha and C. Tschierske*a
aInstitute of Organic Chemistry, Martin-Luther-University Halle-Wittenberg, Kurt-Mothes-Str. 2, D-06120, Halle, Germany. E-mail: carsten.tschierske@chemie.uni-halle.de; Fax: +49 345 552 7346; Tel: +49 345 552 5664
bInstitute of Physical Chemistry, Martin-Luther-University Halle-Wittenberg, Mühlpforte, D-06108, Halle, Germany
First published on 24th November 2006
Abstract
Bent-core molecules based on a bent 2,7-disubstituted naphthalene unit and heptamethyltrisiloxane units at one or both ends of the aliphatic side chains were synthesized and investigated by polarized light microscopy, differential scanning calorimetry, X-ray scattering and electrooptical investigations. The effect of fluorine substitution at the periphery of the rigid core and of the replacement of ether oxygens by carboxylate groups as linking units between the aromatic core and the flexible terminal chains was also investigated. All compounds form broad regions of liquid crystalline phases. Different types of non-polar and polar, i.e. antiferroelectric and ferroelectric switching smectic phases, and columnar phases were observed, depending on the molecular structure. Most interestingly, a temperature dependent transition from an antiferroelectric switching to a ferroelectric switching oblique columnar phase was observed. All double-silylated compounds show switching by a collective rotation around the molecular long axis, a process which switches the superstructural chirality in these LC systems.
1. Introduction
Materials with macroscopic polar order are of significant interest for piezoelectric, pyroelectric and nonlinear optical applications. Fluid polar ordered materials with liquid crystalline properties are of special interest for fast switching electrooptical devices (displays) and liquid crystal over silicon (LCOS) devices, used for spatial light modulators (SLMs) and optical correlators.1Meyer et al. first reported that ferroelectricity can occur in liquid crystalline phases with reduced symmetry.2 This is achieved when enantiomerically enriched chiral molecules are arranged in layers and have an average tilt with respect to the layer normal (e.g. SmC* phases).3,4 In 1996 Niori et al. reported that (anti)ferroelectricity in smectic phases can also be achieved by achiral molecules with a chevron shape, also known as banana molecules or bent-core molecules.5 The high values of the polarization (Ps = 600–1000 nC cm−2), the occurrence of new LC phase types, unknown for other LC materials, the observation of chirality and spontaneous symmetry breaking in some cases, as well as the special switching behavior of some mesophases attracted special interest in this field.6–8
These unique properties are due to the restricted rotation of the bent molecules, giving rise to a polar direction parallel to the layers (see Fig. S1 in ESI†). In order to escape from the macroscopic polar order the bent direction usually alternates in adjacent layers, leading to antipolar (antiferroelectric = AF) ground state structures in most cases. These SmCPA phases show AF switching (see Fig. S1a†). Beside these smectic phases there are also columnar phases composed of ribbon-like segments of these layers which adopt a long range order in a 2D lattice. Rectangular (Colr) and oblique (Colob) lattices can be distinguished in most cases.9 Most of these ribbon phases are non-switchable (Colr or Colob) or show AF switching (ColrPA or ColobPA).10,11 Several attempts have been made to design bent-core molecules with stable polar order in the absence of an electric field, leading to ferroelectric (FE) switching materials (see Fig. S1a†).12 Some FE switching smectic phases and also FE switching columnar phases have been reported (ColrPFE or ColobPFE) occasionally.13 The possible modes of AF and FE organization are summarized in Fig. S1b.†
Beside the polar order, chirality in LC phases of non-chiral molecules and the observation of switching of the chirality sense by external fields gave rise to broad and general scientific interest in bent-core mesogens.7c–e,14 Chirality in the mesophases of non-chiral bent-core molecules is due to the tilted organization of the molecules in the polar layers or ribbons. This combination of tilt and polar direction leads to the loss of mirror symmetry. Hence, layer normal, polar direction and tilt direction define either a right-handed or a left-handed system. Any change of polarization direction or tilt direction changes the chirality sense of the systems, whereas simultaneously changing both polar direction and tilt direction retains the chirality sense (see Fig. S2†).6
Oligo(siloxane) units at one or both ends of bent-core compounds have a large impact upon mesophase type, polar order and chirality.15–17 Several of these materials exhibit surface stabilized FE switching (SmCPFE phases), and in addition show spontaneous achiral symmetry breaking, seen as a spontaneous formation of regions with opposite chirality sense in the so-called dark-conglomerate texture, designated with “[*]” in the phase assignment.7c,8 For this reason a large variety of bent-core molecules based on oligosiloxane and carbosilane segments have been synthesized in recent years.15–17 As exemplified in the series of compounds in Scheme 1, the AF switching smectic phase (SmCPA) of the dialkyl-substituted molecule Bp-H18 is replaced by a smectic phase showing ferroelectric switching (SmCPFE[*]) after introduction of the first heptamethyltrisiloxane unit.15a,c This smectic phase is replaced by a non-polar columnar phase (Colob), an AF switching columnar (ColobPA) and AF as well as FE switching smectic phases (SmCPA, SmCPFE) if a second silyl group is attached to the other end (compound Bp-Si2).17 In addition the mechanism of the switching process is changed by the introduction of silyl units. The switching in the mesophases of Bp-H and Bp-Si1 takes place by a collective rotation of the bent-core molecules on a cone. This process, which is usually observed for bent-core molecules, reverses the polar direction as well as the tilt direction and hence retains the chirality of the layers as well as the macroscopic chirality (Fig. S2a†). In contrast, the switching in all mesophases of compounds Bp-Si2, silylated at both ends, takes place by a collective rotation around the long axis which changes only the polar direction, whereas the tilt direction is not changed. In this switching process the chirality of the layers and hence the mesophase chirality is changed (Fig. S2b†).14,17 These effects of the silyl groups are mainly due to the nano-segregation of the oligo(siloxane) units into distinct sublayers located at the inter-layer interfaces, and due to the size of these units which influences the packing density between the aromatic cores and in addition can lead to a distortion of an organization in flat layers.
![Comparison of the mesomorphic properties of a compound consisting of conventional bent-core molecules (compound Bp-H18) with a related one having one heptamethyltrisiloxane-1-yl unit at one end (compound Bp-Si115a,c) and compound Bp-Si2 having heptamethyltrisiloxane units at both ends.17 In this compound there is an additional p-substituted benzene ring in the bent aromatic core which gives rise to significantly enhanced mesophase stabilities in comparison to compounds Bp-H and Bp-Si1; abbreviations: Cr = crystalline state; Iso = isotropic liquid state; SmCPA = antiferroelectric switching birefringent SmC phase; SmCPFE[*] = ferroelectric switching SmC phase which is optically isotropic and composed of a conglomerate of optically active domains of opposite handedness (dark conglomerate phase); SmCPFE = ferroelectric switching birefringent SmC phase; ColobPA = antiferroelectric switching oblique columnar phase; Colob = non-switchable oblique columnar phase.](/image/article/2007/JM/b614089k/b614089k-s1.gif) | ||
| Scheme 1 Comparison of the mesomorphic properties of a compound consisting of conventional bent-core molecules (compound Bp-H18) with a related one having one heptamethyltrisiloxane-1-yl unit at one end (compound Bp-Si115a,c) and compound Bp-Si2 having heptamethyltrisiloxane units at both ends.17 In this compound there is an additional p-substituted benzene ring in the bent aromatic core which gives rise to significantly enhanced mesophase stabilities in comparison to compounds Bp-H and Bp-Si1; abbreviations: Cr = crystalline state; Iso = isotropic liquid state; SmCPA = antiferroelectric switching birefringent SmC phase; SmCPFE[*] = ferroelectric switching SmC phase which is optically isotropic and composed of a conglomerate of optically active domains of opposite handedness (dark conglomerate phase); SmCPFE = ferroelectric switching birefringent SmC phase; ColobPA = antiferroelectric switching oblique columnar phase; Colob = non-switchable oblique columnar phase. | ||
In nearly all silylated materials the 3,4′-disubstituted biphenyl unit18 was used as the central bent unit (BU) within the bent aromatic core.15,16a–c,f,g,17 In order to improve our understanding of the general structure–property relations of bent-core mesogens, to further modify the mesomorphic properties and to find new LC phases with interesting application properties we have modified the bent aromatic core. Herein we report the effects of replacing the 3,4′-biphenyl core used in compounds Bp-Si2 and Bp-Si1 by a 2,7-disubstituted naphthyl unit,19 leading to compounds 1-Si and 4-Si, respectively. In addition, the effect of lateral substituents at the outer phenyl rings (Y = F, 2-Si) and the type of linking unit X connecting the terminal chains to the bent rigid cores (ether unit in 1-Sivs. ester unit in 3-Si) was investigated for the double silylated bent-core molecule 1-Si.
2. Results and discussion
2.1. Synthesis
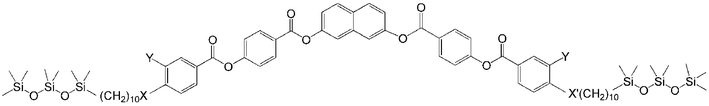
The general synthetic pathway used to obtain compounds 1-Si to 3-Si is outlined in Scheme 2. As shown, the 4-(benzoyloxy)benzoic acids B were obtained by the esterification reaction of 4-(10-undecen-1-yloxy)benzoic acid, of 3-fluoro-4-(10-undecen-1-yloxy)benzoic acid or 4-(9-decen-1-yloxycarbonyl)benzoic acid with 4-hydroxybenzaldehyde to give A, followed by the oxidation of the aldehyde to the corresponding benzoic acid B.20 These substituted benzoic acids were further esterified with naphthalene-2,7-diol to yield the olefinic precursors 1-En to 3-En.19d The hydrosilylation of these olefinic compounds using 1,1,1,3,3,5,5-heptamethyltrisiloxane resulted in the corresponding siloxane substituted compounds 1-Si to 3-Si.17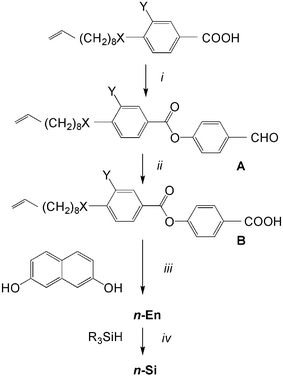 | ||
| Scheme 2 Synthesis of compounds 1-Si to 3-Si (X = CH2O, OOC; Y = H, F; R3SiH = Me3Si(OMe2Si)2H,). Reagents and conditions: i: 4-hydroxybenzaldehyde, DCC, DMAP, dry CH2Cl2, ii: NaClO2, NaH2PO4, resorcinol, t-BuOH, iii: DCC, DMAP, dry CH2Cl2, iv: Karstedt’s cat., dry toluene, 25 °C, 48 h. | ||
The unsymmetrical olefin 4-En with a double bond at only one end was synthesized from the precursor C, similar to earlier reports,19d by esterification with 4-(10-undecen-1-yloxy)benzoic acid. Hydrosilylation with 1,1,1,3,3,5,5-heptamethyltrisiloxane yielded compound 4-Si with only one silyl unit (see Scheme 3). All target compounds were purified using centrifugal thin layer chromatography followed by several crystallizations. The purity was checked by thin-layer chromatography. The chemical structure of all these compounds was confirmed by 1H-, 13C-, 19F-, 29Si-NMR and elemental analysis (see ESI† for details). The mesophase behaviour was investigated by polarized light microscopy, differential scanning calorimetry, electro-optical investigations and X-ray scattering using powder investigations (Guinier film camera) and 2D patterns of aligned samples.
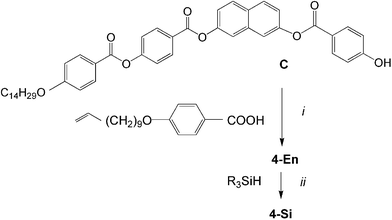 | ||
| Scheme 3 Synthesis of monofunctionalized compounds 4-En and 4-Si (R3SiH = Me3Si(OMe2Si)2H). Reagents and conditions: i: DCC, DMAP, CH2Cl2, ii: Karstedt’s cat., toluene, 25 °C, 48 h. | ||
2.2. Mesomorphic properties of the olefinic precursors
Transition temperatures and associated enthalpies for the intermediate compounds n-En with olefinic double bonds on both the terminals are collated in Table 1. Compound 1-En exhibits an enantiotropic AF switching smectic phase, which is optically isotropic and shows chiral domains of opposite handedness, as seen by slightly uncrossing the polarizers.19d Hence, it belongs to the dark conglomerate phases and this phase is assigned as SmCPA[*]. Though the earlier reports indicate that this mesophase is a non-polar BX phase,19d our observations clearly demonstrate two polarization current peaks indicating an AF switching which is shown in Fig. S3.† Compound 2-En with fluorine substituents at the two outer phenyl rings, in ortho positions to the terminal alkoxy chains, also shows a smectic phase with chiral domains. However for this compound no electro-optical response can be observed up to a voltage of about 400 Vpp (peak-to-peak voltage) for a 5 µm thick cell, the maximum voltage attainable from our experimental set-up. Though no switching is found experimentally, the appearance of a dark conglomerate texture suggests the presence of polar order, but the voltage required for switching is obviously not reached. Therefore this mesophase is assigned as a SmCP[*] phase.| Comp. | Y | X | X′ | Phase transitions | Ref. |
|---|---|---|---|---|---|
| a SmCP[*] = smectic C phase with dark conglomerate texture which does not show electrooptical switching under the used experimental conditions (see text), ColobPFE = ferroelectric switching oblique columnar phase, for the other abbreviations, see Scheme 1; transition temperatures were determined from DSC heating scans (10 K min−1). | |||||
| 1-En | H | CH2O | OCH2 | Cr 126 [37] SmCPA[*] 158 [17] Iso | 19d |
| 2-En | F | CH2O | OCH2 | Cr 154 [65] SmCP[*] 171 [18] Iso | — |
| 3-En | H | OOC | COO | Cr 155 [52] ColobPFE 170 [24] Iso | — |
The most interesting LC phase was observed for compound 3-En, with ester groups linking the terminal chains to the rigid core. The optical texture shown in Fig. 1a indicates the columnar nature of the mesophase. The X-ray diffraction pattern of a partially aligned sample (Fig. 1b,c) can be indexed to an oblique cell. In this way the lattice parameters a = 56.4 Å; b = 46 Å and γ = 114° were obtained (see Table 1 and Table S1†). Most columnar phases of bent-core mesogens represent modulated smectic phases built up by ribbon-like fragments of smectic layers organized in a 2D lattice. The parameter a provides information about the cross section of the ribbons. It was estimated that the total number of molecules per unit cell (ncell) amounts to 8–9, which means that on average 8–9 molecules are located in the cross section of each ribbon (see Table S2†).21 The parameter b is smaller than the molecular length (L = 55 Å)22 which indicates that the molecules are tilted with respect to direction b. The scattering in the wide-angle region with a maximum at d = 4.7 Å is diffuse and indicates the liquid crystalline character of the mesophase. The position of the maxima of the wide angle scattering between meridian and equator and the unequal distribution of the intensity with respect to the meridian reveals a synclinic tilt (tilt angle ≈ 27°) of the molecules in this mesophase (see Fig. 1b and S4†).
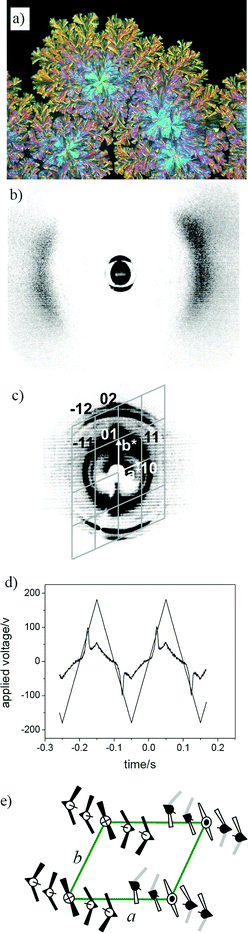 | ||
| Fig. 1 The mesophase of compound 3-En: (a) optical photomicrograph (crossed polarizers) at 168 °C during growing from the liquid state (dark region); (b) XRD pattern obtained for a partially aligned sample at 162 °C, intensity of the isotropic liquid is subtracted to enhance the visibility of the anisotropic distribution of the diffuse scattering (the slightly higher intensity at the top right suggests a synclinic tilt of the molecules); (c) small angle pattern at 162 °C; (d) switching current response attained under a triangular wave field (360 Vpp, 5 Hz, 5 µm, 155 °C); (e) model of the organization in the ribbon phase (only 5 of the 8–9 molecules located in the cross section of the ribbons are shown). | ||
The transition enthalpy (ΔH = 24 kJ mol−1) for the Colob to Iso transition is rather large (Table 1). As no sharp reflections in the wide angle region of the X-ray diffraction pattern can be found (see Fig. 1b) a highly ordered (semi-crystalline) mesophase can be excluded. In this case the high transition enthalpy is a hint of a polar organization in this mesophase. A single polarization current peak was detected in electrooptical experiments only at a very high voltage of more than 300 Vpp in a 5 µm cell (see Fig. 1d). It disappears at the transition to the isotropic liquid state and the appearance of this peak is associated with a change of the texture. Hence, it could be possible that either a FE switching smectic phase is induced at high voltage or FE switching of the columnar phase itself is observed under these conditions. Upon slow cooling under an AC field partially aligned circular domains were obtained which do not change their position upon reversal of the applied electric field.23 In these domains the extinction crosses are inclined with the polarizers, which confirms the synclinic organization of the molecules in this mesophase. Due to the relatively dense packing of the molecules the switching process requires very high voltages. In the case of compound 3-En the dense packing might be due to the presence of the electron deficient terephthalate units which increase the attractive π–π interactions between adjacent aromatic rings. The best packing of the molecules in a ribbon phase with a tilted organization of the molecules is provided in an AF structure where the polar direction changes within the modulated smectic layers along direction a and with a synpolar order along direction b (see Fig. 1e). In such an arrangement all molecules are aligned parallel at the inter-ribbon interfaces. The synpolar order between adjacent ribbons along direction b requires a splay of the polar direction, as shown in Fig. 1e, to reduce the polarization within the layer stacks and to improve the packing of the molecules at the inter-ribbon interfaces.24 Due to the rather large number of molecules organized in the cross section of the ribbons the occurrence of some splay of polarization is likely.25 The fact that related bent-core mesogens with ester bonds interconnecting the rigid core with the alkyl chains show B7 type mesophases with FE switching additionally supports this model.13a,b,26
2.3. Mesomorphic properties of Si-containing compounds
Compound 1-Si in which the 3,4′-disubstituted biphenyl unit is replaced by a 2,7-disubstituted naphthalene central unit shows three different mesophases. The high temperature phase shows a typical SmC schlieren texture, but also a broken fan texture can be observed (Fig. 2a,b). The phase transitions to the other mesophases can be observed optically by slight textural changes (see Fig. 2c,d). The high temperature phase is a tilted smectic phase (d = 44.7 Å, L = 69.5 Å22). The other two mesophases are columnar phases as indicated by XRD of aligned samples (Fig. 2f,g, Fig. 3, Table 4 and Table S1†).
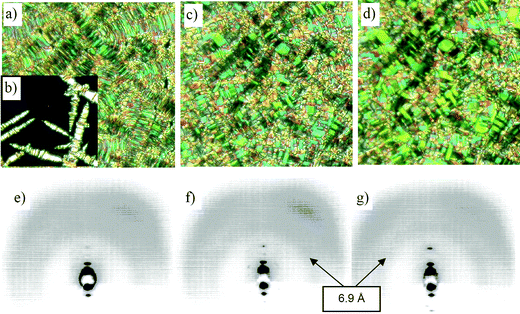 | ||
| Fig. 2 (a)–(d) Optical textures and (e)–(g) XRD patterns of aligned samples of the mesophases of compound 1-Si: (a) SmC phase at 171 °C; (b) Iso–SmC transition at 173 °C; (c) paramorphotic texture of the ColobPA phase at 160 °C; (d) paramorphotic texture of the ColobPFE phase at 140 °C; (e) SmC phase at 171 °C; (f) ColobPA phase at 160 °C; (g) ColobPFE phase at 140 °C. | ||
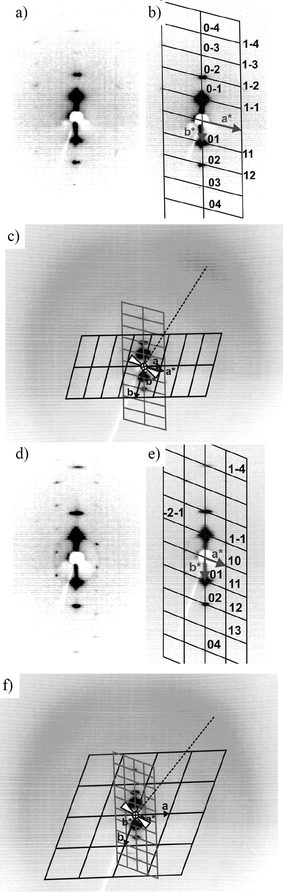 | ||
| Fig. 3 Indexing of the 2D X-ray patterns for a surface-aligned sample of compound 1-Si in the ColobPA (160 °C, (a)–(c)) and in the ColobPFE phase (140 °C, (d)–(f)); (a),(d) original patterns and (b),(e) reciprocal lattices with hk values for the observed reflections in the small-angle region; (c),(f) full scattering range with reciprocal lattice (gray lines), real lattice (black lines), direction of the maximum of the outer diffuse scattering (dashed line), and the most probable orientation of the molecules (wedge shapes) with respect to the axes of the 2D unit cell. | ||
| Comp. | T/°C | Phase type | Lattice parameters/Å; ° | ncell |
|---|---|---|---|---|
| a For details see Table S1 (ESI); abbreviations: ncell = molecules per unit cell, for the calculations see Table S2 (ESI). | ||||
| 3-En | 162 | ColobPFE | a = 56.4; b = 46; γ = 114° | 8–9 |
| 1-Si | 160 | ColobPA | a = 23.8, b = 45.1; γ = 105° | 2–3 |
| 140 | ColobPFE | a = 51.2, b = 47.5; γ = 110.4° | 5–6 | |
| 2-Si | 151 | Colob | a = 84.5; b = 53.2; γ = 113.4° | 10–11 |
| 3-Si | 170 | Colob | a = 87.9, b = 55.5; γ = 114.5° | 11 |
In all diffraction patterns the diffuse scattering in the wide angle region indicates a synclinic tilted organization of the molecules in all three LC phases (tilt angle about 50° in the two phases at higher temperatures, about 45° in the low-temperature phase) with microsegregated oligosiloxane sublayers (diffuse ring at d = 6.9 Å). Electrooptical investigations (see below for a more detailed discussion) indicate a non-polar SmC phase at high temperature, the columnar phase below this smectic phase shows AF switching (ColobPA) which changes to FE at the transition to the low temperature columnar phase (ColobPFE).
The XRD pattern of the high temperature columnar phase (Fig. 2f, 3a) can be indexed to a rectangular cell with the lattice parameters a = 23.8 Å and b = 87 Å. However, the proven synclinic tilted organization of the molecules in this columnar phase (see wide angle region in Fig. 2f which shows a clear maximum of the intensity at the right top) excludes a true rectangular lattice. In fact this mesophase has an oblique lattice with the parameters a = 23.8 Å, b = 45.1 Å and γ = 105° (Fig. 3b), which accidentally coincides with a larger pseudo-rectangular cell, and therefore this mesophase is assigned as ColobPA. In Fig. 4a the pseudo-rectangular lattice is shown in dotted black lines together with an oblique lattice (shown in solid lines) in a possible model of the ColobPA phase. It should be pointed out that this phase assignment, based on non-resonant X-ray scattering, cannot distinguish the polar direction of the columns. The assignment to an overall AF structure is based on electrooptical investigations which indicate an AF switching in this mesophase. There are several different possible modes for such an AF organization. In the most probable model, shown in Fig. 4a, there is an overlapping of the rod-like wings of the bent cores at the interfaces between adjacent ribbons in the modulated layers, as also proposed for the Colob phase of 3-En. However the actual organization is slightly different, as there is no interaction of the aromatic cores across adjacent layers. This avoids any unfavourable interruption of the oligosiloxane sublayers. A change of the polar direction between each ribbon within these modulated layers (along direction a) provides a parallel organization of the rod-like wings at the interfaces between the ribbons (see Fig. 4a, inset). In addition, it is assumed that the correlation between adjacent ribbons is synclinic and synpolar, as typically observed for the preferred organization in the smectic phases of the silylated bent-core molecules.15d,16g,17b This organization would correspond to the monoclinic/oblique layer group p112/a,10b,28 indicated by arrows in Fig. 4a. A splay of polarization is unlikely in this case as the ribbons have only a few molecules in the cross section.
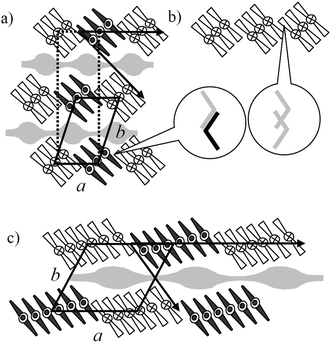 | ||
| Fig. 4 Proposed models for (a) the ColobPA phase of 1-Si (the dotted rectangle indicates the pseudo-rectangular lattice, the solid lines indicate the oblique lattice and the black arrows indicate the settings of the layer group, the light gray areas indicate the position of the oligosiloxane subspaces); (b) FE order within the modulated layers leads to unfavourable inter-ribbon interfaces; the insets in (a) and (b) show the organization of the aromatic cores at the inter-ribbon interfaces in a side view along direction a; (c) model of the AF ground state structure assumed for the ColobPFE phases of 1-Si. | ||
On decreasing temperature, a complete change of the XRD small angle pattern could be observed at the transition to the lowest temperature phase (see Fig. 3d,e) and these reflections can be indexed to an oblique cell with the lattice parameters a = 51.2 Å, b = 47.5 Å and γ = 110.4° (Tables 4 and S1†). Hence, the lattice parameter a is more than doubled whereas the parameters b and γ are only slightly increased. This means that the number of molecules in the cross section of the ribbons is nearly doubled from 2–3 to 5–6 (see Tables 4 and S2†) at the phase transition. As no significant change of the wide angle scattering is observed at the transition between the two columnar phases (see Fig. 2f,g), the tilt direction of the molecules should not be fundamentally changed at this transition. As seen in Fig. 2f and g there is a maximum intensity of the outer scattering at the right side in both mesophases. Such an asymmetric distribution arises from a packing of molecules with synclinic tilt in a sample consisting of polydomains that are not perfectly fibre-like disordered around an axis perpendicular to the alignment surface, but with a preferred orientation. In this case the orientation of the molecules with respect to the axes of the 2D lattice can be deduced from the position of this maximum. The actual tilt angle of the molecules with respect to the b axis (see Fig. 3c,f) may be estimated from the direction of the maximum of the outer scattering with respect to the b* axis on the meridian of the 2D X-ray patterns (= 90° − tilt angle) and the lattice parameter γ as γ − (90° − tilt angle). This tilt is almost the same in both phases and amounts to about 65°. But the tilt of the molecules with respect to direction a (90° − tilt angle) changes from about 40° in the high temperature phase to about 45° in the lower temperature phase, i.e. the molecules become less inclined against the normal to the broken layers, and simultaneously the ribbons become broader at the phase transition.
A model of the ColobPFE phase is shown in Fig. 4c. As will be explained later, the ground state structure of this mesophase is assumed to be AF, though a FE switching is observed experimentally. The organization of the molecules is very similar to that proposed for the ColobPA phase observed for 1-Si at higher temperature with the difference that the ribbons have a larger size.
As already mentioned, the high-temperature columnar phase shows AF switching (ColobPA) which changes to FE in the temperature range of the low temperature Colob phase (ColobPFE, see Fig. 5b). The polarization values are in the range of 200–300 nC cm−2 in both mesophases. On slow cooling from the isotropic liquid under a triangular wave field, circular domains with extinction crosses inclined with the direction of the polarizers were obtained which indicate a synclinic tilt of the molecules in all three phases (Fig. 6). In addition, optical observations imply that the polarization reversal takes place around the long axis of the molecules in the AF as well as in the FE columnar mesophase as indicated by the textures shown in Fig. 6, where no rotation of the extinction crosses can be observed, either in the non-switching SmC phase (Fig. 6a) or in the two polar switching columnar phases (Fig. 6b,c).
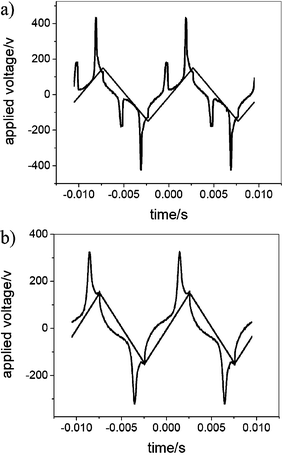 | ||
| Fig. 5 Switching current response traces obtained under a triangular wave (300 Vpp, 100 Hz, 5 µm) for the mesophases of compound 1-Si: (a) ColobPA phase at 155 °C, (b) ColobPFE phase at 135 °C. | ||
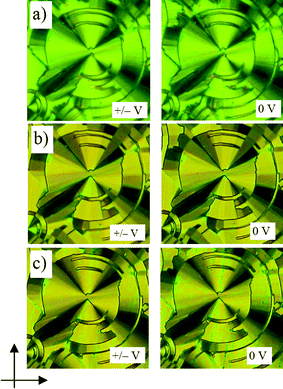 | ||
| Fig. 6 Circular domains with extinction crosses as grown under a triangular wave voltage (300 Vpp, 100 Hz, 5 µm) for compound 1-Si, their position under the applied field (left) is compared with that after switching off the applied field (right): (a) SmC phase at 172 °C; (b) ColobPA phase at 160 °C; (c) ColobPFE phase at 140 °C; the positions of the extinctions are inclined with the polarizers (indicated by arrows) which indicates a synclinic organization in all mesophases; in (a) and (c) the circular domains are smooth indicating a smectic phase, a segmented structure can clearly be seen in (b), there is a slightly lower birefringence in the 0 V states for (b) and (c) which is typically seen for a polar switching by rotation around the long axis. | ||
The behaviour of the ColobPA phase is in line with the observations made with other ColobPA phases of bent-core molecules with silyl groups.15,17 For these mesophases it is assumed that the unfavourable inter-ribbon interfaces occurring in the FE organization along the modulated layers (see Fig. 4b) are responsible for the stabilization of the AF ground state structure, leading to high threshold voltages and relatively low polarization values.15c,17b
The FE switching at lower temperature is more difficult to explain. In principle it is possible that the layer distortion is removed under the alignment field and a smectic phase is induced. In this induced smectic phase the unfavourable inter-ribbon interfaces (stabilizing an AF organization) are largely removed, and hence, surface stabilized FE switching can take place, as typical for the smectic phases (SmCPFE) of other silylated bent-core molecules.15,16g This is in line with the behaviour of other silylated bent core molecules where a transition from ColobPA phases at higher temperature to smectic and undulated smectic phases at lower temperature is often observed.15c,17 This transition is due to the larger thermal expansion coefficient of the oligosiloxane units in comparison to the other parts of the molecules, giving rise to a stronger steric frustration caused by these units at higher temperature. This is in line with the observation that the size of the ribbons is nearly doubled at the phase transition ColobPA to ColobPFE. Also the smooth appearance of the texture seen under an electric field (Fig. 6c), which is similar to that of the SmC phase (Fig 6a) but different from the ColobPA phase (Fig. 6b) is in line with the assumption of a field induced SmCPFE phase. However, in these SmCPFE phases the switching between the FE states usually occurs at very low voltage, closely after zero-voltage crossing and high values of the spontaneous polarization (600–1000 nC cm−2) were measured.15,16a–c,f,g,17b This is clearly not the case for the switching of the ColobPFE phase which is characterized by relatively low polarization values and a rather high threshold voltage. One possible reason could be that in the field induced smectic phase there is still some layer undulation or layer modulation which provides an energetic barrier for the switching between the two field induced FE states.
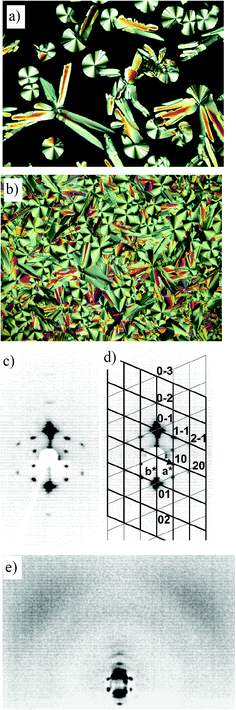 | ||
| Fig. 7 Investigation of compound 2-Si: (a) optical texture (crossed polarizers) obtained at the Iso–Colob transition at 178 °C, (b) natural texture of the Colob phase at 170 °C; (c)–(e) 2D X-ray patterns for a surface-aligned sample of compound 2-Si in the Colob phase (151 °C): (c) small angle region; (d) indexing of the reflections in the small-angle region; reciprocal lattices of the two mainly scattering domains of the fibre-like disordered sample (black and gray lines, respectively) with hk values for the observed reflections and axes given for the black lattice; (e) complete diffraction pattern. | ||
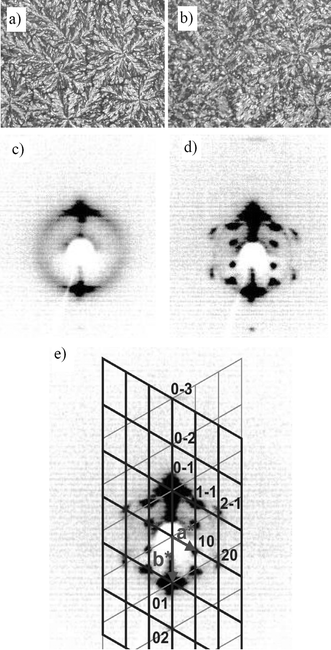 | ||
| Fig. 8 Optical textures (crossed polarizers) and XRD pattern obtained for compound 3-Si: (a) texture of the USmC phase at 178 °C; (b) texture of the Colob phase at 170 °C; (c) small angle XRD patterns of an aligned sample in the USmC phase at 178 °C; (d) in the Colob phase at 150 °C; (e) indexation of the Colob phase showing the reciprocal lattices of the two mainly scattering domains of the fibre-like disordered sample (black and gray lines, respectively) with hk values for the observed reflections and axes given for the black lattice. | ||
2.4. Functionalization of only one end—compounds 4-En and 4-Si
For comparison we have also synthesized the naphthalene derived compound 4-Si with an oligosiloxane unit at only one terminus. This compound shows a completely different mesophase than the double silylated compound 1-Si. The texture is optically isotropic and exhibits chiral domains of opposite handedness under slightly uncrossed polarizers as shown in Fig. 9a–c (dark conglomerate texture). XRD indicates a smectic phase with a layer spacing of d = 45.5 Å at 130 °C which is again smaller than the calculated molecular length (L = 66 Å)22 suggesting a tilted organization. Moreover, this mesophase shows FE switching (see Fig. 9d) with a polarization of about 440 nC cm−2. Hence the mesophase is similar to that observed for the monosilylated biphenyl derivative Bp-Si1 (SmCPFE[*] phase, see Table 2).15c However, the value of the spontaneous polarization is smaller. This is because the aromatic bent core is smaller than that of Bp-Si1. In this case the steric effect of the silyl groups becomes more important. As a consequence, the aromatic cores become more separated and polar order is reduced.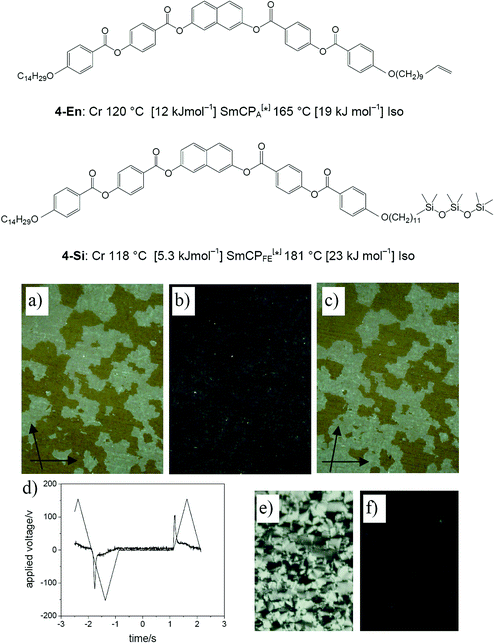 | ||
| Fig. 9 Characterization of the mesophase of compound 4-Si: (a)–(c) textures at 150 °C; (a), (c) were obtained with slightly uncrossed polarizers showing macroscopic chiral domains; (b) dark texture as seen between crossed polarizers; (d) switching current response trace obtained under a modified triangular wave field (300 Vpp, 0.5 Hz, 5 µm) at 130 °C, indicating a surface stabilized FE switching; (e) birefringent textures obtained under these conditions; (f) relaxation to the dark texture within a few seconds after removal of the applied field. | ||
It is also interesting to note that the two very different compounds, the non-silylated compound 4-En and the silylated compound 4-Si, both show the same dark conglomerate textures, though they have a different switching behaviour. Compounds related to 4-En (with decyl and dodecyl chains) were reported to show no switching,19d but in our experiments with a higher voltage (>75 Vpp µm−1) we can indicate an AF switching for 4-En (SmCPA[*] phase). Introduction of a heptametyltrisiloxane segment at one end changes the interlayer interfaces in such a way that only one polarization current peak is observed for the mesophase of 4-Si. This peak does not split under a modified triangular wave voltage (see Fig. 9d) and indicates FE switching. This behaviour is very similar to that observed for the related biphenyl derivatives and suggests that this mesophase is composed of [SmCsPF]aPA layer stacks (see Fig. 10b) as explained earlier.15c,16g,17b For 4-Si the size of these SmCsPF layer stacks is sufficiently large to efficiently stabilize the FE states by polar interaction with the substrate surfaces and hence FE switching is experimentally observed.17b In addition the steric layer distorting effect of only a single silyl group is not sufficient to induce a ribbon structure and therefore FE switching is retained.
![Models showing the modes of organization of compound 4-Si. The assignment of the phases is given at the bottom, where the structure within the layer-stacks (SmCsPF) is given in square brackets and the following subscripts define the correlation of the interfaces between the layer stacks (a = anticlinic, s = synclinic, A = antipolar, S = synpolar); (a) apparently anticlinic and synpolar rac-[SmCsPF]aPS structure as obtained by cooling under an applied AC voltage; (b) the two enantiomeric [SmCsPF]aPA structures as obtained either by cooling from the isotropic liquid state without applied field or in the relaxation process after switching off an applied AC voltage, it seems that the formation of this structure is associated with a layer deformation into a dark texture for which a sponge-like deformation of the layers is proposed, the chiral domains are microscopic in size (no chiral domains can be detected) if the phase is formed by relaxation from structure (a) and macroscopic upon cooling from the isotropic liquid (dark conglomerate texture).17b](/image/article/2007/JM/b614089k/b614089k-f10.gif) | ||
| Fig. 10 Models showing the modes of organization of compound 4-Si. The assignment of the phases is given at the bottom, where the structure within the layer-stacks (SmCsPF) is given in square brackets and the following subscripts define the correlation of the interfaces between the layer stacks (a = anticlinic, s = synclinic, A = antipolar, S = synpolar); (a) apparently anticlinic and synpolar rac-[SmCsPF]aPS structure as obtained by cooling under an applied AC voltage; (b) the two enantiomeric [SmCsPF]aPA structures as obtained either by cooling from the isotropic liquid state without applied field or in the relaxation process after switching off an applied AC voltage, it seems that the formation of this structure is associated with a layer deformation into a dark texture for which a sponge-like deformation of the layers is proposed, the chiral domains are microscopic in size (no chiral domains can be detected) if the phase is formed by relaxation from structure (a) and macroscopic upon cooling from the isotropic liquid (dark conglomerate texture).17b | ||
Interestingly, the birefringent textures induced under an applied electric field are stable only under the field, on terminating the applied field the birefringence disappears rather quickly and the field of view becomes dark as shown in Fig. 9f. It seems that upon field removal the layers relax to a randomly deformed layer structure, similar to the dark conglomerate phase in the ground state. Such a relaxation was also found for the SmCPFE[*] phases of some disilylated bent-core molecules with 3,4′-disubstituted biphenyl based bent-core units17b and it was attributed to a relaxation of the field induced racemic [SmCsPF]aPS structure into a (+)/(−)-[SmCsPF]sPA structure (see Fig. 10) which immediately forms the optically isotropic texture, as explained in ref. 17b. As there is an equal probability for the relaxation either into a (+)-[SmCsPF]sPA or into a (−)-[SmCsPF]sPA structure (follow either i or ii in Fig. 10) the chiral domains are microscopically mixed and the optically isotropic state obtained after field removal does not exhibit macroscopic chiral domains. This kind of relaxation requires a rotation of the molecules around the long axis (see Fig. 10). It seems that in the series of monosilylated compounds this relaxation process is more easy for the 2,7-disubstituted naphthalene derivatives which have a shorter rigid core than for the analogous 3,4′-disubstituted biphenyl derivatives, with a larger rigid core, where it is not observed or significantly slower.15c
This indicates that an oligosiloxane unit at only one of the wings is sufficient to induce FE switching, while retaining the layer structure and polar order. The second siloxane unit in compound 1-Si further increases the steric packing frustration, giving rise to a transition to columnar ribbon phases where the inter-ribbon interfaces destabilize the FE states and change the switching process to AF. A complete loss of polar order is observed in this case at higher temperature.
3. Summary and conclusions
Novel bent-core molecules based on a rigid core incorporating a bent 2,7-disubstituted naphthalene unit with oligosiloxane terminated alkyl chains at one or both ends have been synthesized and their mesophase behaviour has been investigated.Despite the smaller size of the 2,7-naphthyl unit in comparison to the 3,4′-biphenyl unit these compounds exhibit enhanced mesophase stabilities. This is rather unexpected and indicates that probably stronger π-stacking interactions in the aromatic sublayers (flat π-system of the naphthalene core in comparison to the twisted biphenyl unit) are of significant importance for the mesophase stability. This and also the enhanced symmetry of the naphthalene unit leads to increased melting temperatures and this is the major reason for the smaller mesophase ranges observed for the naphthalene derived compounds in most cases (see Table 2). It is also interesting to note that all non-silylated naphthalene derivatives n-En require very high voltages for electrooptical switching. This might also be caused by the relatively strong π-stacking interactions which provide a more dense packing of the bent cores. The bulky silyl groups can reduce the packing density in the aromatic sublayers and this contributes to the reduction of the threshold voltages. However, this reduced packing density also reduces the polarization values and influences the switching mechanism. These effects are stronger for the naphthalene derivatives, because the packing of these shorter bent cores can more easily be distorted.
Bent-core compounds having only one heptamethyltrisiloxane unit are characterized by smectic phases showing surface stabilized FE switching as well as dark conglomerate textures (SmCPFE[*] phases), irrespective of whether a 3,4′-biphenyl or a 2,7-naphthalene structure is incorporated into the bent aromatic core. However, the polarization values are reduced for the naphthalene derivative due to the reduced size of the bent aromatic core.
Introduction of a second heptamethyltrisiloxane unit at the other end completely removes the SmCPFE[*] phases and the polar smectic phases are replaced by a non-polar smectic phase and by columnar phases. This is due to the steric frustration arising from the packing of the bulky silyl groups in a layer structure. It leads to a fragmentation of these layers into ribbon-like segments which adopt a regular organization in a 2D lattice, giving rise to columnar ribbon phases. Only at high temperature can a non-polar smectic phase arise, in which the molecules can freely rotate, as in smectic phases of calamitic molecules. It seems that in this case the increased rotational disorder of the rigid cores at higher temperature (no polar order) increases the effective space required by the aromatic cores and this can partly compensate the steric frustration provided by the silyl units. With decreasing temperature the space occupied by the silyl groups is reduced. As a consequence polar order can set in and due to the more dense packing of the aromatic cores the layer distorting effect of the silyl groups is increased. This leads to the formation of ribbons and in the resulting columnar phase the FE state is destabilized, giving rise to an AF switching oblique columnar phases (ColobPA). With further decreasing temperature the layer frustration is further reduced and this gives rise to an increase of the size of the ribbons, which in turns allows a relatively easy (complete or partial) removal of the layer frustration under an electric field and in this field induced mesophase FE switching takes place (ColobPFE). The introduction of fluorine on the outer phenyl rings (2-Si) as well as introduction of carboxylate groups as linking units (3-Si) removes polar order. This is surprising, as in related non-silylated molecules (e.g.3-En) these units favour a polar packing and FE switching.
As another consequence of the steric frustration and the less dense packing of the aromatic cores the switching process within the polar smectic and columnar phases takes place by a collective rotation around the long axis for all disilylated compounds. This switching process is of special interest as it switches the superstructural chirality in these LC systems.
In summary, these investigations have contributed to a better understanding of the structure–property relationships in bent-core LCs, especially concerning the effects of micro-segregation and packing density upon the mesophase structure and the mode of switching.
Acknowledgements
R. A. Reddy is grateful to the Alexander von Humboldt Foundation for a research fellowship.References
- T. D. Wilkinson, C. D. Henderson, D. Gil Leyva and W. A. Crossland, J. Mater. Chem., 2006, 16, 3359 RSC.
- R. B. Meyer, L. Liebert, L. Strzelecki and P. Keller, J. Phys., 1975, 36, L–69 Search PubMed.
- (a) J. W. Goodby, J. Mater. Chem., 1991, 1, 307 RSC; (b) S. T. Lagerwall, Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, H.-W. Spiess and V. Vill, Wiley-VCH,Weinheim, 1998, vol. 2B, p. 515 Search PubMed.
- K. Miyachi and A. Fukuda, Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, H.-W. Spiess and V. Vill, Wiley-VCH,Weinheim, 1998, vol. 2B, p. 665 Search PubMed.
- (a) T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, J. Mater. Chem., 1996, 6, 1231 RSC; (b) T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, Mol. Cryst. Liq. Cryst., 1997, 301, 337 CrossRef CAS; (c) T. Sekine, Y. Takanishi, T. Niori, J. Watanabe and H. Takezoe, Jpn. J. Appl. Phys., 1997, 36, L1201 CrossRef; (d) T. Sekine, T. Niori, J. Watanabe, T. Furukawa, S. W. Choi and H. Takezoe, J. Mater. Chem., 1997, 7, 1307 RSC.
- D. R. Link, G. Natale, R. Shao, J. E. Maclennan, N. A. Clark, E. Körblova and D. M. Walba, Science, 1997, 278, 1924 CrossRef CAS.
- Recent reviews: (a) G. Pelzl, S. Diele and W. Weissflog, Adv. Mater., 1999, 11, 707 CrossRef CAS; (b) C. Tschierske and G. Dantlgraber, Pramana, 2003, 61, 455 Search PubMed; (c) R. Amaranatha Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907 RSC; (d) M. B. Ros, J. L. Serrano, M. R. De La Fuente and C. L. Folcia, J. Mater. Chem., 2005, 15, 5093 RSC; (e) H. Takezoe and Y. Takanishi, Jpn. J. Appl. Phys., 2006, 45, 597 CrossRef CAS.
- Dark conglomerate phases of non-silylated molecules: (a) J. Thisayukta, Y. Nakayama, S. Kawauchi, H. Takezoe and J. Watanabe, J. Am. Chem. Soc., 2000, 122, 7441 CrossRef CAS; (b) J. Thisayukta, H. Kamee, S. Kawauchi and J. Watanabe, Mol. Cryst. Liq. Cryst., 2000, 346, 63 CrossRef CAS; (c) H. N. Shreenivasa Murthy and B. K. Sadashiva, Liq. Cryst., 2002, 29, 1223 CrossRef; (d) J. Ortega, C. L. Folcia, J. Etxebarria, N. Gimeno and M. B. Ros, Phys. Rev. E, 2003, 68, 011707 Search PubMed; (e) W. Weissflog, M. W. Schröder, S. Diele and G. Pelzl, Adv. Mater., 2003, 15, 630 CrossRef CAS; (f) A. Jakli, Y.-M. Huang, K. Fodor-Csorba, A. Vajda, G. Galli, S. Diele and G. Pelzl, Adv. Mater., 2003, 15, 1606 CrossRef CAS; (g) M. W. Schröder, S. Diele, G. Pelzl, U. Dunemann, H. Kresse and W. Weissflog, J. Mater. Chem., 2003, 13, 1877 RSC; (h) M. W. Schröder, G. Pelzl, U. Dunemann and W. Weissflog, Liq. Cryst., 2004, 31, 633 CrossRef; (i) W. Weissflog, S. Sokolowski, H. Dehne, B. Das, S. Grande, M. W. Schröder, A. Eremin, S. Diele, G. Pelzl and H. Kresse, Liq. Cryst., 2004, 31, 923 CrossRef CAS.
- Hexagonal columnar phases (Colh) are extremely rare in this class of compounds and have distinct structures: (a) E. Gorecka, D. Pociecha, J. Mieczkowski, J. Matraszek, D. Guillon and B. Donnio, J. Am. Chem. Soc., 2004, 126, 15946 CrossRef CAS; (b) S. Kang, J. Thisayukta, H. Takezoe, J. Watanabe, K. Ogino, T. Doi and T. Takahashi, Liq. Cryst., 2004, 31, 1323 CrossRef CAS; (c) H. Takezoe, K. Kishikawa and E. Gorecka, J. Mater. Chem., 2006, 16, 2412 RSC.
- Examples of columnar phases formed by bent-core molecules: (a) J. Watanabe, T. Niori, T. Sekine and H. Takezoe, Jpn. J. Appl. Phys., 1998, 37, L139 CrossRef CAS; (b) K. Pelz, W. Weissflog, U. Baumeister and S. Diele, Liq. Cryst., 2003, 30, 1151 CrossRef CAS; (c) D. Kardas, M. Prehm, U. Baumeister, D. Pociecha, R. Amaranatha Reddy, G. H. Mehl and C. Tschierske, J. Mater. Chem., 2005, 15, 1722 RSC; (d) Y. Takanishi, H. Takezoe, J. Watanabe, H. Takahashi and A. Lida, J. Mater. Chem., 2006, 16, 816 RSC; (e) C. L. Folcia, J. Etxebarria, J. Ortega and M. B. Ros, Phys. Rev. E, 2006, 74, 031702 Search PubMed; (f) C. L. Folcia, I. Alonso, J. Ortega, J. Etxebarria, I. Pintre and M. B. Ros, Chem. Mater., 2006, 18, 4617 CrossRef CAS.
- Polar switching columnar phases: (a) J. Mieczkowski, K. Gomola, J. Koseska, D. Pociecha, J. Szydlowska and E. Gorecka, J. Mater. Chem., 2003, 13, 2132 RSC; (b) H. N. Shreenivasa Murthy and B. K. Sadashiva, J. Mater. Chem., 2003, 13, 2863 RSC; (c) R. Amaranatha Reddy, B. K. Sadashiva and V. A. Raghunathan, Chem. Mater., 2004, 16, 4050 CrossRef CAS; (d) J. P. Bedel, J. C. Rouillon, J. P. Marcerou, H. T. Nguyen and M. F. Achard, Phys. Rev. E, 2004, 69, 061702 Search PubMed; (e) H. N. Sheenivasa Murthy and B. K. Sadashiva, J. Mater. Chem., 2004, 14, 2813 RSC; (f) R. Amaranatha Reddy, B. K. Sadashiva and U. Baumeister, J. Mater. Chem., 2005, 15, 3303 RSC; (g) E. Gorecka, N. Vaupotic, D. Pociecha, M. Cepic and J. Mieczkowski, ChemPhysChem, 2005, 6, 1087 CrossRef CAS.
- Examples of FE switching bent-core materials without silyl units: (a) M. Nakata, D. R. Link, F. Araoka, J. Thisayukta, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Liq. Cryst., 2001, 28, 130; (b) J. P. Bedel, J. C. Rouillon, J. P. Marcerou, M. Laguerre, H. T. Nguyen and M. F. Achard, J. Mater. Chem., 2002, 12, 2214 RSC; (c) R. Amaranatha Reddy and B. K. Sadashiva, J. Mater. Chem., 2002, 12, 2627 RSC; (d) R. Amaranatha Reddy and B. K. Sadashiva, Liq. Cryst., 2003, 30, 1031 CrossRef; (e) S. Rauch, P. Bault, H. Sawade, G. Heppke, G. G. Nair and A. Jakli, Phys. Rev. E, 2002, 66, 021706 Search PubMed; (f) K. Kumazawa, M. Nakata, F. Araoka, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, J. Mater. Chem., 2004, 14, 157 RSC; (g) H. Nadasi, W. Weissflog, A. Eremin, G. Pelzl, S. Diele, B. Das and S. Grande, J. Mater. Chem., 2002, 12, 1316 RSC; (h) S. K. Lee, S. Heo, J.-G. Lee, K.-T. Kang, K. Kumazawa, K. Nishida, Y. Shimbo, Y. Takanishi, J. Watanabe, T. Doi, T. Takahashi and H. Takezoe, J. Am. Chem. Soc., 2005, 127, 11085 CrossRef CAS; (i) K. Nishida, M. Cepic, W. J. Kim, S. K. Lee, S. Heo, J. G. Lee, Y. Takanishi, K. Kishikawa, K.-T. Kang, J. Watanabe and H. Takezoe, Phys. Rev. E, 2006, 74, 021704 Search PubMed; (j) V. Novotna, M. Kaspar, V. Hamplova, M. Glogarova, L. Lejcek, J. Kroupa and D. Pociecha, J. Mater. Chem., 2006, 16, 2031 RSC.
- FE switching undulated and modulated smectic phases: (a) D. M. Walba, E. Körblova, R. Shao, J. E. Maclennan, D. R. Link, M. A. Glaser and N. A. Clark, Science, 2000, 288, 2181 CrossRef CAS; (b) J. P. Bedel, J. C. Rouillon, J. P. Marcerou, M. Laguerre, H. T. Nguyen and M. F. Achard, Liq. Cryst., 2000, 27, 1411 CrossRef CAS; (c) R. Amaranatha Reddy, V. A. Raghunathan and B. K. Sadashiva, Chem. Mater., 2005, 17, 274 CrossRef CAS; (d) W. Weissflog, G. Naumann, B. Kosata, M. W. Schroder, A. Eremin, S. Diele, Z. Vakhovskaya, H. Kresse, R. Friedemann, S. Ananda Ramakrishnan and G. Pelzl, J. Mater. Chem., 2005, 15, 4328 RSC; (e) S. Umadevi, B. K. Sadashiva, H. N. Shreenivasa Murthy and V. A. Raghunathan, Soft Matter, 2006, 2, 210 RSC.
- Examples of switching of suprastructural chirality by collective rotation around the long axis: (a) M. W. Schröder, S. Diele, G. Pelzl and W. Weissflog, ChemPhysChem, 2004, 5, 99 CrossRef; (b) J. Szydlowska, J. Mieczkowski, J. Matraszek, D. W. Bruce, E. Gorecka, D. Pociecha and D. Guillon, Phys. Rev. E, 2003, 67, 031702 Search PubMed; (c) R. Amaranatha Reddy, M. W. Schröder, M. Bodyagin, H. Kresse, S. Diele, G. Pelzl and W. Weissflog, Angew. Chem., Int. Ed., 2005, 44, 774 CrossRef CAS; (d) W. Weissflog, U. Dunemann, M. W. Schröder, S. Diele, G. Pelzl, H. Kresse and S. Grande, J. Mater. Chem., 2005, 15, 939 RSC; (e) M. Nakata, R. F. Shao, J. Maclennan, W. Weissflog and N. A. Clark, Phys. Rev. Lett., 2006, 96, 067802 CrossRef CAS.
- Silicon containing bent-core molecules: (a) G. Dantlgraber, A. Eremin, S. Diele, A. Hauser, H. Kresse, G. Pelzl and C. Tschierske, Angew. Chem., Int. Ed., 2002, 41, 2408 CrossRef CAS; (b) C. Keith, R. Amaranatha Reddy, H. Hahn, H. Lang and C. Tschierske, Chem. Commun., 2004, 1898 RSC; (c) C. Keith, R. Amaranatha Reddy, A. Hauser, U. Baumeister and C. Tschierske, J. Am. Chem. Soc., 2006, 16, 3051 CrossRef; (d) R. Amaranatha Reddy, G. Dantlgraber, U. Baumeister and C. Tschierske, Angew. Chem., Int. Ed., 2006, 45, 1928 CrossRef.
- Silicon containing dimers, oligomers, dendrimers and polymers with bent-core units: (a) G. Dantlgraber, S. Diele and C. Tschierske, Chem. Commun., 2002, 2768 RSC; (b) G. Dantlgraber, U. Baumeister, S. Diele, H. Kresse, B. Lühmann, H. Lang and C. Tschierske, J. Am. Chem. Soc., 2002, 124, 14852 CrossRef CAS; (c) C. Keith, R. Amaranatha Reddy and C. Tschierske, Chem. Commun., 2005, 871 RSC; (d) B. Kosata, G.-M. Tamba, U. Baumeister, K. Pelz, S. Diele, G. Pelzl, G. Galli, S. Samaritani, E. V. Agina, N. I. Boiko, V. P. Shibaev and W. Weissflog, Chem. Mater., 2006, 18, 691 CrossRef CAS; (e) R. Achten, A. Koudijs, M. Giesbers, R. Amaranatha Reddy, T. Verhulst, C. Tschierske, A. T. M. Marcelis and E. J. R. Sudhölter, Liq. Cryst., 2006, 33, 681 CrossRef CAS; (f) H. Hahn, C. Keith, H. Lang, R. Amaranatha Reddy and C. Tschierske, Adv. Mater., 2006, 18, 2629 CrossRef CAS; (g) C. Keith, R. Amaranatha Reddy, U. Baumeister, H. Hahn, H. Lang and C. Tschierske, J. Mater. Chem., 2006, 16, 3444 RSC.
- Bent-core mesogens with two silyl end-groups: (a) C. Keith, R. Amaranatha Reddy, U. Baumeister and C. Tschierske, J. Am. Chem. Soc., 2004, 126, 14312 CrossRef CAS; (b) C. Keith, R. Amaranatha Reddy, U. Baumeister, H. Kresse, J. Lorenzo Chao, H. Hahn, H. Lang and C. Tschierske, Chem. Eur. J. DOI:10.1002/chem.200600876.
- (a) D. Shen, S. Diele, I. Wirth and C. Tschierske, Chem. Commun., 1998, 2573 RSC; (b) D. Shen, A. Pegenau, S. Diele, I. Wirth and C. Tschierske, J. Am. Chem. Soc., 2000, 122, 1593 CrossRef CAS.
- 2,7-Disubstituted naphthalene units as bent units: (a) Amaranatha Reddy and B. K. Sadashiva, J. Mater. Chem., 2004, 14, 1936 RSC; (b) H. N. Shreenivasa Murthy and B. K. Sadashiva, Liq. Cryst., 2004, 31, 1347 CrossRef; (c) V. Kozmik, M. Kuchar, J. Svoboda, V. Novotna, M. Glogarova, U. Baumeister, S. Diele and G. Pelzl, Liq. Cryst., 2005, 32, 1151 CrossRef CAS; (d) V. Kozmik, A. Kovarova, M. Kuchar, J. Svoboda, V. Novotna, M. Glogarova and J. Kroupa, Liq. Cryst., 2006, 33, 41 CrossRef CAS; (e) J. Svoboda, V. Novotna, V. Kozmik, M. Glogarova, W. Weissflog, S. Diele and G. Pelzl, J. Mater. Chem., 2003, 13, 2104 RSC ; see also ref. 8a, 11c,13c.
- R. Achten, A. Koudijs, M. Giesbers, A. T. M. Marcelis and E. J. R. Sudhölter, Liq. Cryst., 2005, 32, 277 CrossRef CAS.
- ncell = molecules per unit cell was estimated from the crystal volumes of the molecules according to A. Immirzi and B. Perini, Acta Crystallogr., Sect. A, 1977, 33, 216 Search PubMed , with a correction for the lower packing density in the mesophase and the volume of a 3D packing unit calculated from the 2D unit cell assuming a height of h = 0.52 nm (for details see ESI† Table S2).
- In all cases the molecular length was determined using CPK models and assuming a 120° bent V-shaped conformation with most extended alkyl chains (all-trans-conformation) and heptamethyltrisiloxane units.
- This can be explained by polarization reversal around the long axis, which would indicate that some layer modulation is retained.
- D. A. Coleman, J. Fernsler, N. Chattham, M. Nakata, Y. Takanishi, E. Korblova, D. R. Link, R.-F. Shao, W. G. Jang, J. E. Maclennan, O. Mondainn-Monval, C. Boyer, W. Weissflog, G. Pelzl, L.-C. Chien, J. Zasadzinski, J. Watanabe, D. M. Walba, H. Takezoe and N. A. Clark, Science, 2003, 301, 1204 CrossRef CAS.
- However, there should be only a slight contribution of splay, at first the number of molecules in the cross section of the ribbons is smaller than in other polarization modulated smectic phases, secondly a splay with a ±90° change of the polar direction is also unlikely, as this should lead to the formation of a rectangular ribbon phase.
- (a) S. Umadevi and B. K. Sadashiva, Liq. Cryst., 2005, 32, 1233 CrossRef CAS; (b) S. Umadevi, A. Jakli and B. K. Sadashiva, Soft Matter, 2006, 2, 875 RSC.
- R. Amaranatha Reddy, U. Baumeister, C. Keith, K. Hahn, H. Lang and C. Tschierske, Soft Matter 10.1039/b615791b.
- V. Kopsky and D. B. Litvin, International Tables of Crystallography, Vol. E, Subperiodic groups, Kluwer Academic Publishers, Dordrecht, 2002 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: additional figures and tables; experimental methods; analytical data. See DOI: 10.1039/b614089k |
| ‡ The HTML version of this article has been enhanced with colour images. |
| § Present address: Dept. of Chemistry & Biochemistry, University of Colorado at Boulder, Boulder, CO 80309-0215, USA. |
| This journal is © The Royal Society of Chemistry 2007 |