Pore architecture affects photocatalytic activity of periodic mesoporous nanocrystalline anatase thin films†
Moises A.
Carreon‡§
a,
Sung Yeun
Choi‡¶
a,
Marc
Mamak||
a,
Naveen
Chopra
b and
Geoffrey A.
Ozin
*a
aMaterials Chemistry Research Group, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada. E-mail: gozin@chem.utoronto.ca; Fax: +1-416-971-2011; Tel: +1-416-978-2082
bXerox Research Centre of Canada, 2660 Speakman Drive, Mississauga, Ontario, Canada
First published on 17th October 2006
Abstract
We report the photocatalytic activity of periodic mesoporous nanocrystalline anatase thin films (denoted meso-nc-TiO2) using Methylene Blue (denoted MB) as a probe of pore architecture effects on reactivity. Specifically, 2D hexagonal and 3D cubic mesoporous nanocrystalline anatase thin films (denoted h-meso-nc-TiO2 and c-meso-nc-TiO2 respectively) annealed at different temperatures were investigated to reveal the effects of different pore architectures on the photocatalytic activity. The adsorption behavior of MB on the films annealed at the same temperature signaled that c-meso-nc-TiO2 has a larger accessible surface area but a lower adsorption surface affinity, compared to h-meso-nc-TiO2. In the case of the solid-state photodegradation of MB, the most efficacious photocatalyst was found to be c-meso-nc-TiO2 annealed at 450 °C. For MB in solution, a 400 °C annealed c-meso-nc-TiO2 was established to have the optimum photocatalytic activity among the samples investigated. The observed superior photocatalytic activity of c-meso-nc-TiO2 relative to both h-meso-nc-TiO2 and nc-TiO2 is believed to originate from the higher photoactivity of anatase nanocrystallites comprising the more open cubic framework. as well as geometrical advantages, such as a larger surface area and less obstructed 3D diffusion paths of guest molecules. It is concluded that the photocatalytic efficiency of periodic mesoporous nanocrystalline anatase thin films depends on the pore architecture.
Introduction
Nanocrystalline titania (denoted nc-TiO2) films have been viewed as a highly attractive material for applications in photovoltaic cells, electrochromic devices, photoconductors, sensors and photocatalysts mainly due to intrinsic properties, such as transparency to visible light, electronic conductivity, high surface affinity towards certain ligands and large surface area.1 Of special note, nc-TiO2 has been recognized as an ideal photocatalyst for the destruction of common organic pollutants in the solid, liquid and vapor phases. This attribute originates from a fortuitous combination of beneficial factors, which include biological and chemical inertness, stability toward photochemical and chemical corrosion, relatively low cost and an electronic band gap that on photoexcitation creates highly oxidizing holes and highly reducing electrons.2,3As mentioned, the photocatalytic activity of titania originates from photogenerated electron–hole pairs produced when it absorbs light having energy equal to or greater than that of its electronic band gap energy. Although most photogenerated electrons and holes are consumed by volume and surface recombination, some proportion of the electrons and holes, which meet an electron donor or acceptor before recombination, can initiate photocatalytic degradation of adsorbed organic species.2 It has been well documented that the recombination rate of photogenerated electron–hole pairs is highly dependent on the crystalline phase, crystallite size and crystallinity of titania, and in this context nanocrystalline anatase is an appealing material.2,4 The high surface to volume ratio of nanocrystalline anatase is advantageous for photocatalysis due to its large surface area and high population of coordinately unsaturated titanium(IV) sites and oxygen(−II) vacancies existing at crystallite corner and edge locations thereby enhancing its photoactivity.
In this regard, recently documented periodic mesoporous forms of titania (denoted meso-TiO2) consisting of organized assemblies of nanocrystalline anatase have gained much attention since this class of materials offer a unique combination of interesting properties that include, high surface area, uniform mesopore size and shape, and a nanocrystalline framework with controllable dimensions and dimensionality.5 The ability to tune the pore architecture and scale of meso-TiO2 is of paramount importance since pore size, shape and dimensionality can exert a profound effect not only on the accessibility, adsorption and diffusion behavior of guest molecules within the pore network but also on the material's mechanical, optical and electrical properties. With the increasing number of publications concerning novel synthesis routes for making meso-TiO2, it is noteworthy that the number of reports which focus on the photocatalytic activity of meso-TiO2 has concomitantly increased.6 Most of these have demonstrated a superior photocatalytic activity for 3D cubic meso-TiO2 compared to nc-TiO2 mainly because of its larger specific surface area and the ordered pore architecture of meso-TiO2 providing facile diffusion pathways to guest molecules. Significantly, the relation between structure and photocatalytic activity for hexagonal and cubic meso-TiO2 remains unknown, although there exists a general consensus that pore architecture is important for fine-tuning the materials properties. In any technology based on meso-nc-TiO2 materials, it is important to elucidate this relationship quantitatively.
Recently, we have reported highly crystalline 2D hexagonal meso-nc-TiO2 synthesized in the novel P123/butanol system.7 The unique and multifaceted role of butanol in this template-based synthesis was emphasized.7 Using this method, we have also succeeded in synthesizing highly crystalline 3D cubic meso-nc-TiO2.8 Herein, we report for the first time the effect of pore architecture on the photocatalytic activity of meso-nc-TiO2 thin films based on the photolytic degradation of methylene blue (denoted MB) utilizing both hexagonal and cubic forms of meso-nc-TiO2 as supports. To obtain an in-depth understanding of the structure–property–reactivity relations in meso-nc-TiO2 the adsorption behavior and photodegradation kinetics of MB on meso-nc-TiO2 both in the solid state and in solution have been investigated. The results establish c-meso-nc-TiO2 to be a better photocatalyst than h-meso-nc-TiO2 not only because of its higher surface area and contiguous 3D pore architecture, but also because of the higher intrinsic photoactivity of anatase nanocrystallites constituting the titania framework.
Experimental
Materials synthesis
Procedures involved in the synthesis of periodic mesoporous titania thin films have been described.7a,8 Briefly, the reactant solution of molar composition 1 Ti(OEt)4 : 2 HCl : 0.013 P123 : 9 1-butanol was prepared for both h-meso-TiO2 and c-meso-TiO2 thin films. The meso-TiO2 thin films were prepared by spin coating (2400 rpm for 20 s for h-meso-TiO2 or 3000 rpm for 15 s for c-meso-TiO2) fresh reactant solution onto a glass substrate for the investigations of photocatalytic activity. The deposited films were aged at 20 °C and 80% relative humidity (RH) for 2 d for h-meso-TiO2 or RT and 95% RH for 18 h for c-meso-TiO2, in a controlled humidity chamber (Laboratory Humidity Chamber LH-1.5, Associated Environmental Systems, Ayer, MA, USA), then subsequently annealed in air by heating at a rate of 1 °C min−1 to 300 °C, holding for 1 h to remove the template and increase cross-linking of the titania framework. These pre-annealed films were thermally treated at 350 °C, 400 °C and 450 °C for 4 h in air to vary the crystallinity and crystallite size of anatase within the frameworks.Conventional nanocrystalline anatase thin films and periodic mesostructured silica thin films were prepared as control and blank samples respectively, based on well-known procedures. A colloidal nanocrystalline TiO2 dispersion was prepared by the Grätzel method described elsewhere.9 After adding polyethylene glycol 20000 (10 wt% equivalent of TiO2, Fluka), the solution was deposited on a glass substrate by spin-coating (3000 rpm for 20 s). Following drying in air for 1 h, the film was thermally treated at 450 °C for 2 h in air. Meso-SiO2 thin films were synthesized using the method described in a previous report.10
HRSEM/STEM analyses were performed using a Hitachi S-5200 (30 kV, 10 mA). All samples for HRSEM were prepared by scraping the thin film samples from the substrate onto carbon film-supported 200 mesh copper grids. XRD data were collected using a PANalytical X'Pert Pro diffractometer equipped with an Anton Paar XRK900 reactor chamber. The crystallinity of thin films was estimated based on the area of the anatase (101) reflection in the XRD pattern obtained from the films deposited on the Si substrate after 4 h annealing at the desired temperature using the diffraction patterns of a blank Si substrate as a background and the diffraction pattern of the same sample obtained after further annealing at 600 °C for 1 h as a 100% crystallinity standard.7c The diffraction data were analyzed using Jade (MDI, Livermore, CA) to profile fit the diffracted and amorphous peaks. Estimated crystallite sizes were extracted from the profile fit of the diffraction peak by the Scherrer formula as implemented in Jade. The instrumental broadening was characterized by use of a monolayer of NIST 640c Si standard reference materials distributed on top of a blank Si substrate.
Adsorption characteristics
The films were immersed in methylene blue solutions with increasing concentrations of 0.5–10 µM for 60 min. The excess of dye was rinsed away with copious amounts of water and anhydrous ethanol. After drying, the extinction of dye on the films was measured using a Perkin Elmer UV/VIS/NIR spectrometer Lambda 900. The peaks in the extinction spectra were deconvoluted using Microcal Origin software to give the extinction of the monomer molecules at 660 nm.Photocatalytic degradation of MB
For the solid-state photodegradation of MB, prior to the photocatalytic degradation experiments, MB was carefully adsorbed to each film in the aqueous solution of which concentrations were calculated based on the Langmuir adsorption isotherm in order to fix the loading amount at a partial coverage of 0.6. MB loaded titania films were exposed to UV irradiation under ambient conditions with an intensity of 100 mW cm−2 produced by an Oriel 40-200 watts UV lamp with Pyrex filters (transmittance >290 nm). The irradiance intensity was measured by a CW Laser Power Meter Model 407A, Spectra-Physics. Photocatalytic degradation experiments were carried out for 2 h.In order to investigate photodegradation of MB in aqueous solution, the films were placed in a small reaction chamber with 5 µM MB aqueous solution (20 ml). After 1 h in the dark to allow MB to adsorb on the films, the photodegradation of MB solution was carried out using the same UV irradiation set-up used in the solid-state experiment. The solution was continuously stirred during UV irradiation.
Results and discussion
Materials
Transparent meso-nc-TiO2 thin films were synthesized using the novel P123/butanol system since this method provides a highly crystalline titania framework along with a well-ordered and large mesopore architecture.7,8 Two types of meso-nc-TiO2 thin film samples were prepared for investigating the effect of pore architecture on the photocatalytic activity of meso-nc-TiO2. These comprise 2D hexagonal meso-nc-TiO2 (denoted h-meso-nc-TiO2) that was produced under film aging conditions of 20 °C and 80% RH while 3D cubic meso-nc-TiO2 (denoted c-meso-nc-TiO2) was prepared under conditions of 25 °C and 95% RH.As seen in Fig. 1a and b, h-meso-TiO2 has a well-defined 1D channel-type pore architecture. The as-synthesized 2D hexagonal mesostructure (p6m) has a hexagonally close-packed arrangement of channels organized parallel to the substrate. The c-meso-TiO2 is comprised of a cubic close-packed array of cage-type pores, which are inter-connected by windows and provide a 3D diffusion path to imbibed guest molecules in contrast to the 2D one of h-meso-TiO2. The symmetry of the as-synthesized c-meso-TiO2 is face-centered-cubic (fm3m) with the [111] direction perpendicular to the substrate.8,11 On thermal post-treatment a unidirectional contraction ensues for meso-TiO2 thin films. This results in a change of symmetry from p6m to c2m for h-meso-TiO2 and fm3m to r![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for c-meso-TiO2.8 As a result, the circular pores of the as-synthesized mesostructures appear elliptical after thermal treatment.
m for c-meso-TiO2.8 As a result, the circular pores of the as-synthesized mesostructures appear elliptical after thermal treatment.
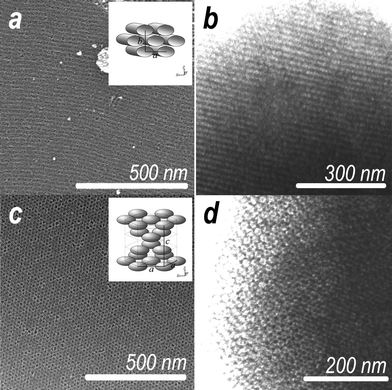 | ||
| Fig. 1 HRSEM and STEM images of h-meso-nc-TiO2 (a and b) and c-meso-nc-TiO2 (c and d) with graphical illustrations depicting pore architectures and lattice parameters (inset). | ||
Representative HRSEM and STEM images of h-meso-TiO2 and c-meso-TiO2 annealed in air at 300 °C are presented in Fig. 1 with accompanying graphical illustrations of the pore architectures including associated lattice parameters (inset). The top surface of h-meso-nc-TiO2 films displayed a swirling pattern originating from topological defects in the precursor mesophase often observed for the channel director of the 2D hexagonal mesostructure (Fig. 1a). The channels are well-ordered and are aligned parallel to the substrate (Fig. 1b). In the case of h-meso-nc-TiO2, the in-plane pore diameter and the wall thickness estimated from STEM images are ∼11 nm and ∼6 nm, respectively. For c-meso-nc-TiO2, the surface of the film displayed hexagonally close-packed open pores (Fig. 1c). STEM images of the kind shown in Fig. 1d confirmed the surface pores comprise the termini of an internal network of 3D interconnected cage-type pores.8,11 The estimated equatorial diameter of cages and the size of interconnecting windows between cages are ∼20 nm and ∼4.5 nm, respectively.
Both types of meso-nc-TiO2 film samples were prepared at three different annealing temperatures, namely 350 °C, 400 °C and 450 °C, leading to three films each having a different surface area, percentage crystallinity and average crystallite size, which have been elucidated (see below). The surface texture of meso-nc-TiO2 coarsened and the pore architecture was slightly deformed with increasing annealing temperature due to titania nanocrystallite growth within the framework during thermal treatment (Fig. S1 in ESI†). Total collapse of the mesostructure, however, was not observed in this temperature region (Fig. S2 in ESI†).7c,8 Conventional nc-TiO2 thin film control samples were prepared using a previously reported method.9 The identity of the crystalline phase, percentage crystallinity and crystallite size of each sample were investigated by XRD, and the film thickness used to estimate the volume of each sample was measured from the HRSEM images of cross-sectional cuts of the films. All samples were established to consist of anatase nanocrystallites in a matrix of amorphous titania. The percentage crystallinity of anatase was estimated using the method described in the Experimental section. The crystallite size was estimated using the FWHM of anatase (101) reflection with the Scherrer equation. The percentage crystallinity, crystallite size and the film volume of samples used in this study are summarized in Table 1.
| Annealing temperature/°C | Crystallinity of anatase (%) | Crystallite size/nm | Film volume/×10−4 cm3 | |
|---|---|---|---|---|
| h-meso-TiO2 | 350 | 53 | 7.0 | 2.88 |
| 400 | 69 | 9.5 | 2.55 | |
| 450 | 86 | 9.9 | 2.44 | |
| c-meso-TiO2 | 350 | 71 | 7.8 | 1.78 |
| 400 | 84 | 9.4 | 1.73 | |
| 450 | 98 | 9.8 | 1.60 | |
| nc-TiO2 | 450 | 100 | 18.5 | 1.16 |
Methylene Blue (denoted MB) was the probe molecule of choice in this study for investigating the comparative photocatalytic activities of meso-TiO2 and nc-TiO2. This choice is predicated on its strong adsorption characteristics on metal oxide surfaces, good resistance to self-photodegradation and its well defined optical absorption at ∼660 nm, which enables facile measurement of the kinetics of photodegradation.12 Typical UV-vis spectra of MB are shown in Fig. 2. The UV-vis spectrum obtained for MB adsorbed in meso-TiO2 film (Fig. 2b) is more-or-less identical to that of an aqueous solution (Fig. 2a) taking account of the spectral background (Fig. 2b′).
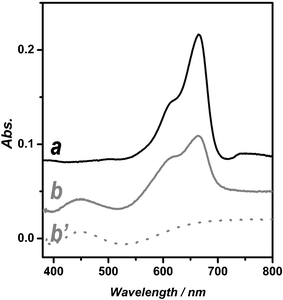 | ||
| Fig. 2 UV-vis spectra of Methylene Blue in aqueous solution (a) and adsorbed on h-meso-nc-TiO2 (b). Note that b′ is the UV-vis baseline spectrum of h-meso-nc-TiO2 before adsorbing Methylene Blue. | ||
Photocatalytic activity of meso-nc-TiO2
When the photocatalytic degradation of organic molecules occurs within the spatial confines of a porous catalyst, the overall process can be described as a series of sequential steps:1. transport of the organic molecules to the active sites on the surface of catalyst through the pores,
2. adsorption of the molecules onto the active sites of the surface,
3. reaction on the surface,
4. desorption of the product(s),
5. transport of the product(s) out of the pores.13
The main factors governing the rate of the overall process of photocatalytic degradation of organic molecules are geometrical factors, such as specific surface area and diffusion pathways, adsorption ability of the catalytic surface toward target molecules and the ability of generating photo-induced electron–hole pairs of active sites. In order to elucidate the importance of these factors the experimental strategy comprised three parts as outlined below.
Adsorption characteristics of Methylene Blue on meso-nc-TiO2
Since the adsorption of organic molecules to the titania surface is a pivotal step in photocatalytic degradation,2 it is of great importance to investigate the adsorption characteristics of MB onto the surface of the differently prepared titania samples. The adsorption characteristics of MB on anatase films can be investigated through the Langmuir adsorption isotherm, which relates the adsorbed concentration of MB to the solution concentration of MB in the equilibrium state. This behavior is described by the expression:| [MB]ads = nmaxK[MB]eq/(1 + K[MB]eq) | (1) |
 | ||
| Fig. 3 Langmuir adsorption parameters: the adsorption affinity K and the saturation adsorption concentration nmax, extracted from the adsorption isotherm of Methylene Blue on h-meso-nc-TiO2 and c-meso-nc-TiO2 thin film samples annealed at different temperatures compared to those of a conventional nc-TiO2 thin film. | ||
The saturation adsorption concentrations, nmax values, of MB obtained for c-meso-nc-TiO2 were larger than those of h-meso-nc-TiO2 for the entire range of annealing temperatures employed in this study. Since MB molecules are adsorbed as a monolayer in this system, the nmax value directly reflects the surface area of titania film. The larger surface area of the cubic structure versus hexagonal structure for similar mesoporous materials is well documented.14 This fact is also supported by comparing their porosity measured by variable angle ellipsometry spectroscopy (VASE) showing that c-meso-nc-TiO2 has 10% higher porosity than h-meso-nc-TiO2 (Fig. S3 in ESI†). With increasing annealing temperature, nmax gradually decreased in both cases. On comparing meso-nc-TiO2 and nc-TiO2, most meso-nc-TiO2 thin films exhibited higher nmax than nc-TiO2. The structural advantages of meso-nc-TiO2 however disappear at 450 °C where one discovers the nmax of c-meso-nc-TiO2 annealed at 450 °C is similar to that of nc-TiO2 while h-meso-nc-TiO2 annealed at 450 °C showed even smaller nmax than that of nc-TiO2.
The adsorption affinity of MB, K, was observed to display an opposite trend to nmax, namely K was observed to increase with higher annealing temperature for both h-meso-nc-TiO2 and c-meso-nc-TiO2 film samples. Here the increased crystallinity of meso-nc-TiO2 with temperature leads to a higher adsorption affinity of the surface. Despite the observation that c-meso-nc-TiO2 samples display higher crystallinity than h-meso-nc-TiO2 over the entire range of annealing temperatures investigated in this study, a higher K was observed for h-meso-nc-TiO2. The fact that crystallinity and K follow opposite trends should alert one that the crystallinity of anatase is not the only factor responsible for determining the adsorption affinity of meso-nc-TiO2. It is well known that adsorption on the surface of titania is ultimately affected by structural and electronic properties of surface states including coordinatively unsaturated surface cations, bridging oxygens, hydroxyl groups and surface adsorbed species, the sensitivity of which depend on the synthesis method used rather than the crystallinity or crystallite size of titania material.2,4 These considerations suggest that the surface properties of c-meso-nc-TiO2 are less favorable for the adsorption of MB than h-meso-nc-TiO2 most probably due to different film aging conditions employed to produce specific pore architectures. It is reasonable to attribute these differences to the employed film aging conditions since both the hexagonal and cubic forms of meso-nc-TiO2 were subjected to identical sol preparation and annealing processes. One should also note that the thermal treatment of meso-nc-TiO2 enhanced the surface adsorption of MB.
Solid-state photocatalytic degradation of Methylene Blue on meso-nc-TiO2
In order to investigate the photocatalytic activities of hexagonal and cubic meso-nc-TiO2 relative to each other as well as control nc-TiO2 films, the solid-state photocatalytic degradation of adsorbed MB was the method of choice. To the best of our knowledge, all photocatalytic studies in the open literature concerning periodic mesoporous titania up until this point in time have been accomplished using an aqueous solution of the probe molecule. In these solution-based systems, the pore architecture is found to highly affect the apparent rate of the decomposition of target molecules since diffusion of both probe molecules and decomposition products is required in order to observe the photocatalytic activity of the system. In contradistinction, the solid-state photodegradation of pre-adsorbed MB allows one to evaluate the photocatalytic activity of the catalyst without the complication of mass transport within the pore network. Furthermore, partial coverage, θMB, was selected as a normalized concentration to obtain the apparent decomposition rate of MB. The common normalization factor employed in the literature is the weight of catalyst, which overlooks the intrinsic geometric factor of catalysts including the porosity and specific surface area.The apparent rate of photocatalytic degradation of MB can be described as a single-component Langmuir–Hinshelwood kinetic equation as expressed below:
| r = −dθMB/dt = kθMB | (2) |
| θMB = [MB]ad/nmax | (3) |
Fig. 4 displays the photocatalytic degradation profiles of MB in h-meso-nc-TiO2 (a) and c-meso-nc-TiO2 (b) film samples together with a nc-TiO2 control film as well as a blank based upon a meso-SiO2 film. Note that meso-SiO2 film was selected as a blank since it is expected to have a very low photocatalytic activity for the photodegradation of MB. The degradation profiles of MB obtained from all samples investigated in this study showed well-defined first order decay kinetics. In both series involving h-meso-nc-TiO2 and c-meso-nc-TiO2 film samples, the decomposition rate of MB increased with annealing temperature. The fastest decomposition of MB was observed in the meso-nc-TiO2 film annealed at 450 °C in both cases. Self-decomposition of MB under the same conditions was negligible, Fig. 4. After two hours, all films with adsorbed MB, with the exception of the blank, turn colorless due to completion of the photodecomposition of MB in the films.
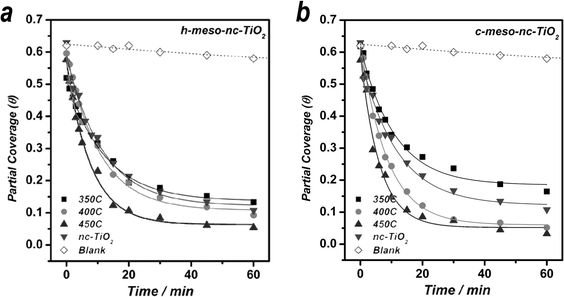 | ||
| Fig. 4 Photocatalytic degradation profiles of MB adsorbed on h-meso-nc-TiO2 (a) and on c-meso-nc-TiO2 (b) films as compared to control nc-TiO2 films at similar MB partial coverage. | ||
The collation of obtained apparent rate constants, k, for the photocatalytic degradation of MB is shown in Fig. 5. The apparent rate constant k increased with increasing annealing temperature for both h-meso-nc-TiO2 and c-meso-nc-TiO2. Since the anatase form of titania has been known as the most photoactive form,2 it is obvious that the increase in crystallinity with increasing annealing temperature led to the increase of the apparent rate constant k. The lower k values observed for h-meso-nc-TiO2 when compared to c-meso-nc-TiO2, throughout the entire range of annealing temperatures, most likely has a common origin, namely the higher crystallinity of c-meso-nc-TiO2 compared to h-meso-nc-TiO2 annealed at the same temperature as presented in the Materials section.
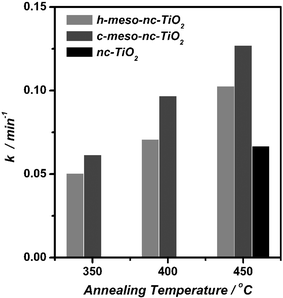 | ||
| Fig. 5 Apparent rate constants (k) obtained for the solid-state photocatalytic degradation of MB in h-meso-nc-TiO2, c-meso-nc-TiO2 and nc-TiO2 annealed at different temperatures. | ||
It is noteworthy that nc-TiO2 showed the lowest k values among samples annealed at 450 °C despite its 100% crystallinity. The k values of c-meso-nc-TiO2 and h-meso-nc-TiO2 were 2 and 1.5 times higher than that of nc-TiO2. Since the amorphous phase of titania does not play a significant role in the photocatalytic process,2 the reason for this difference most likely stems from crystallite size differences.
As mentioned before, most of the photogenerated electron–hole pairs from titania are consumed by volume and surface recombination, and only the charge carriers which meet an electron donor or acceptor before recombination can initiate photocatalytic degradation of adsorbed organic species.2 There are several reports studying the relationship between the recombination rate of photogenerated electron–hole pairs and the particle size of anatase crystallites.15 Serpone et al. investigated the lifetime of charge carriers of titania particles of various sizes using picosecond laser techniques and concluded that ∼10 nm was an optimum size for a photocatalyst, since the lifetime of charge carriers was much shorter in either smaller or larger titania particles due to various recombination processes.15a Their proposal was later substantiated by a report by Zhang et al.15b where they observed that the photocatalytic activity of titania in the photooxidation of CHCl3 increased by decreasing the particle size. The authors interpreted these results to mean that the decrease in particle size results in a reduction of the volume recombination rate and a concomitant increase in the availability of active surface sites. However, below a certain size, they observed a reduction of the photocatalytic activity of titania due to a faster surface recombination rate compared to the interfacial charge carrier process resulting in photodegradation, therefore, an optimal particle size exists: ∼10 nm in the case of titania. This suggestion was reconfirmed by Chae et al. in a photocatalytic study with propanol.15c
The nanocrystallite size of meso-nc-TiO2 used in this study is in the range 7 nm to 10 nm, which is the optimal particle size range defined in the aforementioned reports.15 Therefore, the crystallinity of anatase is the most critical factor governing the photocatalytic efficacy of active sites for these materials. On the other hand, the nanocrystallite size of ∼20 nm in the nc-TiO2 control thin films is too large to expect maximum efficiency due to volume recombination.15b,c Therefore, the higher photocatalytic efficiency of meso-nc-TiO2versus nc-TiO2 film samples in solid-state photocatalytic kinetics can be attributed to the optimized anatase nanocrystallite size rather than any morphological structural advantage.
Photocatalytic degradation of Methylene Blue in solution
In order to evaluate the global photocatalytic activity of meso-nc-TiO2, an investigation of the photocatalytic degradation of MB in aqueous solution with the aforementioned titania films was carried out. A 5 µM MB solution was used for this study since this concentration provides full coverage of active sites on the surface of all kinds of titania thin films used in this study. The photocatalytic degradation profiles of MB in aqueous solution using meso-nc-TiO2 films are depicted in Fig. 6 with nc-TiO2 and meso-SiO2 film samples included for comparison purposes. The degradation profiles of MB obtained for all samples followed first order decay kinetics. Since it is hard to measure the weight of titania thin films, the apparent rate constant of photodegradation of MB obtained from this setup was normalized by the actual film volume used as catalyst. As shown in Fig. 7, the photodegradation of MB in aqueous solution is faster with c-meso-nc-TiO2 than with h-meso-nc-TiO2. In both cases, the film annealed at 400 °C exhibited the largest apparent rate constant. On the other hand, films annealed at 450 °C displayed a similar MB decomposition rate to the film annealed at 350 °C in spite of the much higher photocatalytic efficiency of active sites seen in the solid-state photoreaction. Since the apparent rate constant obtained from the photodegradation of MB in solution is influenced by geometric factors, such as surface area, diffusion ability of the guest molecules, surface properties governing adsorption affinity, and photo-efficiency of active sites, this result can be interpreted that c-meso-nc-TiO2 film annealed at 400 °C is the best catalyst for photodecomposition of MB in aqueous solution among the titania films investigated in this study, even though it has a smaller surface area than the sample annealed at 350 °C and lower photocatalytic efficiency of active sites than the sample annealed at 450 °C.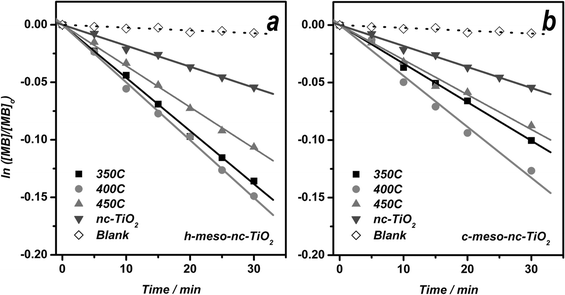 | ||
| Fig. 6 Photocatalytic degradation profiles of MB in aqueous solution for h-meso-nc-TiO2 (a) and c-meso-nc-TiO2 (b) films as compared to nc-TiO2 control films and the meso-SiO2 blank. | ||
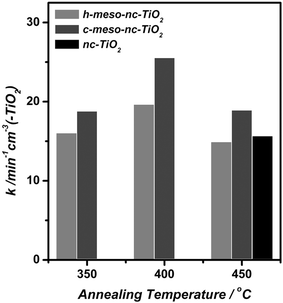 | ||
| Fig. 7 Apparent rate constant (k) normalized by volume of the titania film obtained for the photocatalytic degradation of MB in aqueous solution using h-meso-nc-TiO2, c-meso-nc-TiO2 and nc-TiO2 annealed at different temperatures. | ||
There is one very interesting observation relating to k values obtained for h-meso-nc-TiO2 annealed at 400 °C and nc-TiO2. It was found that h-meso-nc-TiO2 annealed at 400 °C had a similar saturation adsorption concentration of MB on the surface, nmax (Fig. 2) and similar photocatalytic efficiency of active sites (Fig. 5) to nc-TiO2, while its adsorption affinity was much lower than that of nc-TiO2 (Fig. 2). These values suggest that h-meso-nc-TiO2 annealed at 400 °C should display lower photocatalytic activity in aqueous solution. However, the photocatalytic activity of h-meso-nc-TiO2 annealed at 400 °C was found to be slightly higher than that of nc-TiO2. This unexpected photocatalytic activity enhancement of h-meso-nc-TiO2 can be attributed to more facile transport of organic molecules within its pore network. This observation confirms the advantage of the ordered pore architecture of meso-nc-TiO2versus the randomly organized pore network of nc-TiO2 in providing facile diffusion pathways for guest molecules.
Conclusions
Herein we have reported the effect of pore architecture on the photocatalytic activity of meso-nc-TiO2 thin film. The adsorption characteristics and the photocatalytic degradation of Methylene Blue within 2D hexagonal and 3D cubic meso-nc-TiO2 thin films were investigated and compared to nc-TiO2 and meso-SiO2 thin films as control samples. The adsorption characteristics of MB on the titania surface demonstrated that the 3D cubic mesoporous titania has a larger surface area than the 2D hexagonal version while its adsorption affinity is lower. The thermal post-treatment reduced the surface area of meso-nc-TiO2 but modified the surface properties to increase the adsorption affinity. In an investigation of the solid-state photodegradation of MB, designed to evaluate the photocatalytic efficiency of active sites on the titania surface without involving any geometric factors or solvents, it was found that c-meso-nc-TiO2 showed better activity than h-meso-nc-TiO2 and is thought to arise mainly from its higher crystallinity. On the other hand, nc-TiO2 displayed the lowest activity among the titania films annealed at 450 °C mainly due to its larger crystallite size. In an accompanying study of the photodegradation of MB in aqueous solution, it was observed that c-meso-nc-TiO2 showed superior photocatalytic activity to h-meso-nc-TiO2. Moreover, c-meso-nc-TiO2 annealed at 400 °C was the best catalyst among the samples investigated in this study. Based on these results, we can conclude that tuning of the pore architecture of meso-nc-TiO2 affects the material's intrinsic properties, such as adsorption affinity toward guest molecules and photocatalytic efficiency of active sites of nanocrystallites constituting the pore walls as well as accessibility, adsorption, and diffusion behavior of guest molecules within the pore network. Further detailed investigations using more sophisticated photophysical methods to measure the life-time of charge carriers, surface defects and optical density of meso-nc-TiO2 are required for a better understanding of the origin of the observed superior photoefficiency of nanocrystalline anatase within meso-nc-TiO2.Acknowledgements
GAO is Government of Canada Research Chair in Materials Chemistry. The authors thank the Xerox Research Centre of Canada, NSERC, and the University of Toronto for sustained financial support and Dr S. Speakman in ORNL for helping in XRD analysis.References
- (a) F. Campus, P. Bonhote, M. Gratzel, S. Heinen and L. Walder, Sol. Energy Mater. Sol. Cells, 1999, 56, 281 CrossRef CAS; (b) A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49 CrossRef CAS.
- (a) M. A. Fox and M. T. Dylay, Chem. Rev., 1993, 93, 341 CrossRef CAS; (b) M. R. Hoffman, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS; (c) A. L. Linsebigler, G. Lu and J. T. Yates Jr., Chem. Rev., 1995, 95, 735 CrossRef CAS; (d) A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- R. Blossey, Nat. Mater., 2003, 2, 301 CrossRef CAS.
- M. Fernández-Gracía, A. Martinez-Arias, J. C. Hanson and J. A. Rodrigez, Chem. Rev., 2004, 104, 4063 CrossRef CAS.
- (a) F. Schüth, Chem. Mater., 2001, 13, 3184 CrossRef; (b) G. J. A. A. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef; (c) M. Antonietti and G. A. Ozin, Chem.–Eur. J., 2004, 10, 28 CrossRef CAS.
- (a) V. F. Stone Jr. and R. J. Davis, Chem. Mater., 1998, 10, 1468 CrossRef CAS; (b) J. C. Yu, L. Zhang, Z. Zheng and J. Zhao, Chem. Mater., 2003, 15, 2280 CrossRef CAS; (c) M. M. Yussuf, H. Imai and H. Hirashima, J. Sol–Gel Sci. Technol., 2002, 25, 65 CrossRef; (d) J. Yu, J. C. Yu, W. Ho and Z. Jiang, New J. Chem., 2002, 26, 607 RSC; (e) J. C. Yu, X. Wang and X. Fu, Chem. Mater., 2004, 16, 1523 CrossRef CAS; (f) M. Alvaro, C. Aprile, M. Benitez, E. Carbonell and H. J. Garcia, J. Phys. Chem. B, 2006, 110, 6661 CrossRef CAS; (g) Y. Sakatani, D. Grosso, L. Nicole, C. Boissiere, G. J. A. A. Soler-Illia and C. Sanchez, J. Mater. Chem., 2006, 16, 77 RSC.
- (a) S. Y. Choi, M. Mamak, N. Coombs, N. Chopra and G. A. Ozin, Adv. Funct. Mater., 2004, 14, 335 CrossRef CAS; (b) S. Y. Choi, M. Mamak, N. Coombs, N. Chopra and G. A. Ozin, Nano Lett., 2004, 4, 1231 CrossRef CAS; (c) S. Y. Choi, M. Mamak, S. Speakman, N. Chopra and G. A. Ozin, Small, 2005, 1, 226 CrossRef CAS.
- S. Y. Choi, B. Lee, D. B. Carew, M. Mamak, F. C. Peiris, S. Speakman, N. Chopra and G. A. Ozin, Adv. Funct. Mater., 2006, 16, 1731 CrossRef CAS.
- C. J. Barbe, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grätzel, J. Am. Ceram. Soc., 1997, 80, 3157 CAS.
- B. D. Hatton, K. Landskron, W. Whitnall, D. D. Perovic and G. A. Ozin, Adv. Funct. Mater., 2005, 15, 823 CrossRef CAS.
- M. Kuemmel, D. Grosso, C. Boissiére, B. Smarsly, T. Brezesinski, P. A. Albouy, H. Amenitsch and C. Sanchez, Angew. Chem., Int. Ed., 2005, 44, 4589 CrossRef CAS.
- (a) R. W. Matthews, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 1291 RSC; (b) A. Mills and J. Wang, J. Photochem. Photobiol., A, 1999, 127, 123 CrossRef CAS; (c) M. Miyauchi, A. Nakajima, T. Watanabe and K. Hashimoto, Chem. Mater., 2002, 14, 2812 CrossRef CAS.
- N. Serpone and E. Pelizzetti, Photocatalysis; Fundamentals and Applications, John Wiley & Sons, New York, 1989 Search PubMed.
- (a) D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS; (b) M. Kruk, M. Jaroniec, R. Ryoo and J. M. Kim, Chem. Mater., 1999, 11, 2568 CrossRef CAS.
- (a) N. Serpone, D. Lawless, R. Khairutdinov and E. Pelizzetti, J. Phys. Chem., 1995, 99, 16655 CrossRef CAS; (b) Z. Zhang, C.-C. Wang, R. Zakaria and J. Y. Ying, J. Phys. Chem. B, 1998, 102, 10871 CrossRef CAS; (c) S. Y. Chae, M. K. Park, S. K. Lee, T. Y. Kim, S. K. Kim and W. I. Lee, Chem. Mater., 2003, 15, 3326 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: HRSEM images, GISAXS patterns, porosity plots. See DOI: 10.1039/b612550f |
| ‡ These authors contributed equally to this work. |
| § Current address: University of Colorado, Department of Chemical & Biological Engineering, CO, USA. |
| ¶ Current address: Material Science Division, Argonne National Laboratory, IL, USA. |
| || Current address: Plastic Additives Research & Technology, Ciba Specialty Chemicals, NY, USA. |
| This journal is © The Royal Society of Chemistry 2007 |
