Online in-tube solid phase extraction coupled to ICP-MS for in vivo determination of the transfer kinetics of trace elements in the brain extracellular fluid of anesthetized rats
Yuh-chang
Sun
*ab,
Yi-wen
Lu
a and
Yu-teh
Chung
c
aDepartment of Biomedical Engineering and Environmental Sciences, National Tsing-Hua University, Hsinchu, 30043, Taiwan
bNuclear Science and Technology Development Center and Department of Chemistry, National Tsing-Hua University, Hsinchu, 30043, Taiwan
cDepartment of Chemistry, National Tsing-Hua University, Hsinchu, 30043, Taiwan. E-mail: ycsun@mx.nthu.edu.tw; Fax: 886-3-5723883
First published on 16th November 2006
Abstract
Little is known about the in vivo kinetics of trace metal ions in the brain during various neurodegenerative events. In this paper, we describe a method for simultaneously monitoring the kinetics of several trace metal ions in vivo in brain extracellular fluid using microdialysis coupled online with in-tube solid phase extraction (SPE) and ICP-MS detection. To facilitate the online selective separation and ICP-MS detection processes, the trace metal ions in the dialysates, which contained tremendous amounts of salt matrix, were extracted online on the inner wall of a PVC tube—modified with a conditioning solution (10 mg EDTA L–1 of pH 10) in advance—and delivered to the ICP-MS using an eluent containing 20 mg L–1 of EDTA at pH 10. Coupling of the microdialysis sampling with the online in-tube SPE and ICP-MS detection allowed us to measure simultaneously the changes in the concentrations of Zn2+, Cu2+, and Mn2+ in the brain extracellular fluid over time. Using our proposed in-tube SPE process, we obtained online detection limits that were in the sub-microgram-per-litre range (based on 3σ); the temporal resolution was limited to 15 min because of the very low microdialysis perfusion rate (0.5 µL min–1). By combining with microdialysis probe, we also found the long-term stability of the continuous measurement over 5 h of each of the analyte ions was within 10% CV and there was no significant change in the recoveries of analyte ions when using the same PVC tubing with our online system to measure analyte ions at 10 µg L–1. From animal experiments we determined that the basal concentrations of Cu2+, Zn2+, and Mn2+ were 2.19 ± 0.12, 4.07 ± 1.81, and 2.74 ± 0.11 µg L–1, respectively; these values agree well with those found in the literature. Additionally, we observed apparent increases in the levels of zinc and manganese in the extracellular fluid of the brain in vivo after administering MnSO4, ZnSO4, and CuCl2 through intraperitoneal injection. We verified the reliability of this method by analyzing reference urine samples. To the best of our knowledge, our proposed online analytical system is the first example of a minimally invasive technique that is capable of simultaneously investigating the transfer kinetics of several metal ions in the brain in vivo.
Introduction
Many trace metals are essential for maintaining the normal function of the central nervous system (CNS); they also play important roles—as catalysts, secondary messengers, and gene expression regulators—in the functioning of the brain.1 These various metal ions must be maintained at optimal levels because deficiencies or excessive concentrations can result in aberrant CNS function. The blood–brain barrier (BBB) is a specialized system of biological membranes around blood capillaries that protects the brain from harmful substances in the blood stream, while supplying it with the nutrients required for proper functioning. Thus, the transport of metal ions across the BBB is a crucial step in regulating their CNS levels. According to Kaufer et al.,2 breaches in the BBB that result from acute traumatic stress may lead to abnormal metal ion accumulation in the brain and cause post-traumatic stress disorder. Furthermore, progress in brain science has revealed the critical roles that metal ions play during synaptic transmission and memory formation and in the causation and treatment of neurological diseases. Because many neurological disorders are associated with the accumulation and imbalance of metal ions in the nervous system,3 the mechanisms through which abnormal homeostasis of metal ions occur are of great interest in metalloneuroscience. According to Thompson et al.,4 direct evidence is still necessary to elucidate the release and uptake of metal ions during neurological disorders. Because none of the commonly used analytical methods has been able to simultaneously determine several trace metal ions, their kinetics, and their spatial distribution in the brain of living animals, the connection between barrier dysfunction and the etiology of various neurological disorders has remained unclear, and the exploration of physiological roles of various trace metal ions involved in many different behaviors has also remained a demanding challenge.5In vivo microdialysis is used widely for sampling extracellular fluid from specific regions of the brain to monitor the behavior of known transmitters and to search for novel neurotransmitters. A normal saline solution containing 0.9% (w/v) NaCl is frequently used as a perfusate in the microdialysis sampling process. Although ICP-MS is a very powerful technique for trace element analyses, the interference of the molecular ion that is caused by the presence of sodium and chlorine species can interfere with the measurement of several analyte ions [e.g., 58Ni (23Na35Cl) and 63Cu (23Na40Ar)] during the detection process. In addition to spectral interference, systematic errors can occur during these measurement processes as a result of shifting plasma equilibria and salt build-up on the cone tip, which are both caused by the introduction of large amounts of sodium into the plasma.6 To date, only two online FI-AAS systems (microdialysis-FI-ETAAS and microdialysis-FI-FAAS) and one off-line ETAAS method coupled with brain microdialysis have been developed to monitor the dynamic changes of Mg, Mn and Zn species in the brains of anesthetized rats.7–10 These methods could be useful for determining changes in the Mn and Zn concentrations in the microenvironments of animals, but they are difficult to apply to multi-element determination because of the limited detection capability of AAS systems. Therefore, the development of an efficient online analytical system—one that can eliminate the salt matrix from the microdialysate and rapidly measure changes in the levels of desired metal ions in the brains of living animals—should enable the discovery of new ionic neurotransmitters and allow kinetic studies into the homeostasis of metal ions in the brains of anesthetized rats.
To facilitate the long-term monitoring of trace metal ions in minute microdialysate samples (ca. 10 µL), chemical separation is generally necessary to isolate the analyte species of interest (i.e., to remove most of the interfering matrix constituents) prior to ICP-MS analysis. To overcome the contamination that occurs readily during time-consuming, labor-intensive batchwise procedures, various separation techniques have been adapted to FI online separation and preconcentration systems, including sorbent extraction,11,12ion exchange ,13,14coprecipitation ,15,16 and sorption with a knotted reactor17,18 (KR). To achieve a better enhancement factor, KRs have been used widely as online preconcentration systems—coupled with various spectrometric methods,19,20 —because of their low hydrodynamic impedance. The retention efficiencies of KRs are, however, poor (only 30–60%) for most of common analyte species.21 Recently, columns packed with polytetrafluoroethylene (PTFE) beads22 and PTFE turnings23,24 have exhibited improved retention efficiencies relative to those of KR systems.
To separate analyte ions from the microdialysate, high retention efficiencies are essential because only minute volumes of samples are collected from the target tissue; high analytical sensitivity is required for the successful detection of extremely small amounts of analyte ions. Accordingly, the aim of this study was to develop a sensitive method using online coupling of a new FI in-tube SPE system with ICP-MS for the continuous and simultaneous determination of multiple trace elements in the microdialysates collected from the extracellular interstitial fluids of the brains of anesthetized rats. In essence, our proposed in-tube SPE technique consists of driving an aqueous sample through a tract of polyvinyl chloride (PVC) peristaltic tubing, the interior wall of which was previously modified with ethylenediaminetetraacetic acid (EDTA), that was mounted onto a pumping system. The EDTA-modified peristaltic tubing can extract analyte ions quantitatively from the sodium matrix because of the differences between the formation constants of the various metal complexes. The analytes were then desorbed using another EDTA-containing eluent and then detected online through ICP-MS . In conjunction with the in vivo microdialysis sampling technique, our proposed method allows continuous monitoring of the transfer kinetics of trace metal ions in the extracellular interstitial fluids of the brains of anesthetized rats after the simultaneous intraperitoneal (ip) administration of several metal ions. Based on the resulting analytical characteristics, it appears that our proposed system may be useful for assessing the permeability of the BBB to exogenous metal species and to monitoring stress-related metal ion release events.
Experimental
Apparatus
Experiments were performed using the microdialysis/in-tube SPE-ICP-MS hyphenation system depicted in Fig. 1. The microdialysis system was purchased from Carnegie Medicine Associates (CMA, Stockholm, Sweden). The microdialysis sampling system consisted of a microinjection syringe pump (Cole Parmer) and a 24 mm long microdialysis probe (CMA/20) equipped with a 4 mm long, 0.5 mm diameter polycarbonate membrane, which was metal-free and had a molecular mass cut-off of 20 kDa. The connection of the microinjection syringe pump to the inlet of the microdialysis probe and the outlet of the microdialysis probe to the micro-Tee (Alltech Associates, Inc., Deerfield, IL, USA) were both accomplished using fluorinated ethylene polypropylene (FEP) tubing (internal volume: 1.2 µL per 100 mm length; 50 cm long × 0.12 mm id; CMA, Stockholm, Sweden). For the introduction of pure water, the microinjection syringe pump was connected to the micro-Tee through another 100 cm long, 0.12 mm id piece of FEP tubing. The micro-Tee was connected to the sample loop through a 50 cm long, 0.12 mm id piece of FEP tubing. | ||
| Fig. 1 FI manifold for online microdialysis coupled with in-tube SPE-ICP-MS . For details, see the text. P1 and P2: microinjection syringe pumps; P3: peristaltic pump. | ||
The online flow-injection interface comprised a six-port valve (Model 9010, Rheodyne, San Francisco, LA, USA) equipped with a 100 µL sample loop. The connections and conduits were PTFE connecting tubes (11 cm long × 1.0 mm id). The proposed in-tube SPE device consisted of a tract of polyvinyl chloride (PVC) peristaltic tubing (48 cm long × 0.76 mm id, Gilson, USA), the interior wall of which was modified previously using EDTA, that was mounted onto a peristaltic pump. This PVC pump tubing was employed not only for retaining the analyte ions but also for propelling the conditioning solution (10 mg EDTA L–1), the microdialysate, the carrier solution (pure water), and the eluents (20 mg EDTA L–1).
The ICP-MS instrument used was a Micromass Platform (Micromass Ltd., Manchester, UK). A perfluoroalkoxy (PFA) nebulizer (PFA-100, Cetac Technologies Co., Omaha, NE, USA) was employed. The PFA nebulizer was fitted to a Scott-type Ryton double-pass spray chamber. Because similar results were obtained when considering either the peak heights or the peak areas, in this study the samples were quantified only through their peak areas. The instrumental operating conditions selected for optimal sensitivity and low background noise are presented in Table 1.
| Flow injection parameters | |
|---|---|
| Tube material | PVC |
| Length of tube | 48 cm |
| Inner diameter of tube | 0.76 mm |
| pH of EDTA solutions | 10 |
| Concentrations of EDTA solutions | |
| Conditioning solution | 10 mg L–1 |
| Eluent | 20 mg L–1 |
| Liquid flow rates | |
| Conditioning flow rate | 25 µL min–1 |
| Sample flow rate | 25 µL min–1 |
| Eluent flow rate | 50 µL min–1 |
| Sample loop | 100 µL |
| Perfusion rate for microdialysis | 0.5 µL min–1 |
Reagents and containers
EDTA (>99.5%) was purchased from Fluka Co. (Switzerland). The high-purity water used in this study was purified through deionization and double distillation. The perfusion solution (normal saline solution) was prepared by dissolving sodium chloride (0.9 g, Merck, ultrapure grade) in high-purity water (100 mL). Stock solutions (1000 mg L–1) of analytes were purchased from E. Merck Company (Darmstadt, Germany). The working aqueous standards were prepared afresh daily using ultrapure normal saline solution. PTFE and glass containers were used throughout this study; they were cleaned by immersion overnight in conc. HNO3 and then overnight in conc. HCl, followed by steaming successively with HNO3 for 8 h and water vapor for 8 h. The tubes used to connect all of the pieces of apparatus were perfused with high-purity water until the contaminants were eliminated. To avoid contamination of the analyte ions, plastic perfusion syringes (Norm-Ject®, Henke Sass Wolf GMBH, Tufflingen, Germany) were used throughout this study.In vivo experiments
Adult male Sprague–Dawley rats (350–450 g) were obtained from the Laboratory Animal Center of the National Science Council of the Republic of China (Taipei, Taiwan). These animals, which were specifically pathogen-free, were acclimatized to their environmentally controlled quarters (25 °C; 12∶12 h light-dark cycle) for at least 5 days before experimentation and then fasted overnight prior to sacrifice. The rats were fed with standard diet and water and treated under the regulations of the ‘Principles of laboratory animal care’ (NIH publication no. 86-23, revised, 1985). The study was approved by the committee of experimental animals of National Tsing-Hua University.To estimate the in vivo concentrations of the analyte elements, it was necessary to determine the recovery of the microdialysis probe, which was accomplished by placing the microdialysis probe in a solution of a known concentration of tested ions and perfusing at 0.5 µL min–1. The dialysate was online mixed with a stream of pure water at a flow rate of 9.5 µL min–1, collected in a 100 µL loop of the sampling valve, and then transported to the EDTA-premodified PVC tubing for separation and detection. The results of this experiment were compared with those of an experiment run under identical conditions, except that the standard solution was pumped directly into the EDTA-premodified PVC tubing.
The rats were initially anesthetized with urethane (1200 mg kg–1 body weight, ip), and remained anesthetized throughout the experimental period. Following a midline incision of the scalp and mediolateral craniotomy, the brain was exposed and a microdialysis probe (CMA/20, 4 mm dialysis membrane) was inserted. The microdialysis probe was implanted into the brain (0 mm anterior, 5 mm lateral to the bregma, and to a deepness of 5 mm from the brain surface). The probes were perfused using a normal saline solution. The flow rate of the perfusate was 0.5 µL min–1. Basal Mn, Co, Ni, Cu, Zn, and Cd levels were monitored for at least 80 min prior to the administration of MnSO4, ZnSO4, and CuCl2. After intraperitoneal injection (100 mg MnSO4 kg–1, 1000 mg ZnSO4 kg–1 and 1000 mg CuCl2 kg–1 body weight), the levels of Mn, Zn, and Cu were monitored continuously every 15 min.
Results and discussion
Retention of EDTA and metal ions onto hollow PVC tubing
With online preconcentration using knotted reactor techniques, KRs made from PTFE tubing are capable, under appropriate conditions, of retaining hydrophobic inorganic precipitates, ion pairs, and organometallic complexes through surface adsorption.25 The formation of neutral analyte complexes—for example, the ammonium pyrrolidine dithiocarbamate (APDC) complexation system—is frequently used for the isolation of heavy metals. In our present study, initially we compared the extraction efficiencies toward Mn2+ achieved by PVC peristaltic tubing that had been modified with APDC or EDTA. As indicated in Fig. 2, the adsorption of Mn2+ ions was not observed, or it was insignificant, when the unmodified and APDC-modified PVC tubing was used as collecting media. Under identical experimental conditions, the EDTA-modified PVC tubing collected Mn2+ ions effectively on its inner wall. Based on these results, we conclude that, at pH 10, EDTA molecules are more apt to adsorb onto the inner wall of the PVC tubing than are APDC molecules, even through they are all present in anionic forms. In other words, the comparatively complete retention of Mn2+ ions on the EDTA-modified PVC tubing might be attributable to the more favorable adsorption of EDTA molecules during the conditioning process. | ||
| Fig. 2 Retention efficiencies of Mn2+ achieved by PVC tubings modified with APDC and EDTA, respectively. Concentration of Mn2+ was 20 µg L–1. Flow rates of the conditioning solution, sample solution, and eluent were 100 µL min–1; all other conditions were the same as those provided in Table 1. | ||
Because the surface adsorption of a specific species depends strongly on the interactions between the sorbent and the adsorbate—namely, ion–dipole, dipole–dipole, ion-induced dipole, and dispersion or van der Waals forces —the chemical form of EDTA is an important factor determining its adsorption onto the PVC surface. The form of EDTA, which is a synthetic amino acid, is determined by the acidity of the solution;26 therefore, we investigated the effect that the pH of the conditioning solution has on the adsorption of EDTA onto PVC surface. In this experiment, we do not observe the retention of Mn2+ ions when we used PVC tubing that had been modified using conditioning solutions at either pH 2 or 7. In contrary, Mn2+ ion could adsorb quantitatively on the inner wall of PVC tubing when a conditioning solution of pH 10 was used for the modification. Based on the effect of pH on the form of EDTA molecule, EDTA may change form protonated form to fully ionized form when the pH of the solution become higher than 10.26 In addition, because PVC (–[–CH2–CH(Cl)–]n–) is a polar polymer that has a large dipole moment,27,28 we presume that ion–dipole interactions were the main driving force initiating the complexation between fully ionized EDTA and PVC when the pH of the conditioning solution was 10. To optimize the adsorption efficiency of EDTA onto the PVC surface and the extraction efficiency of the metal ions, in subsequent experiments we used an EDTA conditioning solution of pH 10 to modify the inner wall of the PVC tubing prior to introduction of the sample.
Optimization of online in-tube SPE
To simultaneously describe the transfer kinetics of several elements in the extracellular space of the brain, optimizing the online in-tube SPE procedure is critical if we are to obtain reliable long-term results when using ICP-MS to analyze minute microdialysate samples. Thus, we investigated the effects that three main parameters—the acidity of the microdialysate, the sample flow rate, and the concentration of elution solutions—have on the extraction efficiency.Acidity of microdialysate
According to the concept of a conditional formation constant, we investigated the effect of the pH of microdialysate—the controlling factor determining the completeness of the complexation between metal ions and EDTA—upon the extraction efficiency of the analyte ions during the in-tube SPE. Fig. 3 displays the effect of pH on the analyte signals, which were obtained at identical sample volumes and sample flow rates (50 µL min–1) and using identical conditioning (10 mg L–1EDTA; flow rate: 50 µL min–1) and elution (20 mg L–1EDTA; flow rate: 100 µL min–1) solutions. Fig. 3 indicates that when the values of pH of the sample solutions were lower than 6, the extraction efficiencies of all of the tested elements decreased upon increasing the solution acidity. Because the signal intensities of the analyte ions leveled off in the range of pH from 6 to 10, for the sake of simplification and to reduce the blank size, in subsequent experiments we used nearly neutral aqueous solutions, in the pH range from 6.5 to 6.8, without adding any buffer.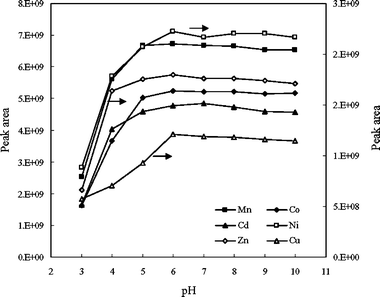 | ||
| Fig. 3 Effect of the acidity of the sample solution on the retention efficiencies of various analyte ions (10 ng mL–1). All other conditions were the same as those presented in Table 1. | ||
Sample flow rate
Because the complete extraction of analyte ions from the microdialysate is vital for improving analytical performance, we studied the effect of the sample flow rate. In our system, the samples were transported using a carrier solution of pure water. Based on the experimental results, the signals we obtained were independent of the flow rate within the range from 25 to 75 µL min–1; i.e., we achieved the maximum retention of analyte ions when the sample passed through the modified PVC tubing at flow rates below 75 µL min–1. Accordingly, in subsequent experiments we used a water stream at a flow rate of 50 µL min–1 to transport the sample solutions into the EDTA-modified tubing.EDTA concentration in elution solution
In this study, we compared the elution efficiencies obtained when using nitric acid and EDTA solutions as eluents. We observed no significant differences between the elution efficiencies achieved when using 0.01 M HNO3 and basic EDTA solution at pH 10, but only if the concentration of EDTA was sufficiently high. A basic solution was found necessary to condition the PVC tubing before we use another 10 mg L–1 of EDTA solution at pH 10 for the stable modification of the inner wall of PVC tubing, when nitric acid solution was used as eluent. To simplify the analytical procedure and to minimize the reagent blank level, in subsequent experiments we used EDTA solutions at pH 10 to desorb the analytes from the PVC tubing. Next, we studied the effect of the concentration of EDTA on the elution efficiencies of the analyte ions in the range from 0.3 to 30 mg L–1. Based on our experimental results, the signals of analytes increase upon increasing the concentration of EDTA up to 15 mg L–1, but then they leveled off. To ensure complete elution of the analytes, in subsequent experiments we used a 20 mg L–1EDTA solution for the desorption.Analytical characteristics
After optimization of the in-tube SPE procedure, we examined the effects that sodium ions present in the microdialysate had on the extraction efficiencies of the analyte ions. We found that some of the analyte signals obtained were suppressed in the presence of 0.9% (w/v) NaCl, but that interference-free signals could be obtained when using normal saline diluted 20-fold. To avoid interference from the salt, a stream of pure water was delivered (at a flow rate of 9.5 µL min–1) by another syringe pump (see Fig. 1) to mix and dilute the microdialysate. In addition, we also examined the long-term stability of the modified tubing by performing SPE measurements continuously for 5 h. We found that there was no significant change in the recoveries of analyte ions when using the same PVC tubing with our online system to measure analyte ions at 10 µg L–1. The repeatability of the continuous measurement over 5 h of each of the analyte ions was within 10% CV. Therefore, this method appears to be practically useful.Because of the extremely low basal concentrations of analyte ions in the extracellular fluids of rat brains7,8 and the limited sample volumes collected during the microdialysis process, it is of primary importance for the system to exhibit a stable and low blank value. In this study we performed blank determination by following established microdialysis and in-tube SPE processes, using normal saline solution as a blank sample, followed by online ICP-MS measurement. Our results indicated that correction for the elemental concentration in the resultant solution was unnecessary, because no apparent changes in the concentration levels of the elements of interest occurred after passing through our proposed online system.
As the system described above was equipped with a 100 µL sample loop, detection limits in the sub-microgram-per-litre regime (Table 2) were obtained for almost all of the analyte ions, based on three times the standard deviation of the baseline noise (n = 7). Because the extracellular concentrations of Mn and Zn in the brain are in the microgram-per-litre range,7,8 this technique appears uniquely suitable for continuously monitoring the dynamic changes that occur in the concentrations of multiple elements simultaneously.
| Element | MDL/µg L–1 | Certified valuea /µg L–1 | Measured value/µg L–1 | Spike recovery (%) |
|---|---|---|---|---|
| a Seronorm urine 009024 reference material . | ||||
| Mn | 0.23 | — | 26.0 ± 4.0 | 90 |
| Ni | 0.56 | 39.3–42.8 | 42.0 ± 2.7 | 101 |
| Co | 0.30 | 9.3–11 | 11.6 ± 1.5 | 117 |
| Cu | 1.50 | 23.9–31.1 | 27.7 ± 1.5 | 120 |
| Cd | 0.71 | 4.7–7 | 5.2 ± 0.3 | 92 |
| Zn | 0.40 | 890–1000 | 979.3 ± 35.2 | 119 |
| Pb | 1.50 | 83–97 | 97.0 ± 3.6 | 110 |
Next, we examined the applicability of our established microdialysis/in-tube SPE-ICP-MS system through the determination of the elements of interest in an acid-digested Seronorm urine 009024 reference material . Table 2 indicates that we obtained a reasonably good agreement between the certified values and our analytical results. We assessed the accuracy of our proposed method by analyzing not only the reference material but also spiked urine samples. The acceptable recoveries that we obtained for the spiked samples indicate that our procedure can be used to accurately determine the concentrations of Mn, Ni, Co, Cu, Cd, Zn, and Pb.
Dynamic changes of trace elements in brain extracellular fluid
As a demonstration of the applicability of our proposed online in-tube SPE procedure, we implanted microdialysis probes to sample the extracellular fluids from the brains of anesthetized rats for in vivo quantification of the kinetics of trace metals, using the system depicted in Fig. 1 and described in the Experimental. Using the methodology described above, Fig. 4 illustrates that the basal levels of Zn, Cu, and Mn in the extracellular fluid from the rat brain were readily measured in a 15 min microdialysis fraction. Initially, a significant increase in the levels of Zn, Cu, and Mn in the dialysates have been observed after the first 30 to 50 min of the implantation of a microdialysis probe into the brain of rats. Similar finding has been interpreted as being a consequence of an initial tissue lesion and rupture of cellular storage compartments.29 After 90 min of the probe implantation, stable basal levels of Zn, Cu, and Mn in the dialysates were obtained and the values of the basal levels (Zn: 4.07 ± 1.81 µg L–1; Mn: 2.74 ± 0.11 µg L–1; Cu: 2.19 ± 0.12 µg L–1) that we obtained using our proposed method are similar to those reported for Zn (4–7 µg L–1)8 and Mn (1.39 µg L–1)7 from sampling using microdialysis techniques. To study the permeability of the BBB to zinc, copper, and manganese, we injected MnSO4, ZnSO4 and CuCl2 (each 100 mg kg–1 of body weight) into the rat abdominal cavity and continuously monitored the levels of the analyte ions in the brain dialysates. Interestingly, after 30 min, we observed apparent increases in the levels of Mn and Zn in the extracellular fluid of the brain. Additionally, because we could observe significant differences in the signals of the analytes after implantation of the microdialysis probes, our proposed method appears to be sufficiently sensitive to observe the basal levels of—and the dynamic changes in—the concentrations of Zn, Cu, and Mn after certain physiological stimulations, such as global ischaemia and brain injury. | ||
| Fig. 4 Time course of the Mn, Zn, and Cu concentrations in the extracelluar space of rat brains following an experimental intraperitoneal injection. Implantation of microdialysis probe into the brain of anesthetized rats; ▼: intraperitoneal injection of MnSO4, ZnSO4 and CuCl2 (each 100 mg kg–1 body weight) individually. The error bars represent standard deviations (n = 3). | ||
Conclusions
The ability to monitor temporary variations in the concentrations of biological substances in certain regions of the brain is critical for the evaluation of various neurological disorders. To allow in vivo monitoring of the release and extravasation of metal ions from neurons and the BBB during various neurological disorders, we have combined microdialysis with a new online in-tube SPE-ICP-MS system for in situ detection of the dynamic changes in the concentrations of trace metal ions in the brain extracellular fluid. Based on a preliminary study of the BBB permeability of metal ions, we found that both zinc and manganese ions, but not copper ions, moved across the BBB and into the extracellular interstitial space after ip administration. Therefore, we believe that the main advantage of this present method over our previous proposed online microdialysis-FI-ETAAS system7 is that it becomes possible to determine several trace elements simultaneously; i.e., more informative and novel observations are possible that may be beneficial to the development of neuroscience and brain science.References
- W. Zheng, M. Aschner and J. F. Ghersi-Egeac, Toxicol. Appl. Pharmacol., 2003, 192, 1 CrossRef CAS.
- D. Kaufer, A. Friedman, S. Seidman and H. Soreq, Nature, 1998, 393, 373 CrossRef CAS.
- I. Angel, A. Bar, T. Horovitz, G. Taler, M. Krakovsky, D. Resnitsky, G. Rosenberg, S. Striem, J. E. Friedman and A. Kozak, Drug Dev. Res., 2002, 56, 300 CrossRef CAS.
- R. B. Thompson, W. O. Whetsell, B. P. Maliwal, C. A. Fierke and C. J. J. Frederickson, Neurosci. Methods, 2000, 96, 35 Search PubMed.
- C. M. Harris, Anal. Chem., 2001, 73, 590A CAS.
- Y. L. Chang and S. J. Jiang, J. Anal. At. Spectrom., 2001, 16, 1434 RSC.
- W. C. Tseng, Y. C. Sun, M. H. Yang, T. P. Chen, T. H. Lin and Y. L. Huang, J. Anal. At. Spectrom., 2003, 18, 38 RSC.
- T. Itoh, T. Saito, M. Fujimura, S. Watanabe and K. Saito, Brain Res., 1993, 618, 318 CrossRef CAS.
- T. Itoh, T. Saito, S. Watanabe and K. Saito, Trace Elem. Electrolytes, 1996, 13, 196 CAS.
- W. C. Tseng, Y. C. Sun, C. F. Lee, B. H. Chen, M. H. Yang and Y. L. Huang, Talanta, 2005, 66, 740 CrossRef CAS.
- B. Saad, C. C. Chong, A. S. M. Ali, M. F. Bari, I. Ab Rahman, N. Mohamad and M. I. Saleh, Anal. Chim. Acta, 2006, 555, 146 CrossRef CAS.
- D. Kara, A. Fisher and S. J. Hill, Analyst, 2005, 130, 1518 RSC.
- J. H. Wang and E. H. Hansen, J. Anal. At. Spectrom., 2001, 16, 1349 RSC.
- S. Hirata, T. Kajiya, M. Aihara, K. Honda and O. Shikino, Talanta, 2002, 58, 1185 CrossRef CAS.
- X. D. Tang, Z. R. Xu and J. H. Wang, Spectrochim. Acta, Part B, 2005, 60, 1580 CrossRef.
- J. E. T. Andersen, Analyst, 2005, 130, 385 RSC.
- K. Benkhedda, B. Dimitrova, H. G. Infante, E. Ivanova and F. C. Adams, J. Anal. At. Spectrom., 2003, 18, 1019 RSC.
- K. Benkhedda, B. Dimitrova, H. Goenaga Infante, E. Ivanova and F. C. Adams, J. Anal. At. Spectrom., 2001, 16, 995 RSC.
- J. H. Wang and E. H. Hansen, Trends Anal. Chem., 2003, 22, 836 CrossRef CAS.
- K. Benkhedda, H. Goenaga Infante, F. C. Adams and E. Ivanova, Trends Anal. Chem., 2002, 21, 332 CrossRef CAS.
- Y. Wang, J. H. Wang and Z. L. Fang, Anal. Chem., 2005, 77, 5396 CrossRef CAS.
- X. B. Long, R. Chomchoei, P. Gala and E. H. Hansen, Anal. Chim. Acta, 2004, 523, 279 CrossRef CAS.
- G. A. Zachariadis, A. N. Anthemidis, P. G. Bettas and J. A. Stratis, Talanta, 2002, 57, 919 CrossRef CAS.
- A. N. Anthemidis, G. A. Zachariadis and J. A. Stratis, J. Anal. At. Spectrom., 2002, 17, 1330 RSC.
- X. P. Yan and Y. Jiang, Trends Anal. Chem., 2001, 20, 552 CrossRef CAS.
- D. A. Skoog, D. M. Weat and F. J. Holler, Fundamentals of Analytical Chemistry, Thomson Learning, Inc., Belmont, CA, USA, 7th edn, 1996 Search PubMed.
- A. Eisenberg and M. Hara, Polym. Eng. Sci., 1984, 24, 1306 CrossRef CAS.
- R. H. Boyd and L. Kesner, J. Polym. Sci., Polym. Phys. Ed., 1981, 19, 375 CrossRef CAS.
- U. Ungerstedt, ‘Introduction to intracerebral microdialysis’ in Microdialysis in the Neurosciences, ed. T. E. Robinson and J. B. Justice, Jr, Elsevier Science, Amsterdam, 1991, pp. 3–22 Search PubMed.
| This journal is © The Royal Society of Chemistry 2007 |
