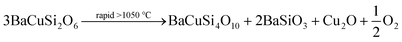The invention of blue and purple pigments in ancient times
Heinz
Berke
Institute of Inorganic Chemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zürich, Switzerland. E-mail: hberke@aci.unizh.ch; Fax: +41 44 635 68 02; Tel: +41 44 635 46 80;
First published on 12th October 2006
Abstract
This tutorial review examines manmade blue and purple pigments appearing in antiquity. They were obtained by chemical synthesis from mineral starting materials and refer to chemical compounds: Egyptian Blue (CaCuSi4O10), Han Blue (BaCuSi4O10) and Han Purple (BaCuSi2O6), Maya Blue (x·indigo·(Mg,Al)4Si8(O,OH,H2O)24) and Ultramarine Blue (Na,Ca)8(AlSiO12)(S, SO4,Cl). The Egyptian and Chinese copper-based pigments are assumed to have been developed independently and are presumably an outcome of the historical developments in glazing techniques. A technology transfer from Egypt into China cannot be fully excluded but, based on the facts acquired up to now, looks less probable.
 Heinz Berke | H. Berke received his Diploma in Chemistry at the University of Erlangen (Germany) in 1971 and his PhD at the University of Tübingen (Germany) in 1974. From 1974–1988 he was at the University of Konstanz (Germany) with an intermediate stay in the Laboratory of R. Hoffmann, Cornell University, Ithaca (USA) in 1977. In 1981 he finished his Habilitation and in 1983 he was awarded the Heisenberg fellowship from the “Deutsche Forschungsgemeinschaft” and the Dozentenpreis of the Fonds der Chemischen Industrie (Germany). In 1987 he was promoted to a C2 Professor at the University of Konstanz before he joined the University of Zürich (Switzerland) in 1988 as a full professor of Inorganic Chemistry. In 1991 he became director of this institute and stayed at this position till present. H. Berke is member of the editorial boards of the journals Dalton Transactions and Mendeleev Communications and is presently president of the Division of Chemical Research of the Swiss Chemical Society. H. Berke's fundamental research activities cover various fields of organometallic chemistry. Major efforts are devoted to the area of transition metal hydrides, which is related to homogeneous catalysis, in particular homogeneous hydrogenations and hydrosilations. Metal–carbon oriented activities concern several catalyses of C–C coupling reactions mediated by transition metal complexes and in addition metallacumulenes, where carbon chained units are sought to space transition metal centers for potential use as single-electron devices. Another research field deals with the archaeometry of ancient, manmade blue and purple pigments. |
1. Blue and purple
1.1 Art, matter and blue in particular
Colours are an intrinsic part of human life. They produce aesthetic stimulation and they fascinate. They are the means of expression in art and they form part of the human culture. The blue colour, however, differs from all the other colours. At all times in history, people ascribed to it a special dimension.1 Yves Klein, the “blue entrepreneur”, who in 1957 started using solely blue for his paintings and sculptures, put it like this: Blue has no dimension, it exceeds everything… All colours evoke associations…, whereas blue is reminiscent of the sea and the sky, which are the most abstract parts of the tangible and visible nature. The opinion of this art expert has not been seconded unanimously; yet, its mere existence may highlight the fact that blue is considered to be different from all other colours.The materialised forms of colour are dyes and pigments. They form the material basis of art. Despite this fundamental role as conditio sine qua non, the role of matter in art was due to a very rigoristic way of thinking only marginally accepted during the European Middle Ages, the Renaissance and still in the early 18th century.
During times of the theological hegemony and according to the viewpoint of spirit–matter dualism, both matter as the material basis and the craft of matter, which is in chemistry, alias alchemy was not ascribed much importance. “Vulgar” chemistry (alchemy) was due to people's disrespect of its ideational properties not sufficiently recognised as a science working in favour of art. Chemistry (alchemy) was made use of in artists' studios, but it lacked prestige, which was also shown by its de facto exclusion from the academic world. A simple example shows, however, to what extent chemistry (alchemy) still managed to have an ideational influence on art: The blue pigment lapis lazuli features, as a mineral, changing amounts of various impurities; thus, it is usually not suitable for use in painting without its undergoing (al)chemical separation processes. Hence, in medieval times, there were several procedures to purify lapis lazuli, some of which were kept secret. The use of chemistry (alchemy) allowed improved colour saturation and brilliance, which resulted in better expression in art.
Only toward the end of the 18th century, did science become liberated from the theological hegemony. Science's coalition in the battle for recognition was rewarded with a massive leap forward.2 People grew more prepared to make use of scientific cognition. And, as chemistry was becoming more rational, there was a growing insight that art and chemistry complement one another with mutual advantages for both sides. As a consequence, the high-quality industrial pigments were developed, some of which had been available since the beginning of the 19th century, and could be used without restraint as of the second part of the 19th century; this was one of the largest benefits chemistry had on art. A silent revolution had taken place and new possibilities for expression advanced the impressionistic style.
In the co-evolution of art and science, both sides generally reaped more benefits from the other than they were willing to acknowledge.3 For ancient cultures, art and science were one entity; this was mainly due to their state of development, which was still defined mostly by the necessities of life and their technical possibilities. The craft of chemistry (alchemy) was, without reservation, used as the material basis for painting. This was the case in particular for the blue colour and the related purple colour, as the material basis in the form of herbal and animal constituents and minerals for these two colours were, as opposed to the elements of all other colours, not sufficiently available. This lack of resources challenged respective craftsmen at the time, being alchemists, to contrive innovative ideas in many ways.
1.2 The blue minerals in ancient and in medieval times
As mentioned previously, the availability of colouring substances played a decisive role in cultural developments and in art. In prehistoric times, only the so-called earth colours, colours provided by the surface soil, could be used as pigments. Blue is not an earth colour and was therefore not available to prehistoric humans as a pigment. Visitors of prehistoric caves, such as the caves of Altamira in Spain or Lasceaux in France, thus notice that in the paintings on the walls of the caves, there is no blue colour.4 In antiquity, the palette of available pigments could be expanded with the help of mining. The trade of mining required specialised mineralogical expertise and technical developments as civilizing achievements, which had been largely unchanged up to the Middle Ages.The gemstone lapis lazuli was the source of the blue minerals obtained through mining in ancient and medieval times (lapis lazuli is in mineralogy called lazurite and contains variable amounts of calcium (Na,Ca)8(AlSiO12)(S,SO4,Cl)). In ancient times, lapis lazuli was valued for its stability and for its brilliance apparent in very pure lapis lazuli. The almost ubiquitous, but unstable azurite is a mineral containing copper (Cu3(CO3)2(OH)2). Depending on its environment, it will eventually transform into malachite, a green pigment, and is unsuitable for outdoor use. Lapis lazuli was in ancient times mined in only one location, situated in the area of present-day Afghanistan (Badakhshan). It should be mentioned that in ancient China, the use of natural lapis lazuli was not as common as in other cultures (Persia, Mesopotamia, Egypt). The factual reasons are not known; however, it may be due to the fact that the Chinese disposed of artificially manufactured blue and azurite, the latter in almost unlimited amounts. Furthermore, minerals containing cobalt that only after a manufacturing process in glasses and glazes transformed into a blue colour were used.
Probably in the early 10th century AD, the blue mineral vivianite (Fe3(PO4)2·8H2O) became a speciality of the European medieval times, mined in areas north of the Alps, and was used as a pigment.5 Vivianite is an unstable iron phosphate mineral with variable iron(II/III) content that is completely colourless if kept in an oxygen-free environment (solely contains iron(II)) and that, if exposed to oxygen, will over time oxidise into blue and eventually brown compounds with a higher iron(III) content. Nowadays, this mineral would not be used as a pigment due to its too low stability. The use in the European Middle Ages can be explained only with the lack of suitable alternatives. Blue vivianite may be found mainly in the vicinity of ore deposits near the Earth's surface and in pegmatite deposit areas if waters containing phosphates reach that area. The mineral oxidation product of vivianite is the brown santabarbaraite pseudomorphus, in which half of the iron atoms are iron(II) and the other half iron(III).
1.3 Necessity has been the mother of invention—especially in ancient times
The notable German art historian J. J. Winckelmann described antique art with the phrase Noble simplicity and quiet grandeur (Edle Einfalt und stille Grösse). He acted on the erroneous assumption that polychromy of art objects was not of real importance for the antique human being. The contrary was the case:6 colour and painted objects were valued greatly in ancient times and their frequent use aggravated the lack of, mainly the rare, blue colour materials. Only by the end of the 18th century was there a fundamental change in the special situation of the scarcity of blue pigments; new blue pigments were invented and later, at the outset of industrialisation, many more new blue and purple materials were created chemically. Also, known natural materials could be produced artificially on a large scale in sufficient amounts. Necessity is the mother of invention!According to the current state of knowledge, there were three geographic areas in ancient times in which special blue and purple pigments were contrived and produced (see also atlas for the distribution of Egyptian Blue and Han Blue and Purple (Fig. 21) and Fig. 1):
• The Mediterranean area, incl. Egypt, as of approx. 3600 BC, the Middle East (Mesopotamia, Persia), and later also the areas of ancient Greece and the Roman Empire, where Egyptian Blue (Fig. 1) was produced7 and cobalt was used to colour glasses and glazes and was also occasionally used as a pigment in a glass-bound form (smalt).
• The area of ancient China in which, according to the current state of knowledge, the synthetic pigments Chinese Blue and Chinese Purple (Fig. 1), also called Han Blue and Han Purple, were produced; the area extends on a relatively limited territory about 200–300 km north of the ancient city of Xian. Today it is thought that, in this area in northern China, Ultramarine Blue (“artificial lapis lazuli”) might also have been produced (∼800 BC) (Fig. 2).
• The area of Middle America with the Indian cultures, which, as of approx. 400 AD, produced Maya Blue (Fig. 2), an intercalation compound of indigo into the white clays of palygorskite.
 | ||
| Fig. 1 Egyptian Blue (CaCuSi4O10, left), Han Blue (BaCuSi4O10, centre) and Han Purple (BaCuSi2O6, right). Under similar conditions, the substances Egyptian Blue and Han Blue strongly resemble one another. The smaller the grain size, the lighter is its appearance. Egyptian Blue is ground whereas Han Blue is granular crystalline. | ||
 | ||
| Fig. 2 A piece of industrially produced Ultramarine Blue (left) and Maya Blue produced from sepiolite (right). The sepiolite stems from commercially available cat litter. | ||
It must be noted that, according to current knowledge, no artificially produced blue pigments were used in ancient India. Mineral pigments like azurite and lapis lazuli were used instead. From a chemical point of view, Egyptian Blue and Han Blue and Purple are very closely related. Being copper silicates with the alkaline earth elements calcium and barium, they are defined chemical compounds of the compositions CaCuSi4O10 (Egyptian Blue), BaCuSi4O10 (Han Blue) und BaCuSi2O6 (Han Purple). Their chemical relationship becomes apparent on the basis of the periodic table of the elements, according to which they differ only in the very similar alkaline earth elements.8–11 Ultramarine Blue, which was probably also produced synthetically by the Chinese, possesses variable compositions and might typically correspond to the formulation Na6.9(Al5.6Si6.4O24)S2.0.12 Likewise, Maya Blue can also not be assigned a defined ratio composition between the two constituents indigo (C16H10N2O2) and the white clay mineral (palygorskite ((Mg,Al)4Si8(O,OH,H2O)24) or more rarely sepiolite).13,14 Smalt, which occurs in the form of glasses and glazes, is not a consistently structured matter and, with the formulation Co(SiO2)n, is not a defined chemical compound.
2. The chemistry of synthetic blue and purple pigments
2.1 The syntheses of Egyptian Blue, Han Blue and Purple, Ultramarine Blue, Maya Blue and Smalt
Egyptian Blue is a defined chemical compound with the formulation CaCuSi4O10. It is the oldest of all the above-mentioned blue and purple pigments11 and it can be obtained relatively easily if the minerals lime (CaCO3), sand (SiO2) and a copper mineral (e.g. malachite (Cu2(CO3)(OH)2) or azurite (Cu3(CO3)2(OH)2)) or metallic copper are exposed to oxygen (O2) and, together with a few percent of a flux such as potassium carbonate (K2CO3), natrium (NaCl) or natrium sulfate (Na2SO4), are heated to temperatures between 800 and 900 °C:In ancient Egypt, quite often trona, a mixture of natrium sulfate, soda (Na2CO3) and sodium chloride, was used for the above-mentioned flux function in syntheses. The presence of oxygen (O2) from air prevents the formation of red cuprite (Cu2O). It probably took generations for humans living at that time to find the right conditions for the creation of high-quality products, including synthesis optimisation by the addition of fluxes. This long process required good technical abilities and expertise, e.g. for controlling the temperature of the furnace and the addition of oxygen, in those times done with blow tubes and later with bellows, which needed to be passed on to future generations in accurate ways.
The chemical conditions for the preparation of Egyptian Blue also needed to be passed on very accurately. Proof that this was done is found in the extraordinary constancy of the chemical composition of the Egyptian Blue elements in art objects dating back more than 2500 years, which we examined according to Table 1.
| Artefact and location | Dynasty | Time | Composition (in oxide percentage) | ||
|---|---|---|---|---|---|
| Ca | Cu | Si | |||
| a Intef, General of Mentuhotep II, 11th Dynasty. | |||||
| Mastaba of Mereruka, Saqqara, Egypt | Old Kingdom | 2575–2134 BC | 15.2 | 21.3 | 63.0 |
| Thomb of Intef,a Theben, Egypt | Middle Kingdom | 2040–1640 BC | 14.9 | 21.5 | 63.8 |
| Nefertete, Berlin, Germany | New Kingdom | 1340 BC | 17.4 | 30.2 | 52.3 |
| Echnaton Temple, Blue of the Talatat Stones, Amarna, Egypt | New Kingdom | 1353 BC | 24.0 | 22.5 | 53.5 |
| Amulet of Bes, origin unknown | Late period | 712–332 BC | 13.6 | 28.5 | 58.3 |
| Mummy coffin, origin unknown | Graeco–Roman period | 332 BC–395 AD | 18.4 | 22.5 | 59.2 |
| Average Composition | 17.3 | 24.4 | 58.4 | ||
| Theoretical Composition | 18.6 | 29.4 | 52.0 | ||
The production of magnificent compact blue bodies of Egyptian Blue (cylinder seals and amulets etc.) required specialised chemical expertise and technical abilities. The production of such items was achieved through various processes. The faience technique was used to produce art objects that featured a primarily blue glazing, which partly contained Egyptian Blue (see section 3. Examined Objects).
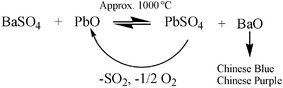
As mentioned above, Han Blue and Purple are compounds based on copper silicates, as well. The production of Han Blue (BaCuSi4O10) and Han Purple (BaCuSi2O6) is generally more difficult than the production of Egyptian Blue. Today, it can be reconstructed easily that, in a first step, a barium mineral (generally barite (BaSO4) or witherite (BaCO3)) was exposed for several hours to quartz (SiO2), a copper mineral and an essential lead salt supplement at a temperature of 900–1000 °C. Fig. 3 shows the thermogravimetric development of a modern synthesis on a micro scale, starting from a mixture of BaCO3, CuO and SiO2. In the case of a temperature rise, at approx. 650 °C there is a mass loss due to the release of CO2 from BaCO3; at 800 °C, the chemical reaction starts, as seen in the bottom DTA curve of the diagram.
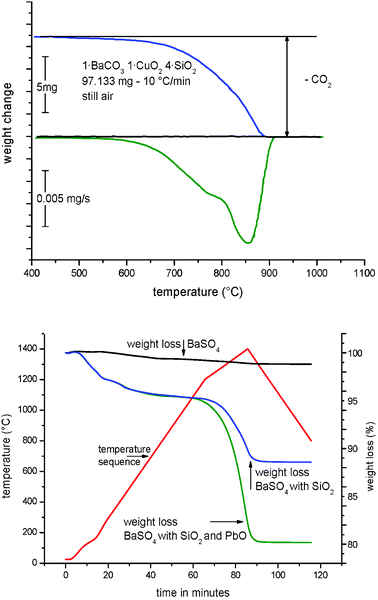 | ||
| Fig. 3 Thermogravimetrics of the transformation of BaCO3, SiO2 with CuO (top). At temperatures of approx. 650 °C, BaCO3 is decomposed and at 800 °C, the chemical reaction for the formation of Han Blue or Purple starts. Thermogravimetrics of the decomposition of BaSO4 in the presence of lead oxide (PbO) and quartz (SiO2) with a reference curve for only BaSO4. The second curve shows the decomposition of the BaSO4–SiO2 mixture. The third curve shows the accelerated PbO-catalysed decomposition of BaSO4 according to the above-mentioned equation (bottom). | ||
For the production of larger amounts of Han Blue, these conditions are not applicable; as mentioned previously and as shown in the following typical equation, the temperature will be higher by approx. 100 °C.
The limited availability and the high stability of the rare barium minerals had a restrictive effect on these syntheses. Thus, it was necessary to reach relatively high temperatures for the synthesis. Reaching the required temperatures was probably facilitated by technical developments such as the invention of the twin bellows, which were also used for other processes, e.g. in ironworks. Furthermore, the decomposition temperature of barite (BaSO4) is very high, which is why it reacts extraordinarily slow in chemical syntheses. The use of barite leads to inferior pigment products if no special additives are applied. Where barite was used as a starting substance, examination of original samples reveals residual sulfur content. The syntheses were considerably more successful when lead salts (lead carbonates, lead oxides) were added, which proved to be an ingenious chemical trick. All samples of Han Blue and Purple that have so far been examined contain lead. Table 2 gives an overview of the compositions of different objects analysed by EDX. Some samples even revealed very high lead contents.
| Sample | Ca | Ba | Cu | Si | Pb | S |
|---|---|---|---|---|---|---|
| a Not analysed due to a too high amount of lead. b See ref. 10. | ||||||
| Octagonal stick,b Freer Gallery, Washington | 2.5 | 35.3 | 5.6 | 37.7 | 11.4 | 2.0 |
| Octagonal stick,b K 4069, Museum of Far Eastern Antiquities, Stockholm | 3.1 | 31.6 | 13.2 | 15.8 | 35.4 | —a |
| Octagonal stick,b K 4070, Museum of Far Eastern Antiquities, Stockholm | 1.0 | 36.5 | 15.6 | 25.9 | 20.7 | — |
| Bead 1 (Fig. 16) | 1.8 | 6.2 | 2.1 | 25.9 | 61.2 | 1.8 |
| Octagonal stick (Fig. 16) | 1.5 | 32.7 | 6.3 | 19.5 | 33.2 | 2.0 |
Lead salt additives serve a chemical double function: on the one hand they assist the catalytic decomposition of barium minerals at lower temperatures and on the other they serve as fluxes in a similar way to the additives in the preparatives of Egyptian Blue.
The progress of a separate decomposition experiment of barium sulfate in the presence of lead oxide was determined with the help of a thermogravimetric experiment and is depicted in Fig. 3. While BaSO4 does not undergo any changes when exposed to higher temperatures. BaSO4, when exposed to approx. 1000 °C in the presence of SiO2, eventually starts evolving gaseous products that indicate the decomposition of BaSO4. If lead oxide is added to this mixture, the reaction will undergo a considerable catalytic acceleration.
As mentioned previously, in the barium-based material system there are several blue or possibly purple chemical compounds, contrary to the calcium copper silicate system. Only Han Blue (BaCuSi4O10), Han Purple (BaCuSi2O6) and another, so far unnamed, but defined, blue compound with the formula BaCu2Si2O7 could principally be used as pigments owing to their facile synthetic availability. In all syntheses, Han Purple can be produced fastest. BaCu2Si2O7 is found yet rarely and only in traces. BaCuSi2O6 is also generated if there is a surplus of quartz aimed at the preparation of Han Blue; Han Blue can, under these circumstances, be obtained only after a longer reaction time.
Recent examinations of pure Han Purple (degree of purity > 99.5%) have shown that Han Purple is not only very difficult to obtain in a pure state, especially using the methods of ancient times, but that it is also, surprisingly, not purple in its pure state, but dark blue. The purple shade of Han Purple comes from the red impurity of copper(I) oxide, Cu2O, (mineral name cuprite), that is slowly generated by the decomposition of Han Purple, which probably happens as outlined in the following chemical equation.
At temperatures of more than 1050 °C, this decomposition takes place at a quite fast rate. The production of copper(I) oxide depends on the conditions of preparation but, based on the traced ancient synthetic procedures, this problem could not be avoided in BaCuSi2O6 production in ancient China. In Fig. 4, the purple or rather reddish blue shades of Han Purple have been simulated by the admixture of copper(I) oxide to pure blue BaCuSi2O6.
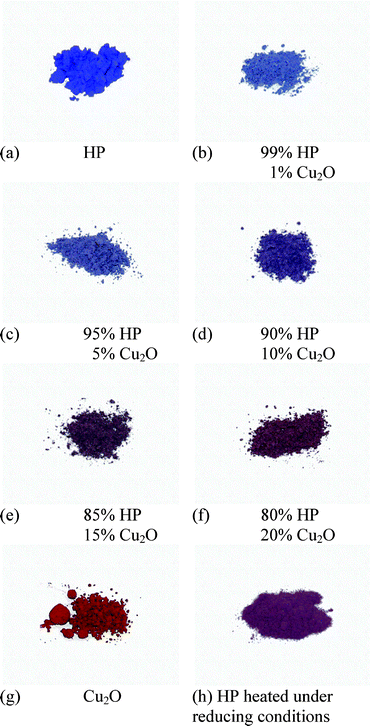 | ||
| Fig. 4 Pure BaCuSi2O6 (Chinese “Purple”) (a), to which cuprite (Cu2O) (g) was gradually added. The samples (b–f) contain increasing amounts of cuprite. According to the increasing amount of cuprite, the shade becomes more reddish. “Normal” Han Purple, which is produced in a synthesis at approx. 1000 °C, comparable to the syntheses conducted in ancient times, is, in terms of the colour shade, similar to sample (c). | ||
Also, ancient objects treated with Han Purple contain variable amounts of copper(I) oxide and therefore display variable purplish shades. E. FitzHugh, the pioneer of the rediscovery of the Chinese pigments, also highlighted this fact, which she ascribed to the various decomposition states of the samples.15 As will be discussed further on, Han Purple is very unstable from a chemical point of view; for that reason, it often shows signs of weathering on excavated historic artefacts. While the copper(I) oxide in Han Purple stayed stable and a decomposition of Han Purple progressed, the purplish colour of the artefacts increased.
Nowadays, Ultramarine Blue can be very easily obtained in the presence of sodium salts (sodium carbonate), sulfur compounds and in alkaline conditions.12 If those conditions are not prevalent, the elements building the alumosilicate frameworks must be available in the form of suitable raw materials such as aluminium and silicon, which may stem from earth minerals. In the case of the examined objects, the blue colouring of sulfur radical ions was probably produced by the reduction of the existing sulfate with spectroscopically detected carbon particles, which were contained in the plant ashes (basically potash K2CO3 and soda Na2CO3). Ultramarine Blue is generated at relatively low temperatures (400–600 °C); this has been done since the early 19th century, when the industrial production process first came into use.16
2.2 Chemical structures and properties of synthetic blue and purple pigments
The microscopic structure of ancient pigments provides information on their chemical and physical features as well as the process by which they were produced.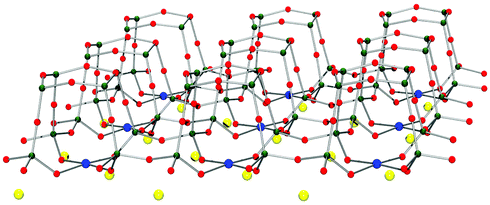 | ||
| Fig. 5 Schematic depiction of an isolated layer of MCuSi4O10 (M = Ca, Ba) with the Cu2+ ions (blue) in a square planar complex. The coordination of the M2+ ions (yellow) is through the bond, with adjoining layers complemented to an eightfold coordination (O red, Si dark green). | ||
These copper ions are the colouring agent (chromophore). They are very tightly bound in the stable silicate matrix and cannot be removed easily by chemical and physical means. This tight binding is the key to the high stability of Egyptian and Han Blue. In contrast to copper, the calcium and barium ions act as relatively independent counterions, positioned between the layers, and therefore do not have only small effects on the colour properties. Heat, strong acids and light cannot harm these two pigments. The chromophore copper is not a very efficient colouring agent, however; consequently, it needs quite a fair amount of material to reach a certain intensity in colour. The layered structure leads to the formation of platelet crystals that feature anisotropic interaction with light. The crystals show dichroism, which can be seen in the substance's lighter appearance when grinding is applied. Different shades of darker or lighter blue can thus be produced (Fig. 1).
Han Purple features a layered structure, as well, but its framework differs greatly from the structure of Han Blue. Its basic units are isolated (SiO)4 four-ring units, whose terminal oxygen atoms bind two connected copper atoms.8–11 This results in the formation of an infinite arrangement of Cu2 units (Fig. 6).
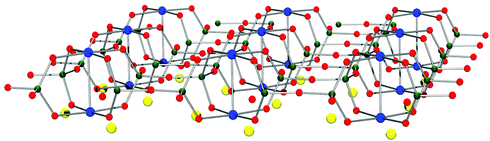 | ||
| Fig. 6 Schematic depiction of the layered structure of Han Purple, BaCuSi2O6. The Cu2 units (blue) are perpendicular to the plane of the layer. They are held together by the four bridging SiO2 units of the silicate four-ring units (Ba yellow, Si dark green). | ||
From a chemical point of view, this copper–copper bond is a very uncommon feature. Metal–metal bonds are in general chemical curiosities if they appear in materials other than metals. The crystals of Han Purple are also dichroitic. As a consequence, the substance's appearance gets lighter when ground and shows otherwise, as a barium–copper-silicate compound, great similarity in various physical properties to Han Blue. Thus, the two substances can be mixed easily, leading to blue/purple colour shades.
Owing to its Cu–Cu bond structure, Han Purple has a low chemical stability. Even weak acids wear and bleach it; hence, a light-blue mixture of Ba/Cu oxalate is formed under the influence of oxalic acid that may occur under natural circumstances, for instance by excretion of certain micro-organisms11 and lead to destruction of pigment layers of paintings containing Han Purple.
Regarding the formation of these compounds in real preparations, a model of progressive silicate condensation has been developed. Starting from the basic structural element SiO44− (orthosilicate), the Si2O76− ion (disilicate) is generated at first. It is represented in the stable BaCu2Si2O7 phase. In the next step, the above-mentioned four-ring unit Si4O128− (cyclo-tetra-silicate) is generated by advancing condensation; this forms the basic silicate unit of Han Purple. The Si4O128− may in turn condense, further building the infinitely connected layers of puckered Si8O20 eight-membered ring units and planar four-membered rings of Egyptian and Han Blue. The reaction scheme of Fig. 7 stresses the fact that, along the pathway of silicate condensations to eventually yield Han Blue, Han Purple is generated as an intermediate product. It is therefore expected to be seen as a first product formed after short reaction times. An “Egyptian Purple” (CaCuSi2O6) is not yet known as a chemical compound.
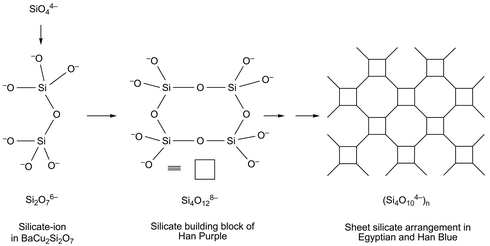 | ||
| Fig. 7 Schematic depiction of the silicate condensation process during the synthesis of Egyptian Blue, Han Blue and Purple. | ||
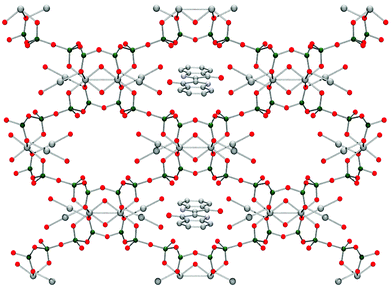 | ||
| Fig. 8 Schematic depiction of the structure of Maya Blue. In the tubular channels of the structural framework of the fibrous clay compound palygorskite ((Mg,Al)4Si8(O,OH,H2O)24), indigo molecules are statistically arranged. The water molecules have been removed from the depiction (Mg, Al grey, Si dark green, O red, N light blue, carbon colourless). | ||
![Schematic depiction of part of the structure of Ultramarine Blue. In the sodalite cages ([Na8Al6Si6O24]2+)—here depicted with sodium ions within the walls of the cage—Cl− ions are enclosed within the sodalite. In the case of Ultramarine Blue, these are replaced by S3− radical ions (Na grey, Si dark green, Al blue, O red, S yellow).](/image/article/2007/CS/b606268g/b606268g-f9.gif) | ||
| Fig. 9 Schematic depiction of part of the structure of Ultramarine Blue. In the sodalite cages ([Na8Al6Si6O24]2+)—here depicted with sodium ions within the walls of the cage—Cl− ions are enclosed within the sodalite. In the case of Ultramarine Blue, these are replaced by S3− radical ions (Na grey, Si dark green, Al blue, O red, S yellow). | ||
3. Examined objects
In the following, a number of examined original objects, that are exemplary for the respective synthetic pigments, will be presented.As a pigment, Egyptian Blue is contained above all in historical paintings, in compact blue art objects and blue Egyptian faience. Amongst the many Egyptian pigment samples examined by our group and partly listed in Table 1, some stemmed from the paint on mummy coffins.9 In Old Egypt, mummy coffins were decorated in exactly the same technical way over thousands of years.19 On the wood of which the coffin is made, there is a layer of Nile mud, which was possibly applied to serve as a binder and filler. Over the layer of Nile mud, the (blue) pigment layer consisting of recarbonated lime was applied, in which the blue platelet-like crystals of Egyptian Blue were incorporated.
A sample of the crown of the famous Bust of Queen Nefertete (Ägyptisches Museum der Staatlichen Museen, Berlin) was examined (Fig. 10). It is dated back to 1340 BC (New Kingdom). The analysis reveals very pure Egyptian Blue with only a few impurities stemming from the sand, lime and flux used for the synthesis.
 | ||
| Fig. 10 Picture of the Bust of Nefertete (Egyptian Museum of the State Museum, Berlin). | ||
The late Egyptian period is documented by the Amulet of the dwarf god Bes (712–332 BC), which was supposed to protect those who wore it (Fig. 11). Judging from how it was produced, it is part of the class of blue compact bodies. The production was probably conducted in two stages. In the first stage, Egyptian Blue was synthesised and in the second stage it was, after being moulded in a dry-press procedure, compacted in another firing with the help of a binder (e.g. gelatine or wax). One stage production would have had the disadvantage of the flux's tendency to migrate to the surface of the formed compact body and thus stop the chemical reaction in the core. In that case, the body would not be Egyptian Blue throughout. The sintering process in the two-stage production process was notably improved by the addition of a small amount of glass powder, which led to the creation of more solid objects.20 It can, with a high probability, be excluded that, in Old Egypt, gypsum was used for the “cold” moulding of blue objects.
 | ||
| Fig. 11 Amulet of the Egyptian dwarf god Bes (712–323 BC). Produced as a compact Egyptian Blue body in a two-stage process (see text). | ||
Another widely used technique of ancient Egypt based on Egyptian Blue is the faience technique, which is one of the oldest techniques, especially for the blue colourisation of art objects. With the faience technique, moulded bodies are glazed in a procedure that has been reconstructed very accurately in several modern experiments.21,22 An impressive modern demonstration of one of the blue faience techniques is shown in Fig. 12. The dried object formed from dry quartz is immersed in the aqueous sludge of burnt cementation powder, which consists of sodium carbonate, calcite, quartz and small amounts of copper oxide and sodium chloride. After the sludge layer has dried, it is burnt for 5 hours at approx. 950 °C. After cooling, a porous outer layer can be crumbled. The quartz body is covered in blue glazing on all sides (Fig. 12).
 | ||
| Fig. 12 Production of blue faience according to the process of wet classification.21 | ||
On a microscopic level, the blue faience glazing usually consists not only of coloured glass, but also of blue, spicular, incorporated crystals; the latter do not have exactly the same composition as Egyptian Blue, as was shown in exemplary experiments on the incorporation of Egyptian Blue.23 A microscopic view of needles of “Egyptian Blue” embedded in glass is shown in Fig. 13.
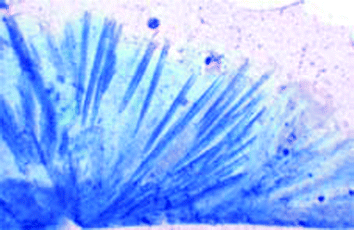 | ||
| Fig. 13 Microscopic view of needles of “Egyptian Blue” embedded in glass. | ||
The glass paste also known as glass frits, which was often used on mummy coffins as a coloured coating incorporated in different binders, is from a chemical point of view very similar to the faience glazings.
In the following, further objects containing Egyptian Blue found in the area of Mesopotamia shall be discussed. The blue building block from Nimrud (1300–700 BC) in present-day Iraq (British Museum, London), (Fig. 14) which in addition to Egyptian Blue also contains large amounts of quartz, is blue throughout and was probably produced just like compact bodies of Egyptian Blue in a two-stage production process, in which, during the second firing, quartz was added. The added quartz content is probably responsible for the higher solidity necessary for the body to be suitable for use as construction material. Further Egyptian Blue samples were investigated from compact blue beads and from cylindrical seals from Nuzi in present-day Iraq.
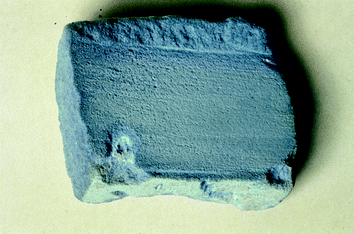 | ||
| Fig. 14 Brick Nimrud. A Mesopotamian building block made from Egyptian Blue and quartz, presumably along the lines of the “compact body procedure” (see text). © Copyright the Trustees of The British Museum. | ||
With most Egyptian Blue objects from Mesopotamia it is difficult to prove whether they had been imported from Egypt or whether they were produced on location. The import hypothesis is supported by some objects that are not typical for Mesopotamia, such as amulets depicting the Egyptian god Bes that were found frequently, even though Bes had no divine significance in Mesopotamia. Domestic production of such artefacts implies technology transfer, which may have taken place as of 1500 BC.
Many Egyptian Blue artefacts from the Roman period were found in Europe, north of the Alps,7 including the areas south of Hadrian's Wall. They were indeed of Roman origin and naturally any such finds were from times earlier than the fall of the Roman Empire. Nevertheless, there are, up to now, two exceptions, where wall paintings were dated to the 9th century AD. One comes from Switzerland, which caught our special interest. The Monastery of Müstair has, in its main church hall, frescoes dated to 860 AD, which indeed contain Egyptian Blue24 (vide infra). It is unknown where this material came from, whether it was a left-over from Roman times or was produced on the spot (Fig. 15). The latter would have required a way to hand down the description of the preparation.
 | ||
| Fig. 15 Picture of the Monastery of Müstair, Switzerland (certified World Cultural Heritage of UNESCO) (top) and part of the lowest layer fresco of the southern wall of the main church of the Monastery (bottom). Copyright by Oskar Emmenegger, Stöcklistrasse, 7205 Zizers, Switzerland. | ||
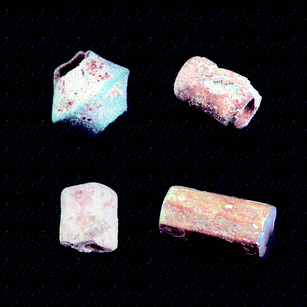 | ||
| Fig. 16 Bead 1 (top left corner), with a faience layer, contains Han Purple and Ultramarine Blue and has a white core. It dates from 777–766 BC.35 Bead 2 (top right corner), with a faience pigment layer, contains Han Blue and Ultramarine Blue and has a coloured core. It dates from the 8th–6th century BC. Origin: the archaeological excavation site Li County (Northwestern China). Bead 3 (bottom left corner) is composed of a heterogeneous, compact blue body (Han Blue) which is part of the class of the sinter minerals that are rich in lead and barium. Excavated and dated: spring and autumn period (770–476 BC).36 The octagonal stick (bottom right corner), dates from 5th–3rd century BC37,38 and is composed of equally coloured sinter material rich in lead and barium, partly crystallised and partly glassy with a decomposed, partly whitish surface.26 | ||
In one bead (approx. 800 BC), all three pigments were identified. It is, so far, the earliest occurrence of a copper silicate pigment.25,26 The synthetic source of Ultramarine Blue could be presumed but not proven. In the case of Han Purple, there was plenty of proof of its occurrence in the period of the Warring States.10 Judging from the artefacts that have been examined so far, a trend can be established, according to which Han Blue was preferred in the early times, whereas later, probably due to a different fashion, more artefacts were made from the pigment Han Purple. From the Eastern Zhou, the Qin and the Han periods, many octagonal sticks were found that were compact bodies made throughout from the same material (Fig. 16). They contain both Han Blue and Han Purple. The Qin and Han periods were the first to produce paints in which Han Blue and Purple could be verified. One example is the Terracotta Army found in the tomb of the first Chinese Emperor Qin Shihuan; amongst other colours, it had also been painted with Han Purple11 (Fig. 17); so far, no Han Blue has been found in the pigment layers of the Terracotta Warriors. The blue colour on the Terracotta Army was, according to the current state of knowledge, rendered mainly through the use of azurite. While traces of Han Blue have been found to accompany polychromic pigment layers of the Han period,27 no accurately dated objects have yet been identified containing Han Blue alone as the blue component. The use of the synthetic barium copper silicate pigments apparently ended with the end of the Han period. A mural painting in the tomb of Bin Wang, in the area of Xian, that had been painted with Han Purple during the Eastern Han period is one of the last pieces of evidence.28
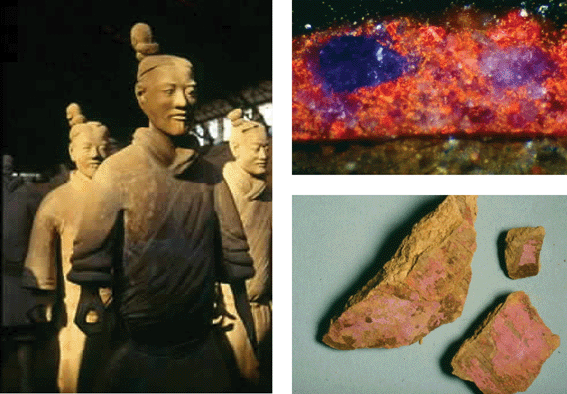 | ||
| Fig. 17 Fragments of the trousers of a Warrior of the Terracotta Army, Xian, China, painted purple (fragments 003–92) (bottom right) and a microscopic cross section through a pigment layer of one of the above-mentioned fragments (top right). Purple fragments correspond with Han Purple. Red particles are composed of vermilion. Horizontal extent: 22 mm. Under the pigment layer, there is a layer of varnish and farther below there is clay. | ||
4. Archaeometry of the pigments of objects with Egyptian Blue, Han Blue and Purple and Ultramarine Blue
In the last two decades, micro-Raman spectroscopy, scanning electron microscopy (SEM and EDX) and X-ray fluorescence spectroscopy have proved to be the best tools for analysing blue pigments in mixtures. EDX delivers micro-element analytical data as part of SEM and X-ray fluorescence, whereas Raman spectroscopy and X-ray powder diffractometry help to conduct a phase analysis. SEM additionally provides information on the surface structure and the heterogeneity of the samples. The sensitivities of all these methods have increased significantly in the last few years. Therefore, it can be assumed that there is a quasi destruction-free situation in archaeometric examinations due to the small sample quantities. All of the above-mentioned analytical methods can be used in a non-destructive way; however, the size and the immobility of the objects often make it impossible to examine them with those tools.The most significant progress in the area of archaeometry in the last few years has been made in Raman spectroscopy, with an enormous increase in the sensitivity of the technology.29 Raman spectroscopy is the most appropriate technique to identify blue pigments.30 All copper silicate pigments and Ultramarine Blue can easily be identified by a characteristic “fingerprint pattern” in the spectrum, which makes it possible to perform a phase analysis (see spectra in Fig. 18 as examples of Egyptian Blue from the Bust of Queen Nefertete and the fresco in Müstair (Switzerland)).
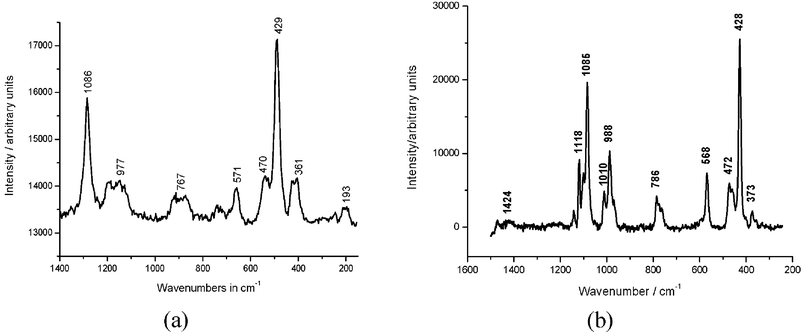 | ||
| Fig. 18 Raman spectrum of virtually pure Egyptian Blue from the crown of the Bust of Nefertete (a) and from a sample of the fresco in the church of the Monastery of Müstair, Switzerland (b) in the range of 200–1400 cm−1 (514 nm). Spectrum (b) also contains bands for SO42− (presumably gypsum) and for CO32− (dolomite). A band at 1118 cm−1 could not yet be assigned. | ||
Examples of the identification of the Chinese pigments are the Raman spectra of Han Blue and Purple and Ultramarine Blue found from various ornamental objects (Fig. 19) as well as those of Han Purple found from samples of the Terracotta Army of the Qin period and found on the tomb of Bin Wang of the Eastern Han period (Fig. 20).
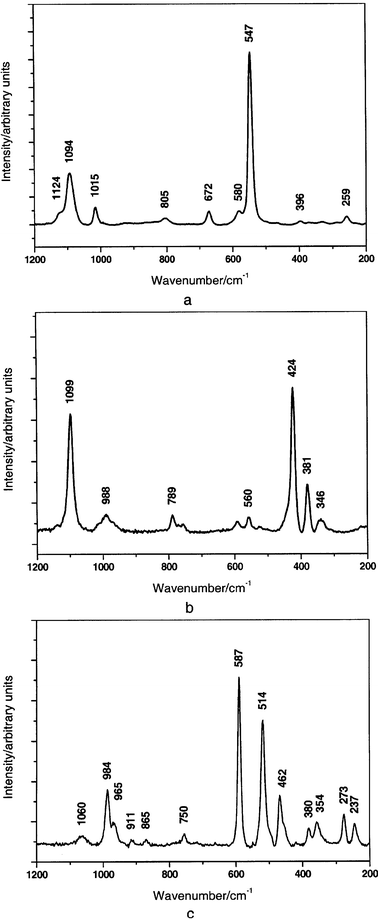 | ||
| Fig. 19 Raman spectra of bead 1 (a) of Han Blue and of bead 3 (b) and of Han Purple of the octagonal stick (c) in the range 1200–200 cm−1.26 Excitation laser 514 nm. | ||
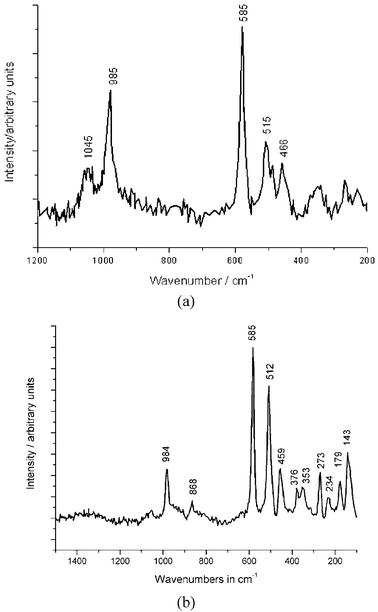 | ||
| Fig. 20 Raman spectra of Han Purple from a sample of the pigment layer of the Terracotta Army in Xian, China (a), and from a sample of the pigment layer of the frescoes from the tomb of Bin Wang (b). Spectral range 1400–400 cm−1 (514 nm). | ||
5. The dissemination of the blue pigments and technology transfer
The various blue pigments that humans have produced give rise to thoughts about whether these developments have taken place independently from one another or whether knowledge and technology transfer have furthered or even made possible some of the achievements. With regard to Maya Blue, there are no indications that e.g. far eastern expertise on indigo processing may have influenced the Indian developments. In the case of Egyptian Blue and the pigments, however, it may not only be conjectured how and when the production of the Chinese pigments may have started, but also whether their chemical similarity and the significantly earlier production of Egyptian Blue give enough indications about whether the Chinese pigments were produced on the basis of Egyptian Blue.The dissemination of knowledge on Egyptian Blue might have sparked the Chinese developments by means of an ancient “technology transfer”. The distribution of Egyptian Blue into the east has, through findings in that area, been verified as far as to the regions of present-day Persia (Fig. 21).
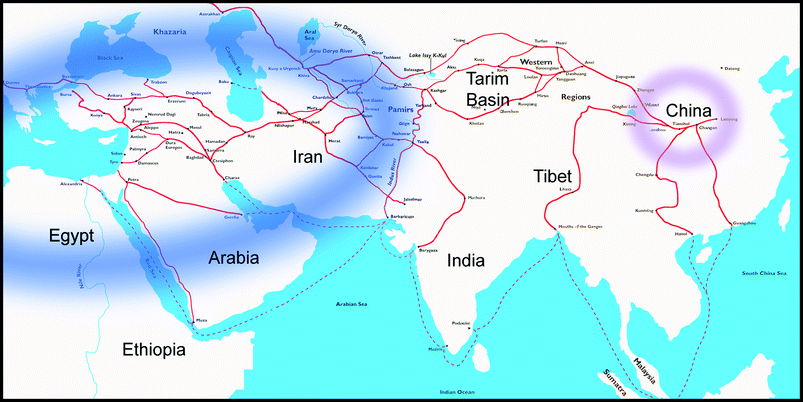 | ||
| Fig. 21 Atlas showing the approximate ancient distribution of Egyptian Blue (blue) and Han Blue and Purple (pink). Red lines indicate the many ways of the silk roads, along which not only trading occurred but also exchange of ideas. | ||
However, it is unclear whether the knowledge about Egyptian Blue really reached Central Asia; further archaeological findings and archaeometric studies will be necessary to answer this question. A geographical overlap of the distribution of the production of Egyptian Blue with the Chinese locations for the barium copper silicate pigments would support, from a chemical point of view, assertion of an ancient technology transfer between the western and eastern worlds; according to current knowledge, however, it cannot conclusively be affirmed.
Therefore, the possibility of a technology transfer seems, from the present-day view, not very likely. It is clear that early inventions, such as the invention of the copper silicate pigments, could not have been sudden discoveries, but requested preceding evolutionary developments. On the basis of an evolutionary process, it can feasibly be demonstrated that the Chinese developments as of approx. 1100 BC were taking place independently from the knowledge about Egyptian Blue. Though, the two historical developments were in many ways taking place similarly. Fig. 22 shows the two independent developments of Egyptian and Han Blue in a flowchart. In both the eastern (China) and the western (Mediterranean area, Egypt, Mesopotamia, Persia) hemispheres, the techniques of glazing stone and clay objects were the first step of the developments. The western hemisphere started out earlier, at first with glazes rich in alkali metal, and later with glazes containing elevated calcium contents. Based on these glazing techniques for the blue colour, the faience and frit techniques were developed and Egyptian Blue emerged and was used in crystalline form.31 In the eastern hemisphere, the glazing techniques were introduced much later, probably even on the basis of transmissions from the west (Fig. 22). As of 1100 BC, however, heavy metal glazes (Pb, Ba) were developed in the east, which eventually led to the creation of early heavy metal glasses and to the production of barium copper silicate pigments.32,33 With regard to the copper silicate pigments, it is probable that they were produced independently from one another as parallel developments in the eastern and western hemispheres.
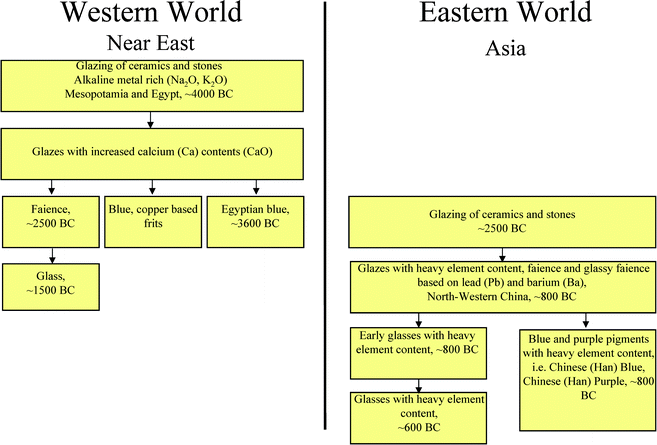 | ||
| Fig. 22 Flowchart showing the development of alkaline earth copper silicate pigments: Egyptian Blue and Han Blue and Purple. | ||
6. Summary of historical considerations
As mentioned previously, the blue pigments were invented from necessity. Humans did not have unlimited access to blue as a pigment, as blue is not an earth colour. About 5500 years ago, with the development of important civilisations, the use of minerals and their chemical transformation probably started. The first synthetic pigment was Egyptian Blue, which the Egyptians, along with the knowledge about its production, transmitted to many cultures in the Mediterranean area and beyond. The Romans were the last to produce Egyptian Blue industrially in factories. With the downfall of the Roman Empire, Egyptian Blue was not passed on any longer and the use of the pigment came to an end.Today, it is assumed that the pigments Han Blue and Purple were used at least since 800 BC probably mostly locally in the smaller north-western Chinese areas. Those are the mineral-rich areas with large copper, barium and lead deposits. As examinations have shown so far, at first Han Blue was used in preference for ornaments, whereas, as of approx. 400 BC, Han Purple replaced it as the preferred pigment. The Terracotta Army of the first Emperor of China, Qin Shihuang, stemming from approx. 220 BC, was painted largely with Han Purple.
It is assumed today that the use of Han Blue and Purple ended in the Han period (220 AD) at the same time as the Chinese Empire was again split; it is probable that, as was the case with Egyptian Blue, political changes stopped the dissemination of the Chinese pigments. Yet, the investigation of the Chinese pigments is far from finished. It is most probable that valuable archaeometric findings will follow and, just as archaeology uncovers more artefacts, shed new light on ancient times, probably above all on the time between 800 BC and 200 AD
In this context, the question arises about the production and dissemination of synthetic Ultramarine Blue produced in ancient times. It is possible that Ultramarine Blue was not only produced in the geographical area of ancient China, but also in other empires such as ancient Mesopotamia, where natural lapis lazuli was frequently used and where there was a high potential for chemical activity. The introduction of Egyptian Blue might have helped to overcome the deficiency of blue pigments and might have stopped any further activities toward the creation of blue pigments. Time will tell if Ultramarine Blue still occurs in archaeological findings of that area.
The historical findings regarding Maya Blue have so far also been too scarce for the scientists to reach definite conclusions. Neither the earliest occurrence of the pigment, nor the time spans in which it was used or disseminated have been confirmed.
The copper silicate pigments have also played a role in recent history. Table 3 provides information on the most important data of the last two centuries, starting with the rediscovery of Egyptian Blue through Napoleon's scientific squad on the Egyptian Expedition.
| 1809 | M. Chaptal, first examination of Egyptian Blue |
| 1814 | Sir Humphrey Davy, identification of Egyptian Blue in samples from Pompeii |
| 1874 | Fontenay, first synthesis of Egyptian Blue |
| 1889 | Fouqui, formulation as (SiO2)4CaOCuO |
| 1900 | Le Chatelier, patent specification no. 112761, Patent Office Berlin, patent for Han Blue and Purple developed from Egyptian Blue |
| 1916 | Bock, industrial production of Egyptian Blue |
| 1959 | Pabst, X-ray structure of Egyptian and Han Blue, synthesis of Han Blue |
| 1983 | FitzHugh, first evidence of Han Blue in ancient artefacts |
| 1989 | Finger, synthesis and X-ray structure of Han Purple |
| 1992 | FitzHugh, first evidence of Han Purple in ancient Chinese samples |
| 1992 | Janczak, correct X-ray structure of Han Purple |
| 1996 | Rhone Poulenc filed a patent for the sol–gel method to produce Han Blue and Purple |
| 2004 | Jaime, Han Purple for the “spin computer” |
The actual scientific investigations did not start until 1809. In 1874, the first synthesis of Egyptian Blue was made and in 1889 Egyptian Blue was defined as an independent compound. The structural determination of Egyptian Blue in 1959 by Pabst is another notable achievement. Han Blue and Purple were, for the first time, recognised as ancient synthetic pigments in the year 1983, when E. FitzHugh and staff members identified Han Blue and Purple in original samples. The “re-invention” of Han Blue and Purple by Le Chatelier in 1900, which was independent from the ancient Chinese knowledge, was another remarkable milestone. In 1900, Le Chatelier filed an application for a patent based on Egyptian Blue with the Patent Office in Berlin.
Le Chatelier unknowingly repeated history with his plan to make new blue and purple pigments available. Necessity is the mother of invention, even though the necessity in this case was not vital but driven rather by a “gap in the market”. As a further milestone in the modern development of Han Blue, Le Chatelier's plan was, in 1996, behind a purely commercial background, improved and optimised with modern methods by the company Rhone Poulenc, which then filed a patent application for its new achievements. The latest cognition regarding the Chinese pigments concerns Han Purple and is still being investigated. Han Purple may have special magnetic properties on an atomic level. The authors of an original publication34 hope that the Cu2 units described might, with their electron spins, be of use in virtual addressing in sub-nano-chips in the so-called “spin computer”, which would lead to an enormous increase in the performance of computers. Hence, the properties of these ancient chemical compounds might continue to be useful for humanity and will further be accompanying human innovations.
7. Chemistry and opportunity
All the pigments described previously have one thing in common: they were all created by human inventive talent, which is apparently strongest when there are situations of deficiency, even if the invention does not necessarily serve vital needs. Colour is an intrinsic part of human life. The human being strives for expression and sensation through colour and thus needs a suitable material basis. Part of this material basis is the pigments, some of which humans created with chemical processes. Chemistry was used as an opportunity. Chemistry was not only an opportunity for the people in ancient times and at the age of industrialisation, but still is an opportunity for the people of today. Seizing opportunities with chemistry means to use silent force and reach copious abundance, according to the notable German chemist Justus von Liebig. In this matter, the ancient civilisations are our role models. Nowadays, however, chemistry is also responsible for its risks.Acknowledgements
First of all, I would like to thank Dr Hans-Georg Wiedemann, Stäfa, Switzerland, who has worked with me on many scientific papers on this subject. I would also like to thank him for providing several illustrations. Furthermore, I am indebted to the many people providing samples who cannot be listed here individually by name, but who are listed in the original publications. With their samples, they provided my group with the essentials to conduct our archaeometric studies. I am particularly indebted to the Egyptian Museum of the State Museum Berlin, Prussian Cultural Heritage, for the sample of the Bust of Queen Nefertete, on which the Raman spectrum was conducted, and to Dr Q. Ma, China National Institute of Cultural Property, Beijing, China, who provided the early Chinese samples and examined them in Zurich. The exact source can be found in the quoted original publications. Furthermore, I would like to thank Ms C. Blänsdorf, Bavarian State Office for the Preservation of Ancient Monuments, Munich, for providing samples, the photographs of the Terracotta Army and the microscopic view. I am also indebted to Dr Susanne Greiff, Roman Germanic Central Museum, Mainz, Germany, for providing a Han Purple sample of the Bin Wang tomb in Baizi, China. We are also grateful to Dr Jürg Goll, Institut für Denkmalpflege ETH Zürich, Switzerland for providing an Egyptian Blue sample of the frescoes in the church of the Monastery Müstair, Switzerland. Finally, I thank the colleagues R. W. P. Wild, A. Portmann, S. Bouherour and T. Corbière for their deep commitment to the projects, on which this paper is based. Their names are also listed in the references. Special thanks go to Dr Ferdinand Wild, whose advice and support significantly contributed to the work on this paper.References
- M. Pastoureau, Blue, the History of a Color, Princeton University Press, Princeton, USA, 2001 Search PubMed.
- Wenn der Geist die Materie küsst, ed. K. Griesar, Deutsch Harri GmbH, Frankfurt am Main, Germany, 2004 Search PubMed.
- P. Ball, Bright Earth: Art and the Invention of Colour, Farrar, Straus and Giroux, New York, USA, 2001 Search PubMed.
- D. Lewis-Williams, The Mind in the Cave: Consciousness and the Origins of Art, Thames and Hudson, London, UK, 2002 Search PubMed.
- E.-L. Richter, Z. Kunsttechnologie Konservierung, 1988, 2, 171–177 Search PubMed; M. Richter, Die Verwendung von Vivianit in der farbigen Fassung und Malerei des Barock und Rokoko, in Historische Polychromie, M. K. Publisher, Munich, Hirmer: 2004, pp. 204–212 Search PubMed.
- D. Knipping, Le Jupiter olympien and the Rediscovery of Polychromy in Antique Sculpture: Quatremère de Quincy between Empirical Research and Aesthetic Ideals, in Proceedings of the conference: The Polychromy of Antique Sculptures and the Terracotta Army of the First Chinese Emperor, Xian, 1999, pp. 89–97 Search PubMed.
- J. Riederer, Egyptian Blue, in Artists’ Pigments: A Handbook of their History and Characteristics, ed. E. W. FitzHugh, Oxford University Press, Oxford, vol. 3, pp. 23–45, 1997 Search PubMed.
- H. Berke, Restauro, 2004, 6, 401 Search PubMed.
- H. Berke, Angew. Chem., 2002, 114, 2595–2600 CrossRef.
- H. Berke and H. G. Wiedemann, East Asian Sci. Technol. Med., 2000, 17, 94–120 Search PubMed.
- H. G. Wiedemann and H. Berke, Chemical and physical investigations of Egyptian Blue and Chinese Blue and Purple, in Proceeding of the conference: The Polychromy of Antique Sculptures and the Terracotta Army of the First Chinese Emperor, Xian, 1999, ed. W. Yonggi, Z. Tinghao, M. Petzet, E. Emmerling and C. Blansdorf, UNESCO International Council on Monuments and Sites, Paris, 2001, pp. 154–170 Search PubMed.
- D. Reinen and G.-G. Lindner, Chem. Soc. Rev., 1999, 28, 75–84 RSC.
- D. Reinen, Chem. Unserer Zeit, 1996, 30, 312–313 Search PubMed.
- G. Chiari, R. Giustetto and G. Ricchiardi, Eur. J. Mineral., 2003, 15, 21–33 CrossRef CAS.
- E. W. FitzHugh and L. A. Zychermann, Stud. Conserv., 1983, 28, 15–23 Search PubMed; E. W. FitzHugh and L. A. Zychermann, Stud. Conserv., 1992, 37, 145–154 Search PubMed.
- H. Wagner, in Pigmente-Herstellung, Eigenschaften, Anwendung, ed. H. Kittel, Wissenschaftliche Verlags-Gesellschaft mbH, Stuttgart, Germany, 1960, pp. 206–236 Search PubMed.
- J. J. Boon, J. van der Weerd, M. Geldof and J. R. J. van Asperen de Boer, Chimia, 2001, 55(11), 952–960 CAS.
- J. Marzahn, Das Iŝtar-Tor von Babylon, in Staatliche Museen zu Berlin – Preussischer Kulturbesitz, Philipp von Zabern, Mainz, Germany, 1995 Search PubMed.
- H. G. Wiedemann, J. Therm. Anal., 1998, 52, 93–107 Search PubMed.
- H. G. Wiedemann, G. Bayer and A. Reller, Egyptian Blue and Chinese Blue – production technologies and applications of two historically important blue pigments. Actes de la Table Ronde: Ravello, Edipuglia, Bari, Italy, 1997, pp. 195–203 Search PubMed.
- R. Busz, G. Sengle and Nil Blau Kassel, Goethe Institute, Alexandria, 2001.
- H. E. Wulff, Archaeology, 1968, 21, 98–107 Search PubMed.
- G. Bayer and H. G. Wiedemann, Sandoz Bull., 1976, 40, 20–39 Search PubMed.
- Die Mittelalterlichen Wandmalereien im Kloster Müstair, in Grundlagen zur Konservierung und Pflege, ed. A. Wyss, H. Rutishauser and M. J. Nay, Vdf Hochschulverlag AG an der ETH Zürich, Zürich, 2002 Search PubMed.
- H. Berke, A. Portmann, S. Bouherour, F. Wild, Q. Ma and H.-G. Wiedemann, Development of Ancient Synthetic Copper-based Blue and Purple Pigments, in Proceedings of the Second International Conference on the Conservation of Grotto Sites, Getty Conservation Institute, Los Angeles, 2006, in press Search PubMed.
- Q. L. Ma, A. Portmann, F. R. P. Wild and H. Berke, Raman and SEM Studies of Ancient Chinese Artifacts Containing Man-made Barium Copper Silicate and Ultramarine Blue Pigments, Stud. Conserv., 2006, 51, 81–89 Search PubMed.
- Unpublished results from analysis of the tomb of Emperor Jing (Han Jingdi) 188–141 BC.
- S. Greiff and S. Yin, Das Grab des Bin Wang, in Malereien der östlichen Han-Zeit in China, Roman-Germanic Central Museum, on consignment with Harrassowitz Verlag Wiesbaden, Mainz, Germany 2002 Search PubMed.
- G. D. Smith and R. J. H. Clark, Raman Microsc. Archeol. Sci., 2004, 31, 1137–1160 Search PubMed.
- S. Bouherour, H. Berke and H. G. Wiedemann, Chimia, 2001, 55(11), 942–951 CAS.
- M. Tite, A. Shortlend and S. Paynter, Acc. Chem. Res., 2002, 35, 585–593 CrossRef CAS.
- R. H. Brill, S. S. C. Tong and D. Dorenwend, Chemical analyses of some early Chinese glasses, in Scientific Research in Early Chinese Glass, ed. R. H. Brill and J. H. Martin, Corning Museum of Glass, Corning, NY, 1991, ch. 4 Search PubMed; P. M. Fenn, R. H. Brill and M. G. Shi, Addendum to ch. 4′, in Scientific Research in Early Chinese Glass, ed. R. H. Brill and J. H. Martin, Corning Museum of Glass, Corning, NY, 1991, pp. 31–64 Search PubMed; R. H. Brill, I. L. Barnes and E. C. Joel, Lead isotope studies of early Chinese glasses, in Scientific Research in Early Chinese Glass, ed. R. H. Brill and J. H. Martin, Corning Museum of Glass, Corning, NY, 1991, ch. 5 Search PubMed; R. H. Brill, M. G. Shi, E. C. Joel and R. J. Vocke, Addendum to ch. 5, in Scientific Research in Early Chinese Glass, ed. R. H. Brill and J. H. Martin, Corning Museum of Glass, Corning, NY, 1991, pp. 65–98 Search PubMed.
- R. H. Brill, Glass and glassmaking in ancient China, and some other things from other places, The Glass Art Society, The Toledo Conference, Glass Art Society, Seattle, 1993, pp. 56–69 Search PubMed.
- M. Jaime, V. F. Correa, N. Harrison, C. D. Batista, N. Kawashima, Y. Kazuma, G. A. Jorge, R. Stern, I. Heinmaa, S. A. Zvyagin, Y. Sasago and K. Uchinokura, Phys. Rev. Lett., 2004, 8, Nr. 087203.
- C. Y. Dai, Wenwu, 2000, 5, 74–80 Search PubMed.
- The Archaeology Team of Baoji, Wenwu, 1993, 10, 1–27 Search PubMed.
- The Archaeology Team of Luoyang City, Wenwu, 1999, 8, 4–13 Search PubMed.
- Museum of Linzi, Wenwu, 1997, 6, 14–26 Search PubMed.
| This journal is © The Royal Society of Chemistry 2007 |