Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices
De-Liang
Long
,
Eric
Burkholder
and
Leroy
Cronin
*
WestCHEM, Department of Chemistry, The University of Glasgow, Glasgow, UK G12 8QQ. E-mail: L.Cronin@chem.gla.ac.uk
First published on 30th October 2006
Abstract
Polyoxometalates represent a diverse range of molecular clusters with an almost unmatched range of physical properties and the ability to form structures that can bridge several length scales. The new building block principles that have been discovered are beginning to allow the design of complex clusters with desired properties and structures and several structural types and novel physical properties are examined. In this critical review the synthetic and design approaches to the many polyoxometalate cluster types are presented encompassing all the sub-types of polyoxometalates including, isopolyoxometalates, heteropolyoxometalates, and reduced molybdenum blue systems. As well as the fundamental structure and bonding aspects, the final section is devoted to discussing these clusters in the context of contemporary and emerging interdisciplinary interests from areas as diverse as anti-viral agents, biological ion transport models, and materials science.
 De-Liang Long | De-Liang Long was born in Hunan, China. He gained his B.Sc. and M.Sc. degrees in chemistry from Wuhan University and completed his Ph.D. under the direction of Professor Xin-Quan Xin at Nanjing University in 1996. After a postdoctoral appointment at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, in 1999 he held the Royal Society KC Wong Fellowship working with Professor Martin Schröder at the University of Nottingham. He is currently a Research Associate in the group of Professor Lee Cronin at the University of Glasgow. His interests are in inorganic synthesis, coordination chemistry, and cluster based materials. |
 Eric Burkholder | Eric Burkholder was born in Pennsylvania, USA. He gained his B.Sc. degree in chemistry from Millersville University and completed his Ph.D. under the direction of Professor Jon Zubieta at Syracuse University in 2004. He is currently a Research Associate with Professor Lee Cronin at the University of Glasgow. His interests are in polyxoxometalate synthesis, coordination chemistry, and inorganic–organic hybrid materials. |
 Leroy Cronin | Lee Cronin graduated with a first class honours degree in Chemistry in 1994 from the University of York, and obtained a D.Phil. in bio-inorganic chemistry in 1997 at the University of York under the supervision of Prof. P. H. Walton. In October 1997 he moved to the University of Edinburgh to take up a postdoctoral fellowship with Dr N. Robertson in macrocyclic ligand design and in the summer of 1998, he took a two-month leave-of-absence to work at the University of Hokkaido, Japan, Institute of Electronic Science with Prof. T. Nakamura in the area of molecular conductors and magnetism. In August 1999 he began an Alexander von Humboldt Research Fellowship with Prof. A. Müller at the University of Bielefeld in Germany on the synthesis and crystallographic analysis of very large polyoxometalate clusters. He then started his independent academic career in 2000 where he was appointed to a lectureship at the University of Birmingham. In 2002 he moved to take up a Lectureship in Glasgow and was promoted to Reader in 2005 and to Full Professor in 2006, and he was recently awarded a five year EPSRC Advanced Research Fellowship. He has wide ranging research interests presently focusing in cluster chemistry, ligand design and supramolecular chemistry. In particular he is interested in assembling functional nanosystems and has an interest in chemical complexity. |
1 Introduction
During the last few years the field of polyoxometalate (POM) chemistry has undergone a revolution fuelled by the availability of extremely fast single crystal data collection strategies. This has allowed the characterisation of ultra-large clusters that have nuclearities as high as 368 metal atoms in a single cluster molecule.1,2 Of course, such discoveries have only been possible thanks to the advances of the instrumentation used to collect the diffraction data coupled with the advent of cheap and powerful computing power for structure solution and refinement. Much of the interest in these molecules has arisen because such clusters represent a paradigm in discovery of systems that can be encouraged to grow from molecular to the nanoscale.3,4 Their versatile nature in terms of structure, size, redox chemistry, photochemistry, and charge distribution means that polyoxometalate chemistry is arguably one of the many areas in inorganic chemistry that is developing most rapidly today. Indeed, since the special thematic issue on polyoxometalates was published in Chemical Reviews in 1998,5 nearly a decade has passed. During this time developments have continued at a rapid pace, and new areas are emerging in POM chemistry that are multidisciplinary, and exploit the great structural and electronic diversity of POM-based systems. In this review we will focus on POM chemistry emerging at these interfaces with the specific idea to place the new discoveries and concepts into context rather than exhaustive coverage of specific application areas (e.g. catalysis which is an extremely important area but will not be covered here explicitly—instead the reader is directed to the following excellent references7). This is especially important now since polyoxometalate chemistry continues its development, both as a pure chemical science, and also with many new dimensions in a multidisciplinary context6 interacting with other aspects like materials,8 nanotechnology,9 biology,10–13 as anti cancer, anti-viral14 and even as insulin mimetic,13 surfaces,10,15 catalysis,16–19 supramolecular materials,20,21 colloid science,4 and electronic materials22 including electro/photo chromic systems,23,24 sensors,25,26 molecular materials27–29 and magnetism.30The large number of structural types in polyoxometalate chemistry31 can be broadly split into three classes. (i) Heteropolyanions: these are metal oxide clusters that include hetero anions such as SO42−, PO43−. These represent by far the most explored subset of POM clusters with over 5000 papers being reported on these compounds during the last four years alone. Much of this research has examined the catalytic properties of POMs with great emphasis on the Keggin {XM12O40} and the Wells–Dawson {X2M18O62} (where M = W or Mo and X = a tetrahedral template) anions which represent the archetypal systems. In particular W-based POMs are robust and this has been exploited to develop W-based Keggin ions with vacancies that can be systematically linked using electrophiles to larger aggregates.32 (ii) Isopolyanions: these are composed of a metal-oxide framework, but without the internal heteroatom/heteroanion. As a result, they are often much more unstable than their heteropolyanion counterparts.33 However they also have interesting physical properties such as high charges and strongly basic oxygen surfaces which means they are attractive units for use as building blocks.9 (iii) Mo-blue and Mo-brown reduced POM clusters: these are related to molybdenum blue type species, which was first reported by Scheele in 1783.34 Their composition was largely unknown until Müller et al. reported, in 1995, the synthesis and structural characterisation of a very high nuclearity cluster {Mo154} crystallised from a solution of Mo-blue, which has a ring topology.1 Changing the pH and increasing the amount of reducing agent along with incorporation of a ligand like acetate facilitates the formation of a {Mo132} spherical ball-like cluster35 and therefore this class of highly reduced POM clusters represents one of the most exciting developments in POM chemistry and with many potential spin off applications in nanoscience.
2 Synthetic strategies
Before outlining the general approach to the synthesis of POM clusters it is informative to consider the most useful synthetic approaches reported for derivatisation and functionalisation of fragments leading to a huge variety of structures. These include (i) the potential of the system to generate a versatile library of linkable units, (ii) the ability to generate intermediates with high free enthalpy to drive polymerisation or growth processes, (iii) the possibility for structural change in the building units or blocks, (iv) the ability to include hetero-metallic centres in the fragments, (v) the possibility to form larger groups which can be linked in different ways, (vi) the ability to control the structure-forming processes using templates, (vii) the ability to generate structural defects in reaction intermediates (e.g. leading to lacunary structures) e.g. by removing building blocks from (large) intermediates due to the presence of appropriate reactants, (viii) the ability to localise and delocalise electrons, (ix) the ability to control and vary the charge of building blocks. In this context it is important to realise that the most stable product in the polymerisation of metal oxo-buildings is the metal oxide itself, see Fig. 1.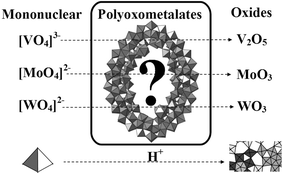 | ||
| Fig. 1 Polyoxometalates are formed in experimental conditions that allow linking of polyhedra. Discrete structures are formed as long as the system is not driven all the way to the oxide. One such example, in this case a part of a {Mo256Eu8} cluster unit,36 is depicted in the square. | ||
Generally, the approaches used to produce high nuclearity POM based clusters are extremely simple, consisting of acidifying an aqueous solution containing the relevant metal oxide anions (molybdate, tungstate and vanadate). In the case of acidification, for example, a solution of sodium molybdate will give rise to metal oxide fragments, which increase in nuclearity as the pH of the solution decreases.6,37
These isopolyanions have been extremely well investigated in the case of molybdenum, vanadium and tungsten. However, the tungsten cases are limited due to the time required for the system to equilibrate (of the order of weeks).37 Heteropolyanions are another class of cluster that can be synthesised when hetero atoms are introduced, and these are extremely versatile. Indeed, heteroanions based on tungsten have been used in the assembly of extremely large clusters.32 In the case of molybdenum the acidification of solutions of molybdate followed by its subsequent reduction yields new classes of clusters with interesting topologies and very large nuclearities.1,35 The synthetic variables of greatest importance in synthesising such clusters are (i) concentration/type of metal oxide anion, (ii) pH and type of acid, (iii) type and concentration of electrolyte, (iv) heteroatom concentration, (v) possibility to introduce additional ligands, (vi) reducing agent (particularly in the case of the Mo systems, (vii) and other basic parameters such as temperature and solvent. Often such syntheses are done in a single pot and this can mask the extraordinary complexity of the assembly event(s) that results with the high nuclearity cluster. As we continue through this review specific reaction variables and considerations will be pointed out in the relevant sections.
In addition to traditional POM syntheses that were normally carried out in aqueous solution at room temperature or at elevated temperatures not higher than the boiling point of the solvent, synthetic strategies based on a hydrothermal approach have been developed recently and this method has been widely applied in the synthesis of POM clusters.38 A few new interesting structures were reported recently. For example, a novel three-dimensional framework formed by [GdMo12O42]9− anions with nine coordinated Gd(III) centres was discovered by Wu et al.39 and a three-electron reduced heteropoly blue [P6Mo18O73]11− with a basket-shaped skeleton was reported;40 this is structurally similar to that of a Dawson structure, but expanded with extra phosphate anions on the shell of the cluster along with a coordinated potassium ion, see Fig. 2.
![A representation of [K⊂(P6Mo18O73)]10−. Colour scheme: Mo, grey; K, purple; O, red. PO4 moieties are shown as orange tetrahedra.](/image/article/2007/CS/b502666k/b502666k-f2.gif) | ||
| Fig. 2 A representation of [K⊂(P6Mo18O73)]10−. Colour scheme: Mo, grey; K, purple; O, red. PO4 moieties are shown as orange tetrahedra. | ||
3 New structure types
3.1 Isopolyanions
Until now only a few (ca. 30) isopolyanions were known and only a few examples have been reported during the last couple of years. For example, Pope et al reported the characterisation of the formation of unsymmetrical polyoxotungstates via transfer of POM building blocks. Indeed, NMR studies support the kinetic stability of the pentatungstate anion, [W5O18]6−, in aqueous solution.41 A {W24} isopolyoxotungstate Cs24(W24O84)(H2O)26 was reported by Bruedgam et al.42 which has a ring structure based around a six-fold axis. Not only is it made up of WO6 octahedra, but it also contains WO5 units, which are very rare. Six vertex-sharing WO6 octahedra form an inner ring and six W3O13 groups are condensed to this ring, again through common octahedral vertices. Also, the W3O13 groups are made up of two vertex-sharing octahedral WO6 and one pyramidal WO5, which is distorted in the direction of a trigonal bipyramid, see Fig. 3.![A representation of the six-fold structure of the cluster [W24O84]24−. Colour scheme: W, light grey; O, dark grey.](/image/article/2007/CS/b502666k/b502666k-f3.gif) | ||
| Fig. 3 A representation of the six-fold structure of the cluster [W24O84]24−. Colour scheme: W, light grey; O, dark grey. | ||
Conventionally, POMs are normally synthesised and isolated using simple counterions such as Na+, K+, and NH4+ and organic tetraalkylammonium cations. Some years ago, through our research, we realised that protonated organic amines with hydroxyl groups can be used as cations and pH buffer systems and could manipulate the assembly of the overall POM, especially for Mo and W systems (these could even be thought of as inverse templates in contrast to the templating role of organo-amines in the formation of certain aluminosilicates and aluminophosphates; the structures of these amines can even be used in the de novo design of such materials).43 For vanadate systems, the use of protonated organic amines as cations is limited as the pH for polyoxovanadate formation is most often around neutral or higher and at these pH values organic amines are unlikely to be protonated. Therefore protonated hexamethylenetetraamine (HMTAH+ or C6H13N4+) was primarily utilised in our work on the synthesis of polyoxomolydates at pH 4, which led to the isolation of quasi-stable clusters such as [H2Mo16O52]10−, see Fig. 4.44 This cluster has been found to have a flat geometry and four of the 16 molybdenum centres are one electron reduced. The shape of the cluster resembles that of a ‘bat’ and its main ‘body’ consists of a central unit with twelve molybdenum atoms and two ‘wings’ each with two molybdenum atoms (giving the formulation [Mo12 + 2Mo2]). In contrast to known polyoxomolybdates of similar nuclearity, [H2Mo16O52]10− has a flat form with dimensions of ca. 13 Å × 11 Å × 6 Å. Of the twelve molybdenum centres that form the main body of the ‘bat’, eight molybdenum centres are placed in two lines of four to form two [Mo4] ‘backbones’. The two protons in the [H2MoV4MoVI12O52]10− anion are involved in the hydrogen bonding interactions inside the cluster (O⋯O distance of 2.732(5) Å).
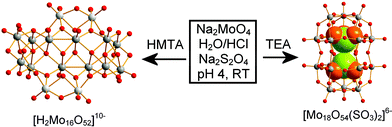 | ||
| Fig. 4 Illustration of cation effect on the formation of POM clusters. Colour scheme: Mo, grey; O, red or orange; S, green. The SO3 moieties are shown in a space filling representation. | ||
The anion [H2MoV4MoVI12O52]10− demonstrates a high nucleophilicity and is capable of binding divalent transition metal ions (FeII, MnII, CoII, NiII, or ZnII) into its framework. This yields a family of isostructural complexes and shows the basic topology found for the [H2MoV4MoVI12O52]10− cluster with the addition of two [MII(H2O)4] moieties yielding a complex of the composition: [MII2(H2O)8H2MoV4MoVI12O52]6− (M = Mn, Fe, Co).45
While [H2MoV4MoVI12O52]10− is diamagnetic because of strong antiferromagnetic exchange within each of the two pairs of MoV2 units, (with χdia/TIP = −1.1 × 10−3 emu mol−1), the magnetic properties of the complexes [M2(H2O)8H2MoV4MoVI12O52]6− (M = Fe, Mn and Co) indicate weak, yet significant, intramolecular antiferromagnetic exchange interactions between the two MII centres despite their wide spatial separation of 1.18 nm. This exemplifies the efficiency of reduced polyoxomolybdates to act as superexchange ligands, where in the present case the superexchange pathways involve at least six centres and both [MoV2] dimers of the [Mo12] framework. The encapsulation of the cluster anion also effectively prevents any significant inter-cluster exchange interactions.
Extending the synthetic strategy utilising organo cations to a tungsten system with protonated triethanolamine (TEAH+), led to the isolation of a new isoplyoxtungstate [H12W36O120]12− with an alkali or alkaline earth metal ion to form metal complexes of the type {M⊂W36} (M = K+, Rb+, Cs+, NH4+, Sr2+ and Ba2+), see Fig. 5.46,47 The {M⊂W36} clusters are approximately C3v-symmetric with a Celtic ring-like shape and comprise three {W11} cluster subunits linked together by three {W1} bridges, see Fig. 5. The {W11} cluster consists of a ring of six basal W positions, an additional W position in the centre of this ring, and four apical W positions in a butterfly configuration. Within the {W11} moieties, two protons form hydrogen bonds between the four central μ3/4-oxo ligands which are visible in 1H NMR at 5.2–5.6 ppm. The Mn+ ion (M = K+, Rb+, Cs+, Sr2+ and Ba2+) located at the cluster centre has a rather distorted coordination geometry and is coordinated by 10 oxygen atoms, of which six are from the Oterminal ligands of the {W36} cluster and four are water ligands.
 | ||
| Fig. 5 Summary of pH effect on the system of tungstate and TEA. Colour scheme: W, grey (the six basal W atoms in the W36 are shown as larger spheres) or blue; O, red or purple. | ||
Most recently we discovered a new family of isopolyoxotungstate [H4W19O62]6−, which was isolated in a similar system to that for {W36} clusters using TEAH+ as cations with only slightly lower solution pH and longer heating time.48 Two isomers α- and γ*-[H4W19O62]6− were obtained and characterised crystallographically; the α form has D3h symmetry and the γ* form D3d symmetry. The α-[H4W19O62]6− comprises the {W18O54} cage framework and interior oxo ligand positions of the conventional Dawson cluster anion α-[W18O54(XO4)2]n− but, contrary to the classical Dawson structure, the two tetrahedral heteroanions are replaced by a triangular-prismatic {WO6} unit and two μ3-oxo ligands, each of which bridges the capping {W3} triangle from inside the cluster. Two tetrahedral ‘voids’ are identified at the positions that are typically occupied by the heteroatoms in conventional Dawson cluster anions α-[W18O54(XO4)2]n−. The γ*-[H4W19O62]6− is based on the geometry of the {W18O54} cage framework and interior oxo ligands, as found in the conventional Dawson cluster anion γ*-[W18O54(SO4)2]4−.19 Again, an additional W position is located at the centre of the cluster and coordinates to six oxo ligands to form a central {WO6} centrosymmetric template of octahedral geometry, see Fig. 5.
One interesting observation we have made in all the work done utilising the TEAH+ cations, as summarised in Fig. 5, is that clusters with 3-fold symmetry are exclusively produced from the reaction systems. These observations may be completely circumstantial, but we have isolated over 4 distinct structure types using the TEAH+ cations, and this has led us to propose that the TEAH+ cation is able to transfer its symmetry onto the clusters in solution. Indeed, supramolecular interactions between the TEAH+ cation and the cluster building blocks via the three hydroxyl groups on the TEAH+ cation, can form H-bonds to intermediate components of the final product and possibly even guide their assembly to the overall cluster architecture.48 In addition, this hypothesis is supported by the observation that a similar reaction, which produces 3-fold symmetric [Mo18O54(SO3)2]6− clusters in the presence of TEAH+, yields low symmetrical-type POM clusters of [H2Mo16O52]10− with different large cations, e.g. protonated hexamethylenetetramine, see Fig. 3.
3.2 Non-conventional Dawson-type clusters
Conventional Dawson-type clusters contain two tetrahedral templates (XO4). There are two kinds of non-conventional Dawson-type clusters: one is where the two tetrahedral templates (XO4) are replaced by one or two triangular pyramid templates (XO3) and the other is where one X atom is missing in the conventional Dawson-type clusters. In the first case the X atoms have been known for As, Sb, Bi prior to 2000.49 Recently examples for Sn have been reported in the cluster [H3SnW18O60]7−.50 The peanut shaped cluster is a non-conventional Dawson cluster with 18 W atoms on the shell. There is only one X (As, Sb, Bi, Sn) atom disordered between two possible sites which are distant by ca. 2.0 Å from each other, which is obviously too short to hold two fully-occupied X atoms due to the large size of the hetero atoms As, Sb, Bi and Sn. In our work we anticipated that the inclusion of the sulfite anion into the Dawson-structure type would represent the first examples of Dawson-like cluster containing two triangular pyramid templates (XO3).51 The first example in this series was a α-[Mo18O54(SO3)2]6− which was isolated as a reduced cluster, produced by the reaction of sodium molybdate with reducing agent Na2S2O4 in the presence of protonated triethanolamine (TEAH+) as cation at a pH of ca. 4, see Fig. 4.This initial work was extended by the discovery of non-reduced both α- and β-[Mo18O54(SO3)2]4−, see Fig. 6.51 The [Mo18O54(SO3)2]4− also has a peanut shape similar to that of [H3SnW18O60]7−.50 However the two encapsulated sulfur atoms are located only 3.2 Å apart from each other inside the cluster shell (this is 0.4 Å less than the 3.6 Å expected from non-bonded S⋯S interactions but still longer than the 2.2 Å found in S2O62−) and could indicate a strong interaction between the two sulfur atoms; this was also suggested by DFT calculations.51 By altering the synthetic conditions we also obtained the first few examples of SO3 based polyoxotungstates, α-[W18O54(SO3)2]4−,52 which is isostructural to α-[Mo18O54(SO3)2]4−,51 and [WVI18O56(SO3)2(H2O)2]8−.52 The latter is described as a “Trojan Horse” in which a structural re-arrangement allows the two embedded pyramidal sulfite (SO32−) anions to release up to four electrons (analogous to the “soldiers” hidden inside the “Trojan Horse”) to the surface of the cluster generating the sulfate-based, deep blue, mixed valence cluster [W18O54(SO4)2]8− upon heating, see Fig. 7. The sulfite anions adopt a radically different orientation in [WVI18O56(SO3)2(H2O)2]8− whereby they each only ligate to seven metal centres: three from the cap and four (out of six) from the “belt” of the cluster framework, see Fig. 7.
 | ||
| Fig. 6 Comparison of the α-SO3- (LEFT), the β-SO3- (MIDDLE), and the SO4-based Dawson clusters (RIGHT) showing that the sulfur atoms of the sulfite clusters are directly adjacent. Colour scheme: O, red; Mo, grey; S, green and O of the SO3, orange (atoms of the sulfite and sulfate anions are shown in larger spheres). | ||
 | ||
| Fig. 7 Scheme showing the change in the metal oxo-framework on one half of the cluster upon oxidation of the internal SO32− ligand to SO42− (shown by the movement of each of the number oxygen atoms on the LHS to the end position on the RHS) which is commensurate with the reduction of the cluster shell by 4 electrons giving rise to the deep blue material from the colourless crystals. | ||
The orientation for the sulfite anions within the cluster type is somewhat like the coordination mode for the tetrahedral templates (XO4y−) in conventional Dawson [M18O54(XO4)2]2y−, i.e. one of the oxo ligand bridges three capping W centres, the remaining oxo ligands each bridge two of the “belt” W centres. Nevertheless, this leaves two “belt” W atoms uncoordinated to the template SO3 moiety as SO3 has one oxo ligand less than XO4. Thus it can be seen that the sulfite ions are grafted onto the bottom side of the cluster, which resembles a “basket” with four “uncoordinated”
“belt” metal centres on the top part and now has a lower C2v symmetry compared to the D3h symmetric cluster α-[Mo18O54(SO3)2]4−. To compensate for the coordination, these unique “uncoordinated”
“belt” W centres (four for the whole cluster) each have two terminal ligands, rather than one as found for the remaining metal centres in the cluster. These are in addition to the four other μ2 bridging oxo (O2−) ligands between metal centres and complete a slightly distorted octahedral coordination geometry for each of the four “uncoordinated”
“belt” metal centres concerned. Single crystal structure analysis revealed that two of the four unique metal centres each have two W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O terminals (W–O ∼1.7 Å) and the other two each have one W
O terminals (W–O ∼1.7 Å) and the other two each have one W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O terminal and one W–OH2 terminal (W–O ∼1.7 Å and ∼2.2 Å, respectively). Furthermore it is interesting that the unique “belt”
μ2 bridging oxo ligands between the pair “uncoordinated”
“belt” W atoms now bends in towards the cluster, rather than outwards as normal and is located ca. 2.9 Å distant from the sulfur centre of the SO3 moiety, whilst the two sulfur centres are positioned 3.6 Å apart at opposite sides of the cluster shell. In this respect the mechanism for the reduction of the cluster shell proposes an interaction between the sulfur atom and the special belt oxo ligand, which then react to form two sulfate anions located within the {W18} cluster shell, see Fig. 7.
O terminal and one W–OH2 terminal (W–O ∼1.7 Å and ∼2.2 Å, respectively). Furthermore it is interesting that the unique “belt”
μ2 bridging oxo ligands between the pair “uncoordinated”
“belt” W atoms now bends in towards the cluster, rather than outwards as normal and is located ca. 2.9 Å distant from the sulfur centre of the SO3 moiety, whilst the two sulfur centres are positioned 3.6 Å apart at opposite sides of the cluster shell. In this respect the mechanism for the reduction of the cluster shell proposes an interaction between the sulfur atom and the special belt oxo ligand, which then react to form two sulfate anions located within the {W18} cluster shell, see Fig. 7.
The structure of non-conventional Dawson cluster, α-[Mo18O54(P2O7)2]4−, has been reported by Körtz and Pope53 in which the coordination of the pyrophosphate anion is quite similar to two sulfite anions in the cluster α-[Mo18O54(SO3)2]4−,51 with the only difference being that an oxygen atom is located in between two phosphorus atoms. This oxygen atom causes the structure to expand in [Mo18O54(P2O7)2]4− around the six equatorial oxygen atoms in the middle. The pyrophosphate anion P2O74− can be described structurally as two corner sharing tetrahedra and each P atom coordinates to the cluster shell via 3 oxo atoms. A cigar-shaped 30-molybdobispyrophosphate [{(P2O7)Mo15O45}2]8− was also reported recently with two P2O74− included inside the cluster.54 The P–O–P bond angle was found to be nearly linear, and it is interesting to conceive of an extended cluster type incorporating many such templates in a row, all templating an outer metal oxo shell, since the resulting structure would resemble a metal oxide tube.
In recent years, POM chemists have noted that one of the two hetero atoms in the conventional Dawson clusters could be “removed” to form semivacant clusters; this was observed in the monolacunary Dawson-type tungstoarsenate [H4AsW17O61]11− and its first-row transition-metal ion derivatives.55 X-Ray determination confirmed the configuration of a semivacant Dawson polyoxotungstate skeleton in the structures of [Ce{X(H4)W17O61}2] (X = P, As) and even indicate the probable location of internal protons. Bond valence sum calculations for these structures show that the four protons required for charge balance in all salts of the {XW18} anions and their lacunary derivatives are almost certainly bound to the oxygen atoms of the empty tetrahedra.56
3.3 Metal complexes of lacunary clusters
Metal complexes of lacuna still remain the most popular focus of POM studies. Hundreds of different types of metal complexes lacunary POMs have been reported in the last eight years. Notably Kim and Pope reported a number of dioxouranate complexes of lacunary polyoxotungstates.57 Lacunary polyoxotungstates are an obvious choice of materials to study with respect to sequestration of radioactive waste due to the thermal stability and the radiation resistant properties of polyoxotungstates, for example, the entrapment of UO22+ cations sandwiched between two {A-PW9O34}9− in the compound Na12[Na2(UO2)2(PW9O34)2]·42H2O, see Fig. 8.57 In addition new plenary and lacunary polyoxotungstate structures have been assembled from {AsIIIW9O33}9− anions and uranyl cations. Since the heteropolytungstate contains AsO33− as the templating anion, with its lone pair electrons, this prevents the formation of sandwich clusters.58 These types of materials have led to a new structural family of heteropolytungstate lacunary complexes with the uranyl cation, UO22+, to be discovered.59![A representation of the structure of [(UO2)2(PW9O34)2]14−. Colour scheme: U, dark grey (large spheres); W, light grey; O, dark grey (small spheres). PO4 moieties are shown as tetrahedra.](/image/article/2007/CS/b502666k/b502666k-f8.gif) | ||
| Fig. 8 A representation of the structure of [(UO2)2(PW9O34)2]14−. Colour scheme: U, dark grey (large spheres); W, light grey; O, dark grey (small spheres). PO4 moieties are shown as tetrahedra. | ||
Lanthanide complexes of lacuna-type clusters continue to yield interesting results relevant to POM chemistry and possibly even offer routes to develop new photo active and responsive materials. Many new structure types have been observed e.g. large cluster formation through multiple substitution with lanthanide cations (La, Ce, Nd, Sm, Eu, and Gd) of the polyoxoanion {(B-α-AsO3W9O30)4(WO2)4}28−, and it has been shown that {(B-α-AsO3W9O30)4(WO2)4}28− has the ability to trap alkali, alkaline earth and lanthanide metal cations.60 The chemistry of lathanides with lacunary POMs is not just limited to reactions with large POM clusters. Recently, it has been demonstrated that pre-formed POMs such as {LnSiW11O39}5− could readily be functionalised via the addition of negatively charged bridging ligands, i.e. acetate, where the ligand would couple two POMs by Ln–O–C–O–Ln linkages forming dimeric [(SiW11O39Ln)2(μ-CH3COO)2]12− (Ln = GdIII, YbIII) complexes.61,62
Along with the f-block elements, there are a considerable number of reports based on reactions of 2nd and 3rd row d-block transition metal complexes of Pd, Pt, Ru, Rh with POM clusters, which are attractive due to potential catalytic properties. A theoretical study by Musaev et al was made on the role of the central template atom in the structure and reactivity of POMs with adjacent d-electron metal sites in the structure γ-[(Xn+O4)RuIII2(OH)2M10O32](8−n)− for M = Mo and W, and X = AlIII, SiIV, PV, and SVI.63 In the study both X and M were analysed for their role in defining electronic states and reactive species. It was shown that the identity of X does significantly impact the lower lying electronic states of POMs and therefore has an important role in determining catalytic activity as it could possibly be used to ‘tune’ catalytic activity. Along with theoretical studies, several Ru–POMs have been fully characterised since detailed structural understanding of pre-catalyst and active catalyst is essential for the advancement of mechanistic chemistry. The Ru-supported heteropolyanions [HXW7O28–Ru(dmso)3]6− (X = P, As) are composed of a Ru(dmso)3 group attached to an unique heptatungstate fragment via a facile interaction with the cluster, see Fig. 9. The Ru atom is bound to the anion by two terminal O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) W bonds and one O–X bond, which represents a fundamentally novel mode of Ru-coordination to a polyoxoanion framework and multinuclear 183W, 31P, 13C, with 1H NMR studies indicating the high stability of these Ru–POMs in solution.64
W bonds and one O–X bond, which represents a fundamentally novel mode of Ru-coordination to a polyoxoanion framework and multinuclear 183W, 31P, 13C, with 1H NMR studies indicating the high stability of these Ru–POMs in solution.64
![A representation of the structure of [HAsW7O28–Ru(dmso)3]6−. Colour scheme: W, grey; Ru, orange; S, yellow; O, red; C, deep grey. AsO4 moiety is shown as a tetrahedron.](/image/article/2007/CS/b502666k/b502666k-f9.gif) | ||
| Fig. 9 A representation of the structure of [HAsW7O28–Ru(dmso)3]6−. Colour scheme: W, grey; Ru, orange; S, yellow; O, red; C, deep grey. AsO4 moiety is shown as a tetrahedron. | ||
The first structurally characterised Pd-substituted tungstosilicate was [Cs2K(H2O)7Pd2WO(H2O)(A-α-SiW9O34)2]9−, which consists of two (A-α-SiW9O34) Keggin moieties linked via a {WO(H2O)}4+ group and two equivalent, square-planar Pd2+ ions leading to a sandwich-type structure with C2v symmetry. The central belt of the structure contains also one potassium and two caesium ions.65 Some other examples of Pd–POM complexes are well described in the palladium(II)-substituted, lone pair containing tungstoarsenates(III) [Na2(H2O)2PdWO(H2O)(α-AsW9O33)2]10− and [CS2Na(H2O)8Pd3(α-AsW9O33)2]9−.66 Some other examples of large POM clusters are well demonstrated in [(As6W65O217)(H2O)7]26−.67 In a very interesting development a late-transition metal oxo complex: [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PtIV(H2O)L2]16− has been discovered where L = [PW9O34]9−, and this is the first complex to include a Pt
PtIV(H2O)L2]16− has been discovered where L = [PW9O34]9−, and this is the first complex to include a Pt![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety,68 see Fig. 10. Furthermore, a second cluster containing the Pd
O moiety,68 see Fig. 10. Furthermore, a second cluster containing the Pd![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety, [PdIVO(OH)WO(OH2)(PW9O34)2]13− was also reported,69 in which the coordination geometry around the Pd centre is described as a distorted octahedron with five Pd–O bond distances of ca. 1.96 Å and one Pd
O moiety, [PdIVO(OH)WO(OH2)(PW9O34)2]13− was also reported,69 in which the coordination geometry around the Pd centre is described as a distorted octahedron with five Pd–O bond distances of ca. 1.96 Å and one Pd![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distance of ca. 1.68 Å.
O bond distance of ca. 1.68 Å.
![The structure of the [OPtIV(H2O)(PW9O34)]16− complex. W = large black spheres, O = small black spheres, Pt = white, P = grey. Note the oxo ligand of the PtO unit is assigned to the oxo site above the Pt ion whereas the site in the ‘cavity’ is assigned as water.](/image/article/2007/CS/b502666k/b502666k-f10.gif) | ||
Fig. 10 The structure of the [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PtIV(H2O)(PW9O34)]16− complex. W = large black spheres, O = small black spheres, Pt = white, P = grey. Note the oxo ligand of the Pt PtIV(H2O)(PW9O34)]16− complex. W = large black spheres, O = small black spheres, Pt = white, P = grey. Note the oxo ligand of the Pt![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit is assigned to the oxo site above the Pt ion whereas the site in the ‘cavity’ is assigned as water. O unit is assigned to the oxo site above the Pt ion whereas the site in the ‘cavity’ is assigned as water. | ||
In recent years the coordination chemistry of the hexavacant tungstophosphate [H2P2W12O48]12− has been very popular and promising, with three good examples reported. The first one [H4P2W12Fe9O56(OAc)7]6− is the coordination compound of [H2P2W12O48]12− with nine FeIII ions.70 Nine iron centres are attached at the hexavacant positions of the [H2P2W12O48]12− and are bridged and protected by seven acetates. The second one is [H12P4W28Fe8O120]16−, which includes four iron centres located in the cluster vacancies, and the molecules are jointed together by Fe–O–Fe bridges.70 The wheel-shaped Cu20 tungstophosphate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25− ion is the third example of a coordination compound of [H2P2W12O48]12−,71 in which four {P2W12} units form a ring and include 20 copper ions inside the cluster.
Other transition metal complexes of lacunary clusters have been also reported. One interesting example is the 15-cobalt-substituted polyoxotungstate [Co6(H2O)30{Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}]5− which has been characterized by single-crystal X-ray analysis. The trimeric polyanion has a core of nine CoII ions encapsulated by three (β-SiW8O31) fragments and two Cl− ligands. The central assembly {Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}17− is surrounded by six antenna-like Co ions.72
3.4 Polyoxovanadates
An area of intense research in POM chemistry is the synthesis of polyoxovanadates that are known to have attractive chemical and physical properties pertaining to catalysis, biochemistry and advance materials science. Recently the focus in synthesis has been vanadate clusters with reduced or mixed-valance vanadium centres. To this avail one synthetic approach that has been adopted has been the one of synergy involving the introduction of organic and anion templating components. The anion is typically imbedded in the vanadate cluster, which can either be covalently or non-covalently bound to the vanadium atoms, used to generate clusters with unique topologies, as in the synthesis of Na6[(PO4)VV6VIV12O39]2·H3PO4·31H2O,73 and [VV13VIV3O42(Cl)]8−,74 which are examples of covalently bonded and non-bonded anions respectively. The anionic cluster, [(PO4)VV6VIV12O39]3−, is similar to other molecular polyoxovanadates encapsulating a VO4 template with six {VO5} square pyramids and 14 octahedral {VO6} units. However, in contrast to other molecular clusters, the [(PO4)VV6VIV12O39]3− clusters are connected by six linear V–O–V bridges into a three-dimensional framework via the six {VO5} square pyramids, see Fig. 11.![Ball and stick representation of the anion network [(PO4)VV6VIV12O39]3− with the sodium cation removed for clarity. Colour scheme: V, orange; P, purple; O, red. The channels of the framework are filled with sodium cations, phosphoric acid and water; interestingly the organic component, barbituric acid, was not incorporated into the structure but co-crystallized as a trimeric barbiturate salt with Na6[(PO4)VV6VIV12O39]2·H3PO4·31H2O.73](/image/article/2007/CS/b502666k/b502666k-f11.gif) | ||
| Fig. 11 Ball and stick representation of the anion network [(PO4)VV6VIV12O39]3− with the sodium cation removed for clarity. Colour scheme: V, orange; P, purple; O, red. The channels of the framework are filled with sodium cations, phosphoric acid and water; interestingly the organic component, barbituric acid, was not incorporated into the structure but co-crystallized as a trimeric barbiturate salt with Na6[(PO4)VV6VIV12O39]2·H3PO4·31H2O.73 | ||
Synthesis of polyoxovandates has classically been done in aqueous solutions or under hydrothermal conditions. Recently new synthetic routes are being explored for the formation of vanadate clusters from organic media with organically soluble vanadium precursors. One example of a vanadium cluster synthesised from organic solvent which has been reported by Hong et al. is {VIV2VV12O36Cl}5− as a tetraethylamine salt that has blue luminescence.75 In this reaction VO2(acac) was used as a source of soluble VO2+ in acetonitrile. The cluster was then crystallised from a DMF solution with [Et4N]5[VIV2VV12O36Cl] being air stable in both solution and in the solid state. The cluster is built up from 14 {VO5} square pyramids however, unlike other polyoxovanadate clusters it is an open cage with two holes in the surface reminiscent of a basket, see Fig. 12. The non-bonded chloride anion occupies the centre of the cavity, with an average vanadium–chloride distance of 3.496 Å, and may offer a path for exchange of the chloride anion with other anions.
 | ||
| Fig. 12 Ball and stick representation of the basket {VIV2VV12O36Cl}5− cluster with chloride in the centre. Colour scheme: V, orange; Cl, green; O, red. | ||
It would appear that polyoxovanadate chemistry still has much to offer and the move from classical aqueous solution to organic solvents via organically soluble vanadium precursors75 may lead to clusters with as of yet unseen structural connectives, as well as new physical properties not previously seen in aqueous based vanadate cluster synthesis.
3.5 Polyoxoniobates
Polyoxoniobate chemistry has been largely limited to isopolyniobates, i.e. [Nb6O18]8− Lindqvist ion. Recently, Nyman et al. have developed a general synthetic protocol for the formation of heteropolyniobates which has presented an important step forward since few heteropolyniobates have been isolated until recently. Heteropolyniobate formation is preferred in hydrothermal reaction at 170–250 °C, in aqueous alkaline mixtures.76 The first heteropolyniobate, K12[Ti2O2][SiNb12O40], was isolated as a Keggin ion, which is not surprising given the pervasiveness of the Keggin structure in all aspects of polyoxometalate chemistry.76 The {SiNb12O40}16− cluster was isolated in the α-Keggin form linked into a one-dimensional chain by TiO6 octahedra shown in Fig. 13.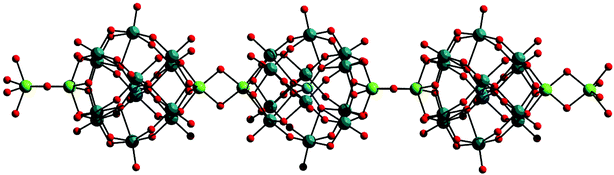 | ||
| Fig. 13 View of the {SiNb12O40}16− and {Ti2O2}4+ chains. Colour scheme: Nb, dark green; Ti, pale green; Si, blue; O, red. | ||
Since the isolation of K12[Ti2O2][SiNb12O40] as a 1-D chain both Na16[SiNb12O40] and Na16[GeNb12O40] have since been isolated as water soluble isolated clusters.77 These clusters, {XNb12O40}16− (X = Si, Ge), have the highest negative charge compared to other Keggins, and lacunary Keggin clusters with such high charge should provide these clusters with unique properties with respect to metal binding. In this context it is important to note that the first lacunary heteropolyniobate, Na15[(PO2)3PNb9O34]·22H2O, which is an A-type trivacant α-Keggin cluster has also been discovered and characterised.78
The surface of the cluster is decorated by three phosphate tetrahedra that are connected to the cluster by two corner sharing interactions with two different NbO6 octahedra shown in Fig. 14. Each of the NbO6 pairs that are linked by the PO4 tetrahedra are also the same NbO6 octahedra that edge-share in the lacuna central belt which is built up from alternating edge- and corner-sharing niobate interactions. The {(PO2)3PNb9O34}15− are then linked by the 15 sodium cations into a three-dimensional framework.
 | ||
| Fig. 14 View of the {(PO2)3PNb9O34}15− cluster showing the surface decoration by three phosphates. Colour scheme: Nb, dark green; P, purple; O, red. | ||
3.6 Mo-blue and Mo-brown clusters
Since the discovery of the {Mo154} ‘big-wheel’1,79 and the {Mo132} ‘big-ball’ or Kepelerate35,80 clusters in the late 1990's this area of chemistry has continued to inspire chemists to both understand and utilise the unique building blocks81 present in these materials.82 Much progress has been made in both the wheel and ball systems including sizing of the clusters,83 removal of inner building block fragments, addition of ligands, electrophiles and even promoting growth processes.33,80 The giant wheels represent nanosensors and nanoreactors, enabling the initiation of chemical processes at different positions, like a “structured landscape”, and can even be used as robust synthons for the construction of compounds with typical solid-state structure, a situation comparable to crystal engineering.79 The potential functionality of these clusters and the use of the spherical systems as functional nanospaces and models for molecular uptake and storage/as artificial cell models is discussed in section 4.4.The reduction of molybdate in the presence of Eu(III) ions at pH 1–2 with ca. 20% of the added molybdate reduced to Mo(V) leads to a giant wheel shaped cluster, similar to the archetypal {Mo154} = [Mo154O462H14(H2O)70]14− but this time the inclusion of the Eu(III) ions leads to the assembly of a giant elliptical cluster dimer {Mo256Eu8} = [{Mo128Eu4O388H10(H2O)81}2]20− with a diameter of ca. 4 nm, see Fig. 15. It appears that the inclusion of the Eu(III) ions causes a dramatic increase in curvature, when compared to the original {Mo154} wheel which results in the elliptical shape of the new cluster.36
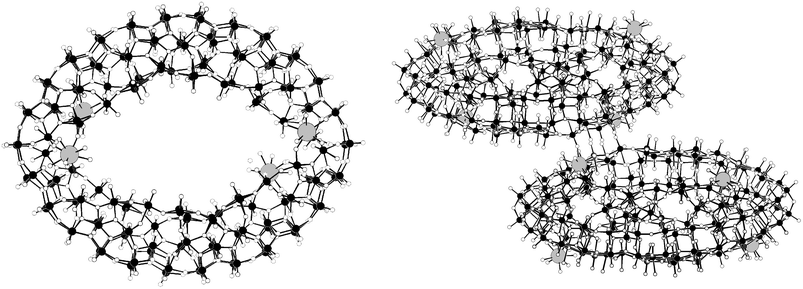 | ||
| Fig. 15 The structure of the elliptical {Mo128Eu4} wheel (LHS) and the structure of the full dimeric {Mo256Eu8} cluster unit (RHS); the Eu(III) ions are depicted as grey spheres, Mo ions as black spheres, and oxygen atoms as white spheres. | ||
In a further dramatic development a new {Mo368} ‘lemon’ shaped cluster was synthesized with the approximate formula [H16Mo368O1032(H2O)240(SO4)48]48−.2 This cluster was synthesized from a solution acidified with sulfuric acid and this allowed the incorporation of the sulfate anion within the cluster framework that dramatically alters the cluster framework compared to the wheel type clusters which are synthesized under similar conditions.146 In this case the ‘lemon’ shaped cluster was synthesised at low pH (1–3) and with 30% of the available molybdate reduced to Mo(V). Thus the ‘lemon’ cluster is more highly reduced than most ‘Mo-blue’ species isolated to date and includes 48 sulfate anions on the inner sphere of the cluster. The cluster incorporates both positively and negatively curved surfaces and has a maximum diameter of ca. 5 nm, see Fig. 16.
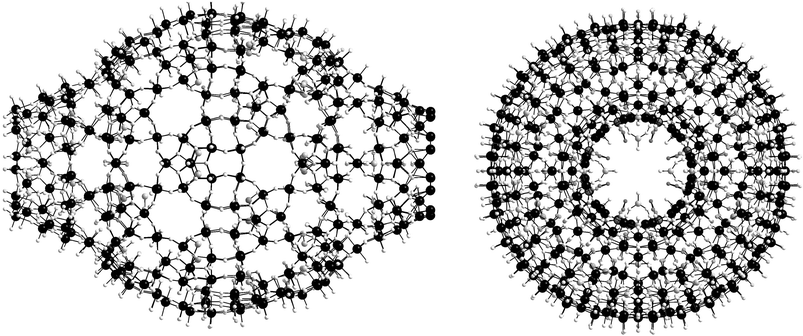 | ||
| Fig. 16 The structure of the {Mo368} ‘lemon’ cluster. The side view is shown (LHS) and the top view is also shown (RHS). | ||
3.7 Related building blocks
The self-condensation of [Mo2O2E2]2+ based building blocks (where E = O or S) has proved to be extremely versatile allowing a large variety of clusters, including cyclic clusters, to be isolated. In the case of the cyclic species the condensation can be performed in the presence or not of guest species. With E = O, the resulting cycles delimit an anionic open cavity, which can be filled by neutral polar molecules such as aqua ligands, or by alkaline cations. With E = S, the resulting cycles open a cationic cavity, which can be filled with neutral molecules, or anions like halides or more sophisticated groups like carboxylates.84–88 For example, decavacant Dawson clusters can be linked to give a compound of the form: [Mo12S12O12(OH)12(H2O)6]. The [Mo2S2O2]2+ building block has been of great utility in the formation of cyclic ring clusters.86 In addition, a polyoxothiomolybdate with a MoVI octahedron has been encapsulated in a reduced Mo–V cyclic octanuclear core89 by self condensation of [Mo2S2O2]2+ with phosphate or arsenate ions to give [(HXO4)4Mo6S6O6(OH)3]5−, X = P, As.90 The condensation of six [Mo2S2O2]2+ building blocks around the 3,5-benzenetricarboxylate anion leads to the new dodecanuclear oxothioanion [Mo12S12O12(OH)12{C6H3(COO)3}]3−, isolated as a tetramethylammonium salt and structurally characterized by single-crystal X-ray diffraction study.91 Further, the influence of a range of carboxylate anions on the self condensation process involving the [Mo2S2O2]2+ building block yields rings with 8, 10 and 12 Mo ions in a ring.92 The new oxothiomolybdate anion [Mo8S8O8(OH)8{HWO5(H2O)}]3− is formed from four [Mo2S2O2]2+ building blocks connected through hydroxo bridges around a central {WO6} octahedron.93 In an extension of this work with [Mo2O2E2]2+ based building blocks it was shown that a supramolecular tetra-Dawson polyoxothiometalate: [(α-H2P2W15O56)4{Mo2O2S2(H2O)2}4 {Mo4S4O4(OH)2(H2O)}2]28− is a polyoxothiometalate-based macrocycle ca. 3 nm in diameter which has been constructed by the connection of complementary cation fragments: [Mo2O2S2]2+ and the basic trivacant α-[P2W15O56]12− ion. The formation of Mo–OH–Mo bridges contributes to the build up of the tetrameric cluster, and shows that it is possible to generate elaborate architectures based on POM-incorporated cyclic oxothiomolybdates, see Fig. 17.94![A representation of the structure of [(α-H2P2W15O56)4{Mo2O2S2(H2O)2}4 {Mo4S4O4(OH)2(H2O)}2]28−. Colour scheme: W, grey; Mo, deep grey; S, yellow; O, red; PO4 moieties are shown as pink tetrahedra.](/image/article/2007/CS/b502666k/b502666k-f17.gif) | ||
| Fig. 17 A representation of the structure of [(α-H2P2W15O56)4{Mo2O2S2(H2O)2}4 {Mo4S4O4(OH)2(H2O)}2]28−. Colour scheme: W, grey; Mo, deep grey; S, yellow; O, red; PO4 moieties are shown as pink tetrahedra. | ||
The sulfite anion has been utilised as an interesting building block for the formation of [Mo12O24(SO3)16]20− and {Na[Mo12O24(SO3)4]2}15−-based framework structures.95,96 In addition, vanadium-based POMs of the form, [(VIVO)6(μ4-O)2(μ3-OH)2(μ3-SO3)4(H2O)2]2+ and [(VIVO)6(μ4-O)2(μ3-OH)2(μ3-SO3)4(H2O)2] have been isolated that exhibit a unique structural motif with a central cubic {VIV4O2(OH)2} fragment and two vanadium(IV) ions located at two of the corners of the cluster.97 Sulfite-based heteropolyoxometalates have also been synthesised with the Dawson-like cage structure, [M18O56(SO3)]4− (M = Mo or W)51 and these and related derivatives were discussed previously in section 2.2.52
4 Materials, biology, devices and nanostructures
4.1 POM-based hybrids and polymers
The derivatisation of POM frameworks by replacing/derivatising the oxo ligands is an important aim since this will allow a much greater degree of control, potentially allowing the simultaneous exploitation of self assembly of the POM fragments, and step wise synthesis to introduce pendant functionalities. One such example is the derivatisation of Lindqvist-type (aryldiazenido)polyoxomolybdates to give compounds of the form (nBu4N)3[Mo6O18(N2Ar)], see Fig. 18.98![Molecular structure of [Mo6O18(N2C6H-p-CO2H)]3−. Colour scheme: Mo, deep grey; O, red; N, blue; C, grey.](/image/article/2007/CS/b502666k/b502666k-f18.gif) | ||
| Fig. 18 Molecular structure of [Mo6O18(N2C6H-p-CO2H)]3−. Colour scheme: Mo, deep grey; O, red; N, blue; C, grey. | ||
Functionalisation has not only been limited to the Lindqvist but also Keggin99 and Dawson100 derivatives have been functionalised. ‘Organometallic’ POM clusters101 have also been produced containing Ru(p-cymene),102 as well as a carbine derivative of a POM, [(PW9O34)2(cis-WO2)(cis-RuLMe2)]13− (LMe = 1,3-dimethylimidazolidine-2-ylidene).103 Tin-based linkers can be used effectively to build complex architectures and this has been done by reacting (CH3)2SnCl2 with Na9[A-PW9O34] to yield [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(A-PW9O34)12]36−, see Fig. 19.104
![A representation of the structure of [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(A-PW9O34)12]36−. Colour scheme: W, grey; Sn, green; O, red; C, black. PO4 moieties are shown as pink tetrahedra.](/image/article/2007/CS/b502666k/b502666k-f19.gif) | ||
| Fig. 19 A representation of the structure of [{Sn(CH3)2(H2O)}24{Sn(CH3)2}12(A-PW9O34)12]36−. Colour scheme: W, grey; Sn, green; O, red; C, black. PO4 moieties are shown as pink tetrahedra. | ||
The Anderson cluster type can also be derivatised by using a ligand that has three pendant hydroxyl groups that can replace the hydroxide groups on the surface of the Anderson. This has produced a variety of tripods including ‘tris’ which has also allowed further derivatisation via imine and peptide bond formation.105,106
POMs can form polymers in many ways: (i) through polymerisation by linkers of M–O–M bonds between POM clusters; (ii) covalently bonded to organic components like acetyl groups which can polymerise; (iii) covalently bonded to organic ligands like polypyridyls which can further form coodination polymers; (iv) POMs as ligands to bind metal ions to form coodination polymers.
In terms of forming direct POM polymers linked by Mo–O–Mo units, it has been observed that in the synthesis of the wheel- and sphere-shaped nanosized molybdenum-oxide based clusters even under one-pot conditions, extensive linking via these types of units can occur. This means that the clusters primarily formed by self-assembly can become further linked in the same phase.107 An example is that the paramagnetic Keplerate “necklaces” synthesized by a novel room-temperature solid-state reaction were characterised in the solid state as metal-oxide-based nanoparticles which formed from controlled linking of {(MoVI)-MoVI5}12Fe30-type Keplerate balls connected by inter-ball Mo–O–Mo bonds.108
Peng's group reported a series of work on {Mo6} imine compounds in which POMs are covalently bonded with terpyridine ligands which can coordinate to other metal ions to form coordination polymers109 and a rational synthesis of covalently bonded organic–inorganic hybrids was described.110 Molecular and polymeric hybrids based on covalently linked POMs and transition-metal complexes has as of yet been limited to the Mo6O19−x(NR)x, x = 1 or 2, cluster type.111 POMs can also be linked through direct condensation or secondary metal ligand coordination complexes.112,113 Other examples of POM polymers, covalent hybrid materials based on nanolatex particles and Dawson clusters was reported recently,114 while coordination polymers with controllable growth of chains and grids from polyoxomolybdate building blocks linked by silver(I) dimers was demonstrated by us.115
POM networks are now ubiquitous, but mesoporous systems may offer some new routes to catalytic materials. For instance a mesoporous hybrid POM based on an inorganic ‘sandwich’ POM, [ZnWZn2(H2O)2(ZnW9O34)2]12− with branched tripodal organic polyammonium salts has been synthesised. Electron microscopy demonstrated that a 3-D porous material was produced (3.6 nm pores) and BET measurements indicated a high surface area (30–50 m2 g−1). These materials behaved as very effective and selective heterogeneous catalysts for the epoxidation of allylic alcohols and oxidation of secondary alcohols to ketones with hydrogen peroxide as oxidant.16 The same ‘sandwich’ POM was also found to be active in the oxidation of alcohols, diols, pyridine derivatives, amines and aniline derivatives with hydrogen peroxide.116
4.2 POM-based materials with magnetic and conducting properties
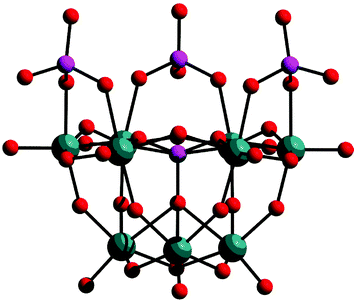
The development of POM-based clusters incorporating paramagnetic centres is an interesting goal since it is possible to utilise existing building blocks/clusters to generate very large magnetic molecules. In fact, it has been shown that it is possible to substitute the {Mo2} ‘linker’ groups present in the Keplerate (Pentagon)12(Linker)30 species with FeIII to yield a {Mo72Fe30} cluster with the formula [Mo72Fe30O252(CH3COO)10{Mo2O7(H2O)}{H2Mo2O8(H2O)}3(H2O)91].83 This cluster is smaller than the parent {Mo132} with an outer diameter of ca. 24 Å and inner diameter of ca. 18 Å. Further, the {Mo72Fe30} cluster is comprised of only MoVI atoms whereas the {Mo72Mo30} cluster contains 36 reduced MoV centres (the 30 linking units are reduced and the remaining 6 centres are delocalised over the 12 pentagonal centres present in the cluster). The presence of the Fe(III) centres, combined with the weak antiferromagnetic exchange between these centres, means that there are 30 mainly uncorrelated spins at room temperature and the cluster therefore has a very large S (approaching 150/2). The Fe(III) centres of the cluster span an icosidodecahedron and the extremely rich and interesting magnetic properties have been investigated using the Heisenberg model.117 In this respect the {Mo72Fe30} has been termed as a mesoscopic paramagnet in which classical behavior extends down to extraordinarily low temperatures. Moving from Fe–Mo to Fe–Mo–V, the self-assembly of molybdate building units in the presence of FeII and VIV produces the first mixed-spin heterometal Keplerate-type clusters displaying ferrimagnetic interactions118 where the clusters have the compostion: {(MoVI)–MoVI5}12M30 where M = VIV, FeIII.119 Further, it appears that when large numbers of paramagnetic metal centres like 30 FeIII or 20 VO2+ are integrated within their structure, extraordinary spin topologies can be realised on a discrete molecular level. Further functionalisation of these systems allows, e.g. to link them forming chains or layers in solid state reactions at room temperature.80Rather than using self assembly of pure POM building blocks, the ligand directed assembly of magnetically interesting POM clusters can also be considered. For instance a {V8O14} cluster can be formed with two ligated 1,3,5-trideoxy-cis-inositol moieties yielding a vanadyl cluster with a large spin ground state arising from strong ferromagnetic interactions within the cluster. High nuclearity copper(II) complexes have been recently produced; a cavity directed {Cu20W48} species, [Cu20Cl(OH)24(H2O)12(P8W48O184)]25− see Fig. 20 and {Cu9} = [{SiW8O31Cu3(OH)(H2O)2(N3)}3–(N3)]19−120 and {Cu14} = {[(SiW9O34)(SiW9O33(OH))(Cu(OH))6Cu]2}23− clusters.121
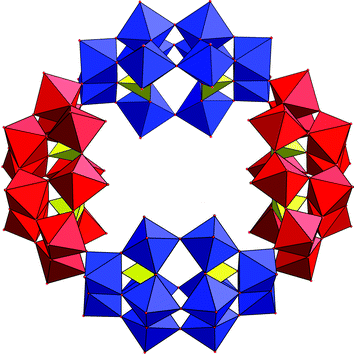 | ||
| Fig. 20 Structure of the {W48} cluster showing the cavity in which the 20 copper(II) ions are complexed; the {W12} hexavacant building blocks are shown in blue and red and the copper(II) ions are omitted for clarity. | ||
The formation of hybrid materials based on POMs with stacks of partially oxidized p-electron donor molecules of tetrathiafulvalene (TTF) has been accomplished to yield conducting POM-based materials. This is interesting because the inorganic POM anion can act as a structural spacer unit, incorporate additional functionality such as a scaffold for paramagnetic ions or to act as an electron acceptor.27 This area is progressing rapidly with the compounds based on [BEDT-TTF]5[H3V10O28]122 and [BEDT-TTF]6[Mo8O26]123 (BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene) which behave as metals down to 50 and 60 K with room temperature conductivities of 360 and 3 S cm−1, respectively. In addition, a POM radical salt with metallic behavior down to 2 K has been synthesized.124 The compound is based on [BEDOTTF]6K2[BW12O40] and is formed from [BW12O40]5− and the organic radical (BEDO-TTF) (=bis(ethylenedioxo)tetrathiafulvalene). The realization of POM–organic conducting hybrids means that devices incorporating both POM clusters and organic conductors and polymers are also accessible. A chiral POM conductor has also been produced using the chiral cluster [H4Co2Mo10O38]6− electrocrystallised with (BEDT-TTF or ET)28 and POM-based materials that have mobile lithium ions have also been realized.93
4.3 Chiral and biologically active polyoxometalates
Interest in the design and synthesis of chiral POMs has been recently gathering momentum driven by applications to materials125,126 and catalysis.127,128 The amplification and transfer of chirality from organic tartrate to a large polytungstate has been achieved by reacting the achiral lacunary Wells–Dawson POM unit, α-[P2W15O56]12−, which has C3v symmetry with D or L-tartrate. Given the unfavourable electrostatic interactions between these fragments a positively charged mediator, in this case, ZrIV is used because of high charge and coordinative flexibility to yield the chiral POM, {α-P2W15O55(H2O)]Zr3(μ3-O)(H2O)(tartH)[α-P2W16O59]}15− (both enantiomers are available and depend on the symmetry ‘printed’ onto the cluster by the tart ligand). This is interesting since the cluster framework is chiral and robust, see Fig. 21. Tartrate has also been used to form POM-based chiral helical polymers with Gd(III).129 The interaction of amino acids with enantiomers of [CeIII(α-P2W17O61)(H2O)x]7− has been investigated using 31P NMR and shows diastereomer formation however no such splitting is observed with glycine or D/L-proline, or when chiral amino acids are added to the corresponding complex of the achiral α-[P2W17O61]10−.130![A representation of the structure of {α-P2W15O55(H2O)[Zr3(μ3-O)(H2O)(tartH)[α-P2W16O59]}15−. Colour scheme: W, grey; Zr, deep grey; O, red; C, black; H, light blue. The PO4 moieties are shown as green tetrahedra.](/image/article/2007/CS/b502666k/b502666k-f21.gif) | ||
| Fig. 21 A representation of the structure of {α-P2W15O55(H2O)[Zr3(μ3-O)(H2O)(tartH)[α-P2W16O59]}15−. Colour scheme: W, grey; Zr, deep grey; O, red; C, black; H, light blue. The PO4 moieties are shown as green tetrahedra. | ||
Perhaps one of the most extraordinary areas of application of polyoxometalate chemistry lies in biology,11 but perhaps is not so surprising given the wide variety of structures, water solubility, anionic nature, electrochemical activity, and recent realisation that large inorganic clusters can penetrate cell walls. Indeed, recent investigation of anti-tumor,131 -viral, and -bacterial activities of POMs shows induced cell-apoptosis, inhibition of virus binding to a receptor, and the enhancement of P-lactam antibiotics, inhibition of bacterial growth, herbicidal action,132 as well as regulation of insulin levels.12,133 Aqueous vanadate and aqueous tungstate have been known to mimic all or most of the actions of insulin in intact cell systems with respect to normalisation of the blood glucose level.134,135 By carrying out oral administration in vivo experiments on the blood glucose level of streptozotocin (STZ)-induced diabetes (STZ mice), the insulin-mimetic (IM) effects of POMs have been examined with and without vanadium substitution. Several homo-POMs (Dawson {M18} and vanadium-substituted (M16V2) showed hypoglycemic effects.13
4.4 Polyoxometalate based nanostructure assemblies … towards devices
The fact that POMs have high charge, well defined structures based on conserved building blocks, and form via self assembly means that they have great potential to bridge multiple length scales. For instance, the {Mo154} wheel cluster, self assembled at low pH under reducing conditions from Na2MoO4, can undergo a further self-assembly process to form hollow spherical structures. These structures are approximately monodisperse with a radius of about 45 nm and are composed of ca. 1200 {Mo154} wheel-shaped clusters in solution; they appear to lie flat homogeneously distributed over a vesicle surface. Unlike conventional lipid vesicles, the structures that were observed are not stabilised by hydrophobic interactions, but rather because of a subtle interplay between short-range van der Waals attraction and long-range electrostatic repulsion, with important further stabilisation arising from hydrogen bonding involving water molecules encapsulated between the wheel-shaped clusters and in the vesicles' interior.4 The cluster ball, {Mo72Fe30}, also undergoes a self assembly process to form hollow, 2.5-nm-diameter, spherical heteropolyoxometalate {Mo72Fe30}-based macro-ions which form into single-layer vesicles (averaging 50–60 nm in diameter).136 Large colloidal aggregates of POMs have also been used to produce 3-D nanostructures.137Given the anionic nature of POM clusters in general, the manipulation of POM-based materials using cation exchange is an extremely important route to the design of new materials. For instance, the partially reduced polyoxomolybdate [H3Mo57V6(NO)6O183(H2O)18]21− has been encapsulated in a shell of dimethyldioctadecylammonium (DODA) surfactant molecules to give (DODA)20(NH4)[H3Mo57V6(NO)6O183(H2O)18] rendering the {Mo57V6} cluster core soluble in organic solvents. Slow evaporation of a solution of the surface encapsulated clusters causes the hydrophobic particles to aggregate into a cubic lattice and the compound forms stable Langmuir–Blodgett films and multilayers.138 In an extension to this work the spontaneous self assembly of the surfactant encapsulated spherical {Mo132}42− cluster has also been achieved and the nanoporous core–shell particles examined revealing a porous supramolecular structure.139,140 Building on this work, ultrathin composite films incorporating the {Mo132}42− cluster have been constructed using a stepwise self-assembly strategy. Alternating adsorption of the Keplerate anions and PAH results in single layers with an apparent surface coverage of approximately 50%.141
The integration of POMs into functional architectures and devices, however, necessitates the development of general methods that allow positioning these clusters in well-defined supramolecular architectures, thin films, or mesophases. This can be done by electrostatic layer-by-layer self-assembly (ELSA) of POMs and a variety of water-soluble cationic species an also be included.21 Using ELSA, the formation of multilayers with polyelectrolytes and nanoscopic POM clusters of different sizes and charges has been investigated. Cyclic voltammetry indicates that the electrochemical properties of the POM clusters are fully maintained in the polyelectrolyte matrix, which opens a route toward practical applications such as sensors or heterogeneous catalysts. Also, the permeability toward electrochemically active probe molecules can be tailored through the multilayer architecture and deposition conditions.142 For instance, the POM cluster [CoII4(H2O)2P4W30O112]16− embedded in a self-assembled polyelectrolyte matrix shows a remarkable pH dependence of its electrochemical response, opening a route for this material to be used as a molecular probe or to fabricate pH microelectrodes.26 Biologically inspired POM–surfactant composite materials using the Keplerate cluster have also been examined140 and the multilayer film composed of POM anion α-[SiW11O39Co(H2PO4)]7− with poly(diallyldimethylammonium chloride) was fabricated and it was shown that the film can immobilize the DNA molecules via a Mg2+-bridging medium.10
The spherical {Mo132}42− Keplerate nanocluster itself has been studied intensely recently. This cluster has the form [{(pent)12(link)30}], e.g. like [{(Mo)(Mo5O21(H2O)6}12{Mo2O4(ligand)}30]n− with binuclear linkers where the 12 central pentagonal units span an icosahedron and the linkers are a distorted truncated icosahedron; the highly charged capsule with sulfate ligands and n = 72 was used very successfully. For instance, the truly nanoscale capsules (inner cavity diameter ca. 2.5 nm) allow different types of encapsulations, e.g. of well-structured large water assemblies (up to 100 molecules) with an ‘onion’ like layer structure enforced by the outer shell.33,143 Most importantly, the capsules have 20 well-defined pores and the internal shell functionalities can be tuned precisely since the nature of the bidentate ligands can be varied. In the special case of binuclear MoV2O42+ linkers the pores are {Mo9O9} rings with a crown-ether function (diameters 0.6–0.8 nm) which can be reversibly closed, e.g. by guanidinium cations non-covalently interacting with the rings via formation of hydrogen bonds.144 In a related smaller capsule with mononuclear linkers the {Mo6O6} pores can get closed/complexed correspondingly by smaller potassium ions.145
The most intriguing and exciting property of the highly negatively charged capsules is that they can mediate cation transfer from the solution to the inner nanocavity. Indeed, reaction of the above-mentioned highly charged capsule with different substrates/cations such as Na+, Cs+, Ce3+, C(NH2)3+, and OC(NH2)NH3+ in aqueous solution leads to formations/assemblies which exhibit well-defined cation separations at, above, or below the capsules channel-landscapes (‘nano-ion chromatograph’ behaviour).146 Taking this one step further a temperature-dependent equilibrium process that involves the uptake/release of Li+ ions through the capsule pores has been observed: the porous capsule behaves as a semi-permeable inorganic membrane open for H2O and small cations.147 Furthermore, the 20 pores of the same capsule ‘shut’ by protonated urea as “stoppers”, can be opened in solution thus allowing calcium(II) ion uptake while later closing occurs again (see Fig. 22).148 Remarkably, “pore gating”—just modelling biological ion transport—can illustratively be demonstrated: after initial cation uptake, subsequent cations are found hydrated above the pores due to a decrease of negative capsule charge.149
![Space-filling representation demonstrating a simplified view of the Ca2+ ion uptake based on the capsule [{(Mo)(Mo5O21(H2O)6}12{Mo2O4(SO4)}30]72−. Initially the pores are closed, but treating a solution of the capsule with Ca2+ ions leads to cation uptake (left) while in the final product the pores again are closed (right; Mo-blue, O-red, C-black, N/O(urea)-green, Ca2+-pink, yellow arrows indicate direction of motion).](/image/article/2007/CS/b502666k/b502666k-f22.gif) | ||
| Fig. 22 Space-filling representation demonstrating a simplified view of the Ca2+ ion uptake based on the capsule [{(Mo)(Mo5O21(H2O)6}12{Mo2O4(SO4)}30]72−. Initially the pores are closed, but treating a solution of the capsule with Ca2+ ions leads to cation uptake (left) while in the final product the pores again are closed (right; Mo-blue, O-red, C-black, N/O(urea)-green, Ca2+-pink, yellow arrows indicate direction of motion). | ||
5 Conclusions and perspectives in polyoxometalate chemistry: From biology to nanotechnology
In recent years there has been an unprecedented rise in the number of interesting POM clusters that have been structurally characterised by single crystal X-ray crystallography and, of course, the absolute size of the molecules characterised. Indeed, the elucidation of the structure of {Mo368} represents the largest non-biologically derived macromolecule to be structurally characterised to date and there is no reason to suppose that the discoveries in this area will stop at this nuclearity.Polyoxometalates, with all their relevance to catalysis, reactivity, electronic structure, materials science and medicine are set to become a paradigm for those working in nanoscale science. This is because the POM clusters described here are ideal candidates for the development of a new type of supramolecular chemistry based upon the building-block ideas already established; using these ideas it should be possible to work towards designer nanomolecules of ever increasing size and complexity. Such clusters, for instance, are being proposed as models for biological ion transport pathways, as well as allowing the oxo-wall to be smashed in the isolation of a Pt![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species. They also are finding application as potential components for nanoscale computing devices. Maybe such systems could be used to probe and even discover new physics at the quantum classical limit or examine molecular growth processes. In addition, researchers could ask fundamental questions about the self assembly processes that underpin the creation of such structures and ask what influence could our concept of building geometrical and real building blocks have in the design of these molecules. If we are able to devise some rules for cluster design then it maybe possible to design new types of molecular hostage complexes that could be used in information storage or even in light harvesting systems, as well as potential application as robust sequestration agents. For example, there is potential to design POM clusters that can selectively assemble into a given structural type in the presence of the ion to be sequestered, e.g. actinide ions, to produce a system for sequestering radioactive elements. The design/discovery of POM nano-tubes and junctions would also be an important aspect and provide an interesting alternative to the carbon-based analogues. Whatever the future one thing is sure—that researchers will be captivated and motivated by the beauty and complexity of POM structures that will be discovered during the next years.
O species. They also are finding application as potential components for nanoscale computing devices. Maybe such systems could be used to probe and even discover new physics at the quantum classical limit or examine molecular growth processes. In addition, researchers could ask fundamental questions about the self assembly processes that underpin the creation of such structures and ask what influence could our concept of building geometrical and real building blocks have in the design of these molecules. If we are able to devise some rules for cluster design then it maybe possible to design new types of molecular hostage complexes that could be used in information storage or even in light harvesting systems, as well as potential application as robust sequestration agents. For example, there is potential to design POM clusters that can selectively assemble into a given structural type in the presence of the ion to be sequestered, e.g. actinide ions, to produce a system for sequestering radioactive elements. The design/discovery of POM nano-tubes and junctions would also be an important aspect and provide an interesting alternative to the carbon-based analogues. Whatever the future one thing is sure—that researchers will be captivated and motivated by the beauty and complexity of POM structures that will be discovered during the next years.
Acknowledgements
The authors would like to thank the EPSRC, Leverhulme Trust and the Royal Society for financial support.References
- A. Müller, E. Krickemeyer, J. Meyer, H. Bögge, F. Peters, W. Plass, E. Diemann, S. Dillinger, F. Nonnenbruch, M. Randerath and C. Menke, Angew. Chem., Int. Ed. Engl., 1995, 34, 2122 CrossRef
.
- A. Müller, E. Beckmann, H. Bögge, M. Schmidtmann and A. Dress, Angew. Chem., Int. Ed., 2002, 41, 1162 CrossRef CAS
.
- A. Müller and S. Roy, Coord. Chem. Rev., 2003, 245, 153 CrossRef CAS
.
- T. B. Liu, E. Diemann, H. L. Li, A. W. M. Dress and A. Müller, Nature, 2003, 426, 59 CrossRef CAS
.
- C. L. Hill, Chem. Rev., 1998, 98, 1 CrossRef CAS
.
- For example papers covering polyoxometalates in catalysis please see: N. M. Okun, T. Anderson and C. L. Hill, J. Mol. Catal., 2003, 197, 283–290 Search PubMed
; K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi and N. Mizuno, Science, 2003, 300, 964 Search PubMed
; A. M.. Khenkin and R. Neumann, J. Am. Chem. Soc., 2002, 124, 4198 CrossRef CAS
; E. F. Kozhevnikova and I. V. Kozhevnikov, J. Catal., 2004, 224, 164 CrossRef CAS
.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef
.
- M. I. Khan, J. Solid State Chem., 2000, 152, 105 CrossRef CAS
.
- D. L. Long and L. Cronin, Chem.–Eur. J., 2006, 12, 3698 CrossRef CAS
.
- H. Y. Ma, J. Peng, Z. G. Han, X. Yu and B. X. Dong, J. Solid State Chem., 2005, 178, 3735 CrossRef CAS
.
- T. Yamase, J. Mater. Chem., 2005, 15, 4773 RSC
.
- B. Hasenknopf, Front. Biosci., 2005, 10, 275 Search PubMed
.
- K. Nomiya, H. Torii, T. Hasegawa, Y. Nemoto, K. Nomura, K. Hashino, M. Uchida, Y. Kato, K. Shimizu and M. Oda, Journal of Inorganic Biochemistry, 2001, 86, 657 Search PubMed
.
- J. T. Rhule, C. L. Hill and D. A. Judd, Chem. Rev., 1998, 98, 327 CrossRef CAS
.
- R. J. Errington, S. S. Petkar, B. R. Horrocks, A. Houlton, L. H. Lie and S. N. Patole, Angew. Chem., Int. Ed., 2005, 44, 1254 CrossRef CAS
.
- M. V. Vasylyev and R. Neumann, J. Am. Chem. Soc., 2004, 126, 884 CrossRef CAS
.
- R. Neumann, A. M. Khenkin and I. Vigdergauz, Chem.–Eur. J., 2000, 6, 875 CrossRef
.
- N. Mizuno, K. Yamaguchi and K. Kamata, Coord. Chem. Rev., 2005, 249, 1944 CrossRef CAS
.
- I. M. Mbomekalle, B. Keita, L. Nadjo, P. Berthet, K. I. Hardcastle, C. L. Hill and T. M. Anderson, Inorg. Chem., 2003, 42, 1163 CrossRef CAS
.
- D. Volkmer, B. Bredenkotter, J. Tellenbroker, P. Kögerler, D. G. Kurth, P. Lehmann, H. Schnablegger, D. Schwahn, M. Piepenbrink and B. Krebs, J. Am. Chem. Soc., 2002, 124, 10489 CrossRef CAS
.
- S. Q. Liu, D. Volkmer and D. G. Kurth, J. Cluster Sci., 2003, 14, 405 CrossRef CAS
.
- G. Chaidogiannos, D. Velessiotis, P. Argitis, P. Koutsolelos, C. D. Diakoumakos, D. Tsamakis and N. Glezos, Microelectron. Eng., 2004, 73–74, 746 CrossRef CAS
.
- S. Q. Liu, H. Mohwald, D. Volkmer and D. G. Kurth, Langmuir, 2006, 22, 1949 CrossRef CAS
.
- S. Q. Liu, D. G. Kurth, H. Mohwald and D. Volkmer, Adv. Mater., 2002, 14, 225 CrossRef CAS
.
- S. Q. Liu, D. Volkmer and D. G. Kurth, Anal. Chem., 2004, 76, 4579 CrossRef CAS
.
- S. Q. Liu, D. G. Kurth and D. Volkmer, Chem. Commun., 2002, 976 RSC
.
- E. Coronado, C. Gimenez-Saiz and C. J. Gomez-Garcia, Coord. Chem. Rev., 2005, 249, 1776 CrossRef CAS
.
- E. Coronado, S. Curreli, C. Gimenez-Saiz, C. J. Gomez-Garcia and J. Roth, Synth. Met., 2005, 154, 241 CrossRef CAS
.
- L. Xu, E. B. Wang, Z. Li, D. G. Kurth, X. G. Du, H. Y. Zhang and C. Qin, New J. Chem., 2002, 26, 782 RSC
.
- M. Luban, F. Borsa, S. Bud'ko, P. C. Canfield, S. Jun, J. K. Jung, P. Kögerler, D. Mentrup, A. Müller, R. Modler, D. Procissi, B. J. Suh and M. Torikachvili, Phys. Rev. B: Condens. Matter, 2002, 66
.
-
M. T. Pope, Isopolyanions and Heteropolyanions, Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard, and J. A. McCleverty, Pergamon Press, Oxford, 1987, vol. 3, p. pp. 1023–1058 Search PubMed
.
- K. Wassermann, M. H. Dickman and M. T. Pope, Angew. Chem., Int. Ed. Engl., 1997, 36, 1445 CrossRef CAS
.
-
L. Cronin, in High Nuclearity Clusters, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2004 Search PubMed
.
-
W. Scheele, In Sämtliche Physische und Chemische Werke, ed. D. S. F. Hermbstädt, M. Sändig oHG, Niederwalluf/Wiesbaden, 1971 Search PubMed
.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann and F. Peters, Angew. Chem., Int. Ed., 1998, 37, 3360 CAS
.
- L. Cronin, C. Beugholt, E. Krickemeyer, M. Schmidtmann, H. Bögge, P. Kögerler, T. K. K. Luong and A. Müller, Angew. Chem., Int. Ed., 2002, 41, 2805 CrossRef CAS
.
-
M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983 Search PubMed
.
-
J. Zubieta, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2004, vol. 1, p. 697 Search PubMed
.
- C. D. Wu, C. Z. Lu, H. H. Zhuang and J. S. Huang, J. Am. Chem. Soc., 2002, 124, 3836 CrossRef CAS
.
- X. M. Zhang, H. S. Wu, F. Q. Zhang, A. Prikhod'ko, S. Kuwata and P. Comba, Chem. Commun., 2004, 2046 RSC
.
- N. Belai, M. Sadakane and M. T. Pope, J. Am. Chem. Soc., 2001, 123, 2087 CrossRef CAS
.
- I. Bruedgam, J. Fuchs, H. Hartl and R. Palm, Angew. Chem., Int. Ed., 1998, 37, 2668 CrossRef CAS
.
- D. W. Lewis, D. J. Willock, C. R. A. Catlow, J. M. Thomas and G. J. Hutchings, Nature, 1996, 382, 604 CrossRef CAS
.
- D. L. Long, P. Kögerler, L. J. Farrugia and L. Cronin, Angew. Chem., Int. Ed., 2003, 42, 4180 CrossRef CAS
.
- D. L. Long, P. Kögerler, L. J. Farrugia and L. Cronin, Dalton Trans., 2005, 1372 RSC
.
- D. L. Long, H. Abbas, P. Kögerler and L. Cronin, J. Am. Chem. Soc., 2004, 126, 13880 CrossRef CAS
.
- D. L. Long, O. Brücher, C. Streb and L. Cronin, Dalton Trans., 2006, 2852 RSC
.
- D. L. Long, P. Kögerler, A. D. C. Parenty, J. Fielden and L. Cronin, Angew. Chem., Int. Ed., 2006, 45, 4798 CrossRef CAS
.
- Y. Jeannin, C. R. Acad. Sci., Ser. IIc: Chim., 2001, 4, 683 CrossRef CAS
.
- B. Krebs, E. Droste, M. Piepenbrink and G. Vollmer, C. R. Acad. Sci., Serie IIc: Chim., 2000, 3, 205 Search PubMed
.
- D. L. Long, P. Kögerler and L. Cronin, Angew. Chem., Int. Ed., 2004, 43, 1817 CrossRef CAS
.
- D. L. Long, H. Abbas, P. Kögerler and L. Cronin, Angew. Chem., Int. Ed., 2005, 44, 3415 CrossRef CAS
.
- U. Körtz and M. T. Pope, Inorg. Chem., 1994, 33, 5643 CrossRef CAS
.
- U. Körtz, Inorg. Chem., 2000, 39, 623 CrossRef CAS
.
- I. M. Mbomekalle, B. Keita, Y. W. Lu, L. Nadjo, R. Contant, N. Belai and M. T. Pope, Eur. J. Inorg. Chem., 2004, 4132 CrossRef CAS
.
- N. Belai, M. H. Dickman, M. T. Pope, R. Contant, B. Keita, I. M. Mbomekalle and L. Nadjo, Inorg. Chem., 2005, 44, 169 CrossRef CAS
.
- K. C. Kim and M. T. Pope, J. Am. Chem. Soc., 1999, 121, 8512 CrossRef CAS
.
- K. C. Kim and M. T. Pope, J. Chem. Soc., Dalton Trans., 2001, 986 RSC
.
- A. J. Gaunt, I. May, R. Copping, A. I. Bhatt, D. Collison, O. D. Fox, K. T. Holman and M. T. Pope, Dalton Trans., 2003, 3009 RSC
.
- K. Wassermann and M. T. Pope, Inorg. Chem., 2001, 40, 2763 CrossRef CAS
.
- P. Mialane, A. Dolbecq, E. Riviere, J. Marrot and F. Secheresse, Eur. J. Inorg. Chem., 2004, 33 CrossRef CAS
.
- P. Mialane, L. Lisnard, A. Mallard, J. Marrot, E. Antic-Fidancev, P. Aschehoug, D. Vivien and F. Secheresse, Inorg. Chem., 2003, 42, 2102 CrossRef CAS
.
- D. Quinonero, Y. Wang, K. Morokuma, L. A. Khavrutskii, B. Botar, Y. V. Geletii, C. L. Hill and D. G. Musaev, J. Phys. Chem. B, 2006, 110, 170 CrossRef CAS
.
- L. H. Bi, M. H. Dickman, U. Körtz and I. Dix, Chem. Commun., 2005, 3962 RSC
.
- L. H. Bi, U. Körtz, B. Keita, L. Nadjo and H. Borrmann, Inorg. Chem., 2004, 43, 8367 CrossRef CAS
.
- L. H. Bi, U. Körtz, B. Keita, L. Nadjo and L. Daniels, Eur. J. Inorg. Chem., 2005, 3034 CrossRef CAS
.
- U. Körtz, M. G. Savelieff, B. S. Bassil and M. H. Dickman, Angew. Chem., Int. Ed., 2001, 40, 3384 CrossRef CAS
.
- T. M. Anderson, W. A. Neiwert, M. L. Kirk, P. M. B. Piccoli, A. J. Schultz, T. F. Koetzle, D. G. Musaev, K. Morokuma, R. Cao and C. L. Hill, Science, 2004, 306, 2074 CrossRef CAS
.
- T. M. Anderson, R. Cao, E. Slonkina, B. Hedman, K. O. Hodgson, K. I. Hardcastle, W. A. Neiwert, S. X. Wu, M. L. Kirk, S. Knottenbelt, E. C. Depperman, B. Keita, L. Nadjo, D. G. Musaev, K. Morokuma and C. L. Hill, J. Am. Chem. Soc., 2005, 127, 11948 CrossRef CAS
.
- B. Godin, J. Vaissermann, P. Herson, L. Ruhlmann, M. Verdaguer and P. Gouzerh, Chem. Commun., 2005, 5624 RSC
.
- S. S. Mal and U. Körtz, Angew. Chem., Int. Ed., 2005, 44, 3777 CrossRef CAS
.
- B. S. Bassil, S. Nellutla, U. Körtz, A. C. Stowe, J. van Tol, N. S. Dalal, B. Keita and L. Nadjo, Inorg. Chem., 2005, 44, 2659 CrossRef CAS
.
- W. B. Yang, C. H. Lu, Q. Z. Zhang, S. M. Chen, X. P. Zhan and J. H. Liu, Inorg. Chem., 2003, 42, 7309 CrossRef CAS
.
- M. I. Khan, S. Ayesh, R. J. Doedens, M. H. Yu and C. J. O'Connor, Chem. Commun., 2005, 4658 RSC
.
- L. Chen, F. L. Jiang, Z. Z. Lin, Y. F. Zhou, C. Y. Yue and M. C. Hong, J. Am. Chem. Soc., 2005, 127, 8588 CrossRef CAS
.
- M. Nyman, F. Bonhomme, T. M. Alam, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996 CrossRef CAS
.
- M. Nyman, F. Bonhomme, T. M. Alam, J. B. Parise and G. M. B. Vaughan, Angew. Chem., Int. Ed., 2004, 43, 2787 CrossRef CAS
.
- M. Nyman, A. J. Celestian, J. B. Parise, G. P. Holland and T. M. Alam, Inorg. Chem., 2006, 45, 1043 CrossRef CAS
.
- A. Müller and C. Serain, Acc. Chem. Res., 2000, 33, 2 CrossRef CAS
.
- A. Müller, P. Kögerler and A. W. M. Dress, Coord. Chem. Rev., 2001, 222, 193 CrossRef CAS
.
- A. Müller and P. Kögerler, Coord. Chem. Rev., 2000, 199, 335 CrossRef CAS
.
- L. Cronin, C. Beugholt and A. Müller, THEOCHEM, 2000, 500, 181 CrossRef CAS
.
- A. Müller, S. Sarkar, S. Q. N. Shah, H. Bögge, M. Schmidtmann, S. Sarkar, P. Kögerler, B. Hauptfleisch, A. X. Trautwein and V. Schunemann, Angew. Chem., Int. Ed., 1999, 38, 3238 CrossRef
.
- F. Secheresse, A. Dolbecq, P. Mialane and E. Cadot, C. R. Chim., 2005, 8, 1927 CrossRef CAS
.
- V. Bereau, E. Cadot, H. Bögge, A. Müller and F. Secheresse, Inorg. Chem., 1999, 38, 5803 CrossRef CAS
.
- E. Cadot, B. Salignac, S. Halut and F. Secheresse, Angew. Chem., Int. Ed., 1998, 37, 611 CrossRef CAS
.
- E. Cadot, B. Salignac, T. Loiseau, A. Dolbecq and F. Secheresse, Chem.–Eur. J., 1999, 5, 3390 CrossRef CAS
.
- E. Cadot, M. J. Pouet, C. R. Robert-Labarre, C. du Peloux, J. Marrot and F. Secheresse, J. Am. Chem. Soc., 2004, 126, 9127 CrossRef CAS
.
- A. Dolbecq, E. Cadot and F. Secheresse, Chem. Commun., 1998, 2293 RSC
.
- E. Cadot, A. Dolbecq, B. Salignac and F. Secheresse, Chem.–Eur. J., 1999, 5, 2396 CrossRef CAS
.
- A. Dolbecq, E. Cadot and F. Secheresse, C. R. Acad. Sci., Serie IIc: Chim., 2000, 3, 193 Search PubMed
.
- B. Salignac, S. Riedel, A. Dolbecq, F. Secheresse and E. Cadot, J. Am. Chem. Soc., 2000, 122, 10381 CrossRef CAS
.
- A. Dolbecq, C. du Peloux, A. L. Auberty, S. A. Mason, P. Barboux, J. Marrot, E. Cadot and F. Secheresse, Chem.–Eur. J., 2002, 8, 350 CrossRef
.
- E. Cadot, M. A. Pilette, M. Marrot and F. Secheresse, Angew. Chem., Int. Ed., 2003, 42, 2173 CrossRef
.
- M. J. Manos, J. D. Woollins, A. M. Z. Slawin and T. A. Kabanos, Angew. Chem., Int. Ed., 2002, 41, 2801 CrossRef
.
- H. N. Miras, J. D. Woollins, A. M. Slawin, R. Raptis, P. Baran and T. A. Kabanos, Dalton Trans., 2003, 3668 RSC
.
- M. J. Manos, H. N. Miras, V. Tangoulis, J. D. Woollins, A. M. Z. Slawin and T. A. Kabanos, Angew. Chem., Int. Ed., 2003, 42, 425 CrossRef CAS
.
- C. Bustos, B. Hasenknopf, R. Thouvenot, J. Vaissermann, A. Proust and P. Gouzerh, Eur. J. Inorg. Chem., 2003, 2757 CrossRef CAS
.
- C. Dablemont, A. Proust, R. Thouvenot, C. Afonso, F. Fournier and J. C. Tabet, Inorg. Chem., 2004, 43, 3514 CrossRef CAS
.
- S. Bareyt, S. Piligkos, B. Hasenknopf, P. Gouzerh, E. Lacote, S. Thorimbert and M. Malacria, Angew. Chem., Int. Ed., 2003, 42, 3404 CrossRef CAS
.
- R. Villanneau, R. Delmont, A. Proust and P. Gouzerh, Chem.–Eur. J., 2000, 6, 1184 CrossRef CAS
.
- D. Laurencin, R. Villanneau, P. Herson, R. Thouvenot, Y. Jeannin and A. Proust, Chem. Commun., 2005, 5524 RSC
.
- V. Artero, A. Proust, P. Herson, F. Villain, C. C. D. Moulin and P. Gouzerh, J. Am. Chem. Soc., 2003, 125, 11156 CrossRef CAS
.
- U. Körtz, F. Hussain and M. Reicke, Angew. Chem., Int. Ed., 2005, 44, 3773 CrossRef CAS
.
- S. Favette, B. Hasenknopf, J. Vaissermann, P. Gouzerh and C. Roux, Chem. Commun., 2003, 2664 RSC
.
- B. Hasenknopf, R. Delmont, P. Herson and P. Gouzerh, Eur. J. Inorg. Chem., 2002, 1081 CrossRef CAS
.
- A. Müller and S. Roy, Eur. J. Inorg. Chem., 2005, 3561 CrossRef
.
- A. Müller, S. K. Das, M. O. Talismanova, H. Bögge, P. Kögerler, M. Schmidtmann, S. S. Talismanov, M. Luban and E. Krickemeyer, Angew. Chem., Int. Ed., 2002, 41, 579 CrossRef CAS
.
- B. B. Xu, Z. H. Peng, Y. G. Wei and D. R. Powell, Chem. Commun., 2003, 2562 RSC
.
- Z. H. Peng, Angew. Chem., Int. Ed., 2004, 43, 930 CrossRef CAS
.
- J. Kang, B. B. Xu, Z. H. Peng, X. D. Zhu, Y. G. Wei and D. R. Powell, Angew. Chem., Int. Ed., 2005, 44, 6902 CrossRef CAS
.
- W. Ouellette, V. Golub, C. J. O'Connor and J. Zubieta, Dalton Trans., 2005, 291 RSC
.
- E. Burkholder, V. Golub, C. J. O'Connor and J. Zubieta, Inorg. Chem., 2004, 43, 7014 CrossRef CAS
.
- C. Cannizzo, C. R. Mayer, F. Secheresse and C. Larpent, Adv. Mater., 2005, 17, 2888 CrossRef CAS
.
- H. Abbas, A. L. Pickering, D. L. Long, P. Kögerler and L. Cronin, Chem.–Eur. J., 2005, 11, 1071 CrossRef CAS
.
- D. Sloboda-Rozner, P. Witte, P. L. Alsters and R. Neumann, Adv. Synth. Catal., 2004, 346, 339 CrossRef CAS
.
- A. Müller, M. Luban, C. Schröder, R. Modler, P. Kögerler, M. Axenovich, J. Schnack, P. C. Canfield, S. Bud'ko and N. Harrison, ChemPhysChem, 2001, 2, 517 CrossRef CAS
.
- B. Botar, P. Kögerler, A. Müller, R. Garcia-Serres and C. L. Hill, Chem. Commun., 2005, 5621 RSC
.
- A. Müller, A. M. Todea, J. van Slageren, M. Dressel, H. Bögge, M. Schmidtmann, M. Luban, L. Engelhardt and M. Rusu, Angew. Chem., Int. Ed., 2005, 44, 3857 CrossRef
.
- P. Mialane, A. Dolbecq, J. Marrot, E. Riviere and F. Secheresse, Chem.–Eur. J., 2005, 11, 1771 CrossRef CAS
.
- P. Mialane, A. Dolbecq, J. Marrot, E. Riviere and F. Secheresse, Angew. Chem., Int. Ed., 2003, 42, 3523 CrossRef CAS
.
- E. Coronado, J. R. Galan-Mascaros, C. Gimenez-Saiz, C. J. Gomez-Garcia, E. Martinez-Ferrero, M. Almeida and E. B. Lopes, Adv. Mater., 2004, 16, 324 CrossRef CAS
.
- A. Lapinski, V. Starodub, M. Golub, A. Kravchenko, V. Baumer, E. Faulques and A. Graja, Synth. Met., 2003, 138, 483 CrossRef CAS
.
- E. Coronado, C. Gimenez-Saiz, C. J. Gomez-Garcia and S. C. Capelli, Angew. Chem., Int. Ed., 2004, 43, 3022 CrossRef CAS
.
- H. Y. An, E. B. Wang, D. R. Xiao, Y. G. Li, Z. M. Su and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 904 CrossRef CAS
.
- M. Lu, J. H. Kang, D. G. Wang and Z. H. Peng, Inorg. Chem., 2005, 44, 7711 CrossRef CAS
.
- W. Adam, P. L. Alsters, R. Neumann, C. R. Saha-Moller, D. Seebach, A. K. Beck and R. Zhang, J. Org. Chem., 2003, 68, 8222 CrossRef CAS
.
- G. Maayan, R. H. Fish and R. Neumann, Org. Lett., 2003, 5, 3547 CrossRef CAS
.
- C. D. Wu, C. Z. Lu, X. Lin, D. M. Wu, S. F. Lu, H. H. Zhuang and J. S. Huang, Chem. Commun., 2003, 1284 RSC
.
- M. Sadakane, M. H. Dickman and M. T. Pope, Inorg. Chem., 2001, 40, 2715 CrossRef CAS
.
- P. P. Fu, X. L. Wang, E. B. Wang, C. Qin and L. Xu, Chem. Res. Chin. Univ., 2005, 21, 509 Search PubMed
.
- S. J. Xue, S. Y. Ke, L. Yan, Z. J. Cai and Y. G. Wei, J. Inorg. Biochem., 2005, 99, 2276 CrossRef CAS
.
- D. C. Crans, J. J. Smee, E. Gaidamauskas and L. Q. Yang, Chem. Rev., 2004, 104, 849 CrossRef CAS
.
- Y. Zhang, X. D. Yang, K. Wang and D. C. Crans, J. Inorg. Biochem., 2006, 100, 80 CrossRef CAS
.
- P. Buglyo, D. C. Crans, E. M. Nagy, R. L. Lindo, L. Q. Yang, J. J. Smee, W. Z. Jin, L. H. Chi, M. E. Godzala and G. R. Willsky, Inorg. Chem., 2005, 44, 5416 CrossRef CAS
.
- G. Liu and T. B. Liu, Langmuir, 2005, 21, 2713 CrossRef CAS
.
- S. Polarz, B. Smarsly and M. Antonietti, ChemPhysChem, 2001, 2, 457 CrossRef CAS
.
- D. G. Kurth, P. Lehmann, D. Volkmer, H. Colfen, M. J. Koop, A. Müller and A. Du Chesne, Chem.–Eur. J., 2000, 6, 385 CrossRef CAS
.
- D. Volkmer, A. Du Chesne, D. G. Kurth, H. Schnablegger, P. Lehmann, M. J. Koop and A. Müller, J. Am. Chem. Soc., 2000, 122, 1995 CrossRef CAS
.
- D. G. Kurth, P. Lehmann, D. Volkmer, A. Müller and D. Schwahn, J. Chem. Soc., Dalton Trans., 2000, 3989 RSC
.
- D. G. Kurth, D. Volkmer, M. Ruttorf, B. Richter and A. Müller, Chem. Mater., 2000, 12, 2829 CrossRef CAS
.
- S. Q. Liu, D. G. Kurth, B. Bredenkotter and D. Volkmer, J. Am. Chem. Soc., 2002, 124, 12279 CrossRef CAS
.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, B. Botar and M. O. Talismanova, Angew. Chem., Int. Ed., 2003, 42, 2085 CrossRef CAS
.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, S. Roy and A. Berkle, Angew. Chem., Int. Ed., 2002, 41, 3604 CrossRef CAS
.
- A. Müller, B. Botar, H. Bögge, P. Kögerler and A. Berkle, Chem. Commun., 2002, 2944 RSC
.
- A. Müller, S. K. Das, S. Talismanov, S. Roy, E. Beckmann, H. Bögge, M. Schmidtmann, A. Merca, A. Berkle, L. Allouche, Y. S. Zhou and L. J. Zhang, Angew. Chem., Int. Ed., 2003, 42, 5039 CrossRef
.
- A. Müller, D. Rehder, E. T. K. Haupt, A. Merca, H. Bögge, M. Schmidtmann and G. Heinze-Bruckner, Angew. Chem., Int. Ed., 2004, 43, 4466 CrossRef
.
- A. Müller, L. Toma, H. Bögge, C. Schaffer and A. Stammler, Angew. Chem., Int. Ed., 2005, 44, 7757 CrossRef
.
- A. Müller, Y. Zhou, H. Bögge, M. Schmidtmann, T. Mitra, E. T. K. Haupt and A. Berkle, Angew. Chem., Int. Ed., 2006, 45, 460 CrossRef
.
| This journal is © The Royal Society of Chemistry 2007 |
