Nanostructural control of cup-stacked carbon nanotubes with 1-benzyl-1,4-dihydronicotinamide dimer via photoinduced electron transfer†
Kenji
Saito
,
Masataka
Ohtani
and
Shunichi
Fukuzumi
*
Department of Material and Life Science, Division of Advanced Science and Biotechnology, Graduate School of Engineering, Osaka University, SORST, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871, Japan. E-mail: fukuzumi@chem.eng.osaka-u.ac.jp; Fax: +81 6-6879-7370; Tel: +81 6-6879-7368
First published on 15th November 2006
Abstract
The photoinduced electron-transfer reduction of cup-stacked carbon nanotubes (CSCNTs) with 1-benzyl-1,4-dihydronicotinamide dimer [(BNA)2] results in the electrostatic destacking of CSCNTs to afford CSCNTs with uniform size.
Among the wide range of nanocarbon materials prepared to date, carbon nanotubes (CNTs) stand out because of their unique physical and chemical properties, arising from their one-dimensionality.1 However, the main hurdle to the many specialized applications of CNTs is the control of their diameter and size. Although attempts have been made to fabricate nanotube samples with uniform length or diameter,2,3 a more accommodating and efficient approach is highly desired, particularly for commercially available samples. In this context, the cup-stacked carbon nanotubes (CSCNTs), consisting of truncated conical graphene layers, have merited special attention.4 The cup-stacked structure provides a hollow tubular morphology, composed of cup-shaped carbon units with diameters ranging from 50 to 150 nm and lengths of up to 200 µm,4 in contrast with conventional carbon nanotubes made of multiseamless cylinders in a hexagonal carbon network. The availability of the inner and outer edges of these stacked-cups to chemical functionalization or surface modification opens up new ways to utilize them in electronic, catalytic, and photovoltaic applications.5,6 The ball milling processes are reported to result in a decrease in the average lengths of pristine CSCNTs (up to 200 µm) to 7 µm after 24 h milling.7 However, CSCNTs with uniform size have yet to be obtained.
We report herein our discovery that the photoinduced electron transfer from 1-benzyl-1,4-dihydronicotinamide dimer [(BNA)2]8 to CSCNTs yields electrostatically destacked CSCNTs, and thus affords CSCNTs with controlled diameter and size (i.e., cup-shaped carbons), as shown in Scheme 1. This approach opens an easy access toward the development of a novel nanocarbon material with uniform size, using a mild reductant that is stable in air.
 | ||
| Scheme 1 | ||
The CSCNTs used in this study were a gift from GSI Creos Corporation, Japan. They were purified according to the previously reported procedure.9 The CSCNTs with larger diameters (>ca. 50 nm) were removed as follows: purified CSCNTs were suspended in CHCl3 (5 mg mL−1, 10 mL) by mild sonication (i.e., 70 W for 15 min). After centrifugation for 15 min at 1880g, the supernatant was filtered over a PTFE membrane (pore size: 0.1 µm). (BNA)2 was prepared according to literature procedures.8b,10
The CSCNTs with smaller diameters (<ca. 50 nm) were treated with (BNA)2 under photoirradiation to obtain destacked CSCNTs. The progress of the photoinduced electron transfer from (BNA)2 to CSCNTs was detected by monitoring the ultraviolet-visible-near infrared (UV-vis-near-IR) absorption spectrum. Irradiation of 3.1 mL of an acetonitrile (MeCN) suspension containing CSCNTs (0.05 mg) and (BNA)2 (2.1 × 10−7 mol, λmax = 348 nm) with UV-vis light (λ > 340 nm) results in the disappearance of the absorbance due to (BNA)2, accompanied by the appearance of a new absorption band at λ = 260 nm due to BNA+, with a clean isosbestic point.† The absorption spectral changes indicate the consumption of (BNA)2 (ε = 6.9 × 103 mol−1 dm3 cm−1) and generation of nearly 2 equiv. of BNA+ (ε = 6.3 × 103 mol−1 dm3 cm−1), as shown in Fig. 1. The reaction is started by photoinduced electron transfer from (BNA)2 to CSCNTs to produce (BNA)2˙+ and reduced CSCNTs (Scheme 1). This step is followed by a fast cleavage of the C–C bond of the dimer to produce N-benzylnicotinamide radical (BNA˙) and BNA+.8 The subsequent electron transfer from BNA˙ to CSCNTs also occurs efficiently, because BNA˙ is known to act as a strong electron donor.8,11 Consequently, (BNA)2 acts as a two-electron donor to produce 2 equiv. of BNA+ (vide supra). No reaction occurs in the dark. Thus, CSCNTs can be reduced by photoinduced electron transfer with (BNA)2 in deaerated MeCN.
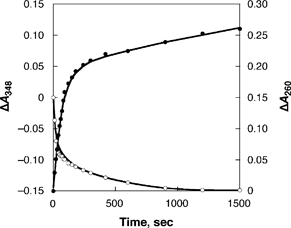 | ||
| Fig. 1 Time course of the spectral changes at 260 (●) and 348 (○) nm in the photoreduction of CSCNTs with (BNA)2 in deaerated MeCN. | ||
We have also examined the solid-state electron spin resonance (ESR) spectrum of reduced CSCNTs to confirm the reduction of CSCNTs by (BNA)2 (Fig. 2).‡ The pristine CSCNTs were virtually ESR silent at 298 K. This indicates that no paramagnetic impurity is contained in the CSCNT sample. In contrast, the reduced CSCNTs exhibit a broad ESR signal at 298 K together with a sharp signal (g = 2.0018), which is virtually the same as observed in K-doped graphite (g = 2.0027).12 The broad ESR signal and its small g value, as compared to the free spin value (2.0023), are characteristic of the delocalized electron on the nanocarbon material.13
 | ||
| Fig. 2 Solid-state ESR spectrum of CSCNTs (0.020 g) after chemical reduction by (BNA)2. | ||
The scanning electron microscopy (SEM) images of pristine CSCNTs revealed long straight carbon nanofibers with lengths of up to submicrometre (Fig. 3a).§ On the other hand, pristine CSCNTs were disassembled after the photoinduced electron-transfer reduction with (BNA)2 to form destacked CSCNTs (Fig. 3b).
 | ||
| Fig. 3 SEM images of (a) pristine CSCNTs and (b) reduced CSCNTs. | ||
The structural changes in CSCNTs induced by the photoreduction of CSCNTs with (BNA)2 were also checked using the transmission electron microscopy (TEM) measurements shown in Fig. 4.¶ The cup-stacked structures of pristine CSCNTs (Fig. 4a) are disassembled to cup-shaped carbons with relatively similar diameters (ca. 50 nm) and lengths (ca. 200 nm) after the photoinduced electron-transfer reduction with (BNA)2 (Fig. 4b).
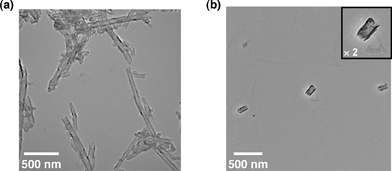 | ||
| Fig. 4 TEM images of (a) pristine CSCNTs and (b) reduced CSCNTs. | ||
Dynamic light scattering (DLS) measurements provide a powerful tool for the detection of the electrostatically destacked carbons generated by the photoinduced electron-transfer reduction with (BNA)2 in solution.|| An MeCN suspension containing a small amount of CSCNTs (0.05 mg per 3.1 mL) was adopted due to monitoring the photoreduction with (BNA)2. DLS in MeCN (Fig. 5) reveals that destacked cup-shaped carbons after the photoreduction with (BNA)2 show a narrow size distribution, with a mean size of 210 ± 57 nm, which is much smaller than pristine CSCNTs (850 ± 330 nm). This value is in good agreement with that observed in the TEM measurement in Fig. 4b. The mean size of the CSCNTs decreases with increasing (BNA)2 concentration (b to c in Fig. 5). This observation indicates that the amount of injected electrons from the (BNA)2 to CSCNTs, via photoinduced electron transfer, plays an important role in the disassembly process, because the electrostatic repulsion of the nanocarbon increases with an increase in the amount of injected electrons. We have examined the occurrence of restacking after oxidation by oxygen (Fig. S2†). The mean size of reduced CSCNTs increased after oxidation (from 270 ± 90 nm to 540 ± 90 nm). This may result from the occurrence of aggregation between cup-shaped carbons together with restacking.
![Size distribution diagrams of (a) dispersion of pristine CSCNTs, (b) reduced CSCNTs ([(BNA)2] = 2.1 × 10−8 mol), and (c) reduced CSCNTs ([(BNA)2] = 2.1 × 10−7 mol) in deaerated MeCN at 298 K.](/image/article/2007/CC/b614181a/b614181a-f5.gif) | ||
| Fig. 5 Size distribution diagrams of (a) dispersion of pristine CSCNTs, (b) reduced CSCNTs ([(BNA)2] = 2.1 × 10−8 mol), and (c) reduced CSCNTs ([(BNA)2] = 2.1 × 10−7 mol) in deaerated MeCN at 298 K. | ||
The elemental analysis afforded a chemical formula of C577(C12H13N2O)·26(H2O) and this indicates that one BNA+ counterion is attached per 577 carbon atoms.** Recently, Petit et al. reported methodology for the advanced dissolution of single walled carbon nanotubes by thermal electron-transfer reduction using sodium naphthalenide.14 In this case, the carbon nanotubes are soluble with a stoichiometry of one negative charge per 10 carbon atoms in polar aprotic solvents, which is much higher than that of our result, i.e., one negative charge per 577 carbon atoms.†† Although the cup-shaped carbons are still insoluble in solvents, even in the case of higher concentration of (BNA)2, we have succeeded in developing highly dispersible nanocarbon materials with uniform size in solution by photoinduced electron-transfer reduction with a mild organic electron donor.
In conclusion, we have demonstrated an accommodating and efficient approach to obtain novel nanocarbon materials with uniform size. CSCNTs were successfully destacked by the photoinduced electron-transfer reduction with a mild electron donor, (BNA)2, to produce cup-shaped carbons with uniform size.
This work was partially supported by a Grant-in-Aid (No. 16205020) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. TEM measurements were performed using a facility in the Research Center for Ultrahigh Voltage Electron Microscopy, Osaka University. We thank Prof. H. Mori and Dr T. Sakata for helpful advice and discussion regarding the TEM measurements. K. S. expresses his special thanks to the center of excellence (21COE) program “Creation of Integrated EcoChemistry of Osaka University”.
Notes and references
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- (a) S. M. Bachilo, L. Balzano, J. E. Herrera, F. Pompeo, D. E. Resasco and R. B. Weisman, J. Am. Chem. Soc., 2003, 125, 11186 CrossRef CAS; (b) K. Hata, D. N. Futaba, K. Mizuno, T. Namai, M. Yumura and S. Iijima, Science, 2004, 306, 1362 CrossRef CAS; (c) Y.-Q. Xu, E. Flor, M. J. Kim, B. Hamadani, H. Schmidt, R. E. Smalley and R. H. Hauge, J. Am. Chem. Soc., 2006, 128, 6560 CrossRef CAS.
- (a) D. Chattopadhyay, S. Lastella, S. Kim and F. Papadimitrakopoulos, J. Am. Chem. Soc., 2002, 124, 728 CrossRef CAS; (b) M. Zheng, A. Jagota, M. S. Strano, A. P. Santos, P. Barone, S. G. Chou, B. A. Diner, M. S. Dresselhaus, R. S. Mclean, G. B. Onoa, G. G. Samsonidze, E. D. Semke, M. Usrey and D. J. Walls, Science, 2003, 302, 1545 CrossRef CAS; (c) D. A. Heller, R. M. Mayrhofer, S. Baik, Y. V. Grinkova, M. L. Usrey and M. S. Strano, J. Am. Chem. Soc., 2004, 126, 14567 CrossRef CAS; (d) K. J. Ziegler, D. J. Schmidt, U. Rauwald, K. N. Shah, E. L. Flor, R. H. Hauge and R. E. Smalley, Nano Lett., 2005, 5, 2355 CrossRef CAS.
- M. Endo, Y. A. Kim, T. Hayashi, Y. Fukai, K. Oshida, M. Terrones, T. Yanagisawa, S. Higaki and M. S. Dresselhaus, Appl. Phys. Lett., 2002, 80, 1267 CrossRef CAS.
- (a) C. Kim, Y. J. Kim, Y. A. Kim, T. Yanagisawa, K. C. Park, M. Endo and M. S. Dresselhaus, J. Appl. Phys., 2004, 96, 5903 CrossRef CAS; (b) M. Endo, Y. A. Kim, M. Ezaka, K. Osada, T. Yanagisawa, T. Hayashi, M. Terrones and M. S. Dresselhaus, Nano Lett., 2003, 3, 723 CrossRef CAS.
- T. Hasobe, S. Fukuzumi and P. V. Kamat, Angew. Chem., Int. Ed., 2006, 45, 755 CrossRef CAS.
- Y. A. Kim, T. Hayashi, Y. Fukai, M. Endo, T. Yanagisawa and M. S. Dresselhaus, Chem. Phys. Lett., 2002, 355, 279 CrossRef CAS.
- (a) M. Patz, Y. Kuwahara, T. Suenobu and S. Fukuzumi, Chem. Lett., 1997, 26, 567 CrossRef; (b) S. Fukuzumi, T. Suenobu, M. Patz, T. Hirasaka, S. Itoh, M. Fujitsuka and O. Ito, J. Am. Chem. Soc., 1998, 120, 8060 CrossRef CAS; (c) S. Fukuzumi, T. Suenobu, T. Hirasaka, N. Sakurada, R. Arakawa, M. Fujitsuka and O. Ito, J. Phys. Chem. A, 1999, 103, 5935 CrossRef CAS; (d) S. Fukuzumi, K. Ohkubo, M. Fujitsuka, O. Ito, M. C. Teichmann, E. Maisonhaute and C. Amatore, Inorg. Chem., 2001, 40, 1213 CrossRef CAS.
- I. W. Chiang, B. E. Brinson, A. Y. Huang, P. A. Willis, M. J. Bronikowski, J. L. Margrave, R. E. Smalley and R. H. Hauge, J. Phys. Chem. B, 2001, 105, 8297 CrossRef CAS.
- K. Wallenfels and M. Gellerich, Chem. Ber., 1959, 92, 1406 CrossRef CAS.
- S. Fukuzumi, S. Koumitsu, K. Hironaka and T. Tanaka, J. Am. Chem. Soc., 1987, 109, 305 CrossRef CAS.
- P. Lauginie, H. Estrade, J. Conard, D. Guerard, P. Lagrange and M. El Makrini, Physica B+C (Amsterdam), 1980, 99, 514 CrossRef CAS.
- J. Stinchcombe, A. Pénicaud, P. Bhyrappa, P. D. W. Boyd and C. A. Reed, J. Am. Chem. Soc., 1993, 115, 5212 CrossRef CAS.
- A. Pénicaud, P. Poulin, A. Derré, E. Anglaret and P. Petit, J. Am. Chem. Soc., 2005, 127, 8 CrossRef CAS.
- K. Saito, M. Ohtani and S. Fukuzumi, J. Am. Chem. Soc., 2006, 128, 14216 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: UV-vis spectrum and dynamic light scattering data. See DOI: 10.1039/b614181a |
| ‡ The ESR spectra were recorded on a JEOL X-band spectrometer (JES-RE1XE) with a quartz ESR tube (4.5 mm i.d.). The ESR spectra were measured under nonsaturating microwave power conditions. The magnitude of modulation was chosen to optimize the resolution and the signal-to-noise (S/N) ratio of the observed spectra. The g values were calibrated with an Mn2+ marker. |
| § SEM images were taken using a JEOL JSM-6700F microscope. The specimens for SEM measurements were prepared by drop casting of a dispersion to the sample holder via a piece of adhesive carbon tape (DTM 9101, JEOL Datum). |
| ¶ TEM images were collected on a HITACHI model H-800 transmission electron microscope operating at an accelerating voltage of 200 kV. TEM samples were prepared by depositing a drop of dispersion on carbon-coated copper grids (250 mesh). |
| || DLS measurements were performed using a LB-500 particle size analyzer (Horiba, Japan). The DLS instrument used in this study has a range between 1 and 6000 nm, and thereby any structures over this limit cannot be detected. |
| ** Anal. Calcd for C577(C12H13N2O)·26(H2O): C, 93.06; H, 0.89; N, 0.37. Found: C, 90.86; H, 0.85; N, 0.36%. |
| †† In the case of “thermal” electron-transfer reduction of CSCNTs using sodium naphthalenide, the stoichiometry is one negative charge per 161 carbon atoms to provide individual cup-shaped carbons with a diameter of 50 nm and length of 100 nm (see: ref. 15). |
| This journal is © The Royal Society of Chemistry 2007 |
