Iron–sulfur cluster stability. Kinetics and mechanism of ligand-promoted cluster degradation†
Shu-pao
Wu‡
and
J. A.
Cowan
*
Evans Laboratory of Chemistry, Ohio State University, 100 West, 18th Ave, Columbus, OH 43210, USA. E-mail: cowan@chemistry.ohio-state.edu
First published on 18th October 2006
Abstract
Reactivity studies of iron–sulfur cluster proteins with chelating ligands model the reactivity of cluster scaffold proteins such as ISU, and suggest a rate law [kobs = k2[ligand]n/{[ligand]n + KD}] consistent with formation of a pre-reaction complex between the Fe–S protein and one chelate ligand.
Iron–sulfur cluster proteins are widely distributed in nature, and play important physiological roles in electron transfer, metabolic reactions, and the regulation of gene expression.1–3 Extensive studies of native and mutant ferredoxins (Fds),4–7 and high potential iron proteins (HiPIPs),8–10 have shown that the number and orientation of hydrophobic residues surrounding the cluster determine the extent of solvent accessibility, and consequently influence the stability of protein-bound iron–sulfur centers. Understanding the factors that contribute to iron–sulfur cluster stability has taken on increased importance in the light of recent findings that scaffold proteins such as ISU11–13 and ISA14,15 form templates for the assembly of labile Fe–S clusters that are subsequently transferred to more stable cluster environments in specific target proteins. The impact of non-physiological ligands on the stability of protein-bound clusters is also of significance in the biomedical context. Several types of iron chelators have been developed for use in the treatment of iron overload and their major target proteins are iron delivery proteins, such as transferrin.16,17 However, the influence of iron chelators on iron–sulfur proteins remains unknown. In this paper, the reactions of Fe–S cluster proteins with iron chelating agents tiron (4,5-dihydroxy-m-benzenedisulfonic acid) and bipy (2,2′-bipyridine) are quantitatively investigated. The reactions of iron–sulfur proteins with iron chelators provide a convenient measure of cluster stability and the results obtained suggest a mechanistic framework for understanding cluster degradation or transfer pathways.
Tiron forms a complex with ferric ion that yields a strong and broad absorption from 400 nm to 600 nm, with λmax ≈ 480 nm (ε = 6200 M−1 cm−1).18,19 In contrast, bipy forms a transient complex with ferric ion that is autoreduced by solvent as a result of the high reduction potential (0.98 V) of Fe(bipy)33+, and the product Fe(bipy)32+ shows λmax ≈ 520 nm (8400 M−1 cm−1).20,21 Consequently, the reaction chemistry of iron–sulfur proteins with iron chelating agents can be monitored spectrophotometrically. Up to two distinct paths were evident and the reaction profiles were accommodated by fitting to eqn (1), where Y corresponds to the absorbance at a defined wavelength, Y0 is the absorbance at time zero, and A and B are the absorbance changes that arise from each distinct phase of the reaction. One kinetic phase derives from degradation of the intact protein-bound iron–sulfur cluster. A distinct phase derives from iron that is either a free solution ion, or adventitiously protein-bound iron that results from degradation of the protein-bound cluster. The latter phase was most prominent for those proteins carrying a previously documented labile cluster and absorbance changes are consistent with the degraded cluster as the source of this iron component. The formation of iron chelate complexes from the second class of iron is faster, and is well described by an exponential process. As expected, observed rate constants kobs2 were seen to follow a linear dependence on ligand concentration over the entire concentration range used.
| Y = Y0 + Aexp(−kobs1t) + Bexp(−kobs2t) | (1) |
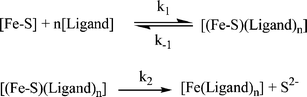 | ||
| Scheme 1 | ||
The mechanism for reaction of stable protein-bound iron–sulfur clusters with iron chelating agents (ligands) is described in Scheme 1. The reaction pathway assumes formation of a pre-reaction complex between the iron–sulfur protein and the chelate ligand, where [Fe–S] corresponds to the concentration of the iron–sulfur protein and the ligand is an iron chelator. A 200-fold excess of iron chelating agent typically was used in experiments to measure the rates of reaction of Fe–S cluster proteins with tiron and bipy. The observed rates are described by eqn (2) and (3) (assuming k−1 > k2), where [Fe(ligand)3] represents the concentration of the iron chelate complex, and KD is equal to k−1/k1. When either [ligand]n ≫ KD, or k1 ≫ k−1, then kobs1 ≈ k2.
| d[Fe(ligand)3]/dt = kobs1[Fe–S] | (2) |
| kobs1 = k2[ligand]n/{[ligand]n + KD} | (3) |
Fig. 1 shows spectra recorded during the reaction of Homo sapiens Fd with bipy. Absorbances at 414 nm and 458 nm are commonly observed for protein-bound Fe–S clusters,6 while the new peak at 520 nm is a MLCT band that results from formation of the Fe(bipy)32+ complex.21 The time-dependent absorbance change was fit to eqn (1), allowing for variations in extinction coefficient. Consistent kinetic results were obtained from each wavelength monitored. The reaction of Fd with bipy displayed biphasic exponential decay kinetics when monitoring the appearance of Fe(bipy)32+ at 520 nm, with the faster phase, defined by kobs2, corresponding to the cluster degradation product. Single phase behavior was observed at 414 nm or 458 nm, reflecting cluster loss (Fig. 1). Earlier reports have suggested that oxygen ligation has a destabilizing influence on a protein-bound cluster,22 while the iron–sulfur cluster scaffold protein ISU is also likely to possess a non-cysteinyl (most likely oxygen) ligand. Accordingly, the reactivities of Cys/Ser substituted Fds were examined and found to be larger than that of native Fd. Similarly, reactions of holo Fds (50 µM) with tiron (10 mM) were followed spectrophotometrically and larger rate constants were obtained for Cys/Ser mutants (Table 1 and ESI†).
 | ||
| Fig. 1 Reaction of Fd with bipy. Native holo Hs Fd (50 µM in 1 mL 100 mM Tris-HCl, pH 7.4) was mixed with 10 mM bipy (in 20 µL ethanol) at 25 °C. The reaction of Fd with bipy was monitored by UV-vis spectroscopy at 414 and 520 nm. | ||
| Fe–S proteinsa | Tiron kobs1b/min−1 | Tiron kobs2b/min−1 | Bipy kobs1b/min−1 | Bipy kobs2b/min−1 |
|---|---|---|---|---|
| a Sp = Schizosaccharomyces pombe; Hs = Homo sapiens. b Observed rate constants determined by fitting to eqn (1). | ||||
| Sp Fd | 0.03 | 0.17 | 5.5 × 10−3 | 0.14 |
| Hs Fd | 0.02 | 0.20 | 4.5 × 10−3 | 0.14 |
| C42S Sp Fd | 0.07 | 0.46 | 0.03 | 0.36 |
| C48S Sp Fd | 0.08 | 0.50 | 0.03 | 0.45 |
| C51S Sp Fd | 0.10 | 0.76 | 0.03 | 0.43 |
| C88S Sp Fd | 0.10 | 0.85 | 0.04 | 0.54 |
| Hs ISU | 0.07 | 0.49 | 0.04 | 0.25 |
| D37A Hs ISU | 0.06 | 0.38 | 0.03 | 0.25 |
Tiron (a catechol-type ligand) and bipy (a pyridine-type ligand) belong to distinct classes of iron chelating ligands. With saturating concentrations of ligand (for kobs1), reactions with tiron are seen to show larger kobs1 values than those with bipy (Table 1). For example, kobs1 values for iron removal from Cys/Ser substituted Sp Fds by tiron are two-fold larger than those measured for reactions with bipy. Tiron is a catechol-type ligand and has two sulfonate groups that carry negative charges in a basic solution. Studies of iron removal from transferrin by iron chelators have shown that anionic ligands, such as enterobactin and its analogs, can promote protein conformational change and facilitate iron removal.17,23 Other chelating ligands that lack negative charges, such as desferrioxamine B, are thermodynamically capable of removing iron from transferrin but are deficient in triggering the prerequisite conformational change. Bipy is a pyridine-type iron chelator that carries no negative charge in a basic solution, and therefore it is likely that conformational changes triggered by bipy are less efficient than those observed for tiron, resulting in smaller rate constants for iron removal by bipy.
Plots of kobs1versus ligand concentration for reactions of C48S Sp Fd (Fig. 2) also show that the reaction with tiron is faster than that with bipy. A saturation state is observed when the concentration of the ligand is high (>10 mM), consistent with eqn (3), where kobs1 ≈ k2 when [ligand]n ≫ KD. Fitting to eqn (3) yielded the values determined for the parameters n, k2 and KD that are summarized in Table 2. The k2 value for tiron is larger than that for bipy, but the KD values for both ligands are similar (Table 2). The rate constant k2 defines the rate of degradation of the iron–sulfur cluster caused by iron chelating agents. Smaller k2 values reflect the difficulty of iron extraction by the ligand. Bipy shows a smaller k2 value, consistent with a lower extraction efficiency. The KD and “n” values for tiron and bipy are similar (∼1), consistent with both ligands following a similar mechanism for iron–sulfur cluster extraction. As a consequence of steric constraints, only one ligand has access to the protein-bound iron–sulfur cluster-binding pocket.
 | ||
| Fig. 2 Plots of kobs1versus the concentration of ligands (tiron and bipy) for the reactions of C48S Sp Fd (50 µM) with tiron and bipy (1–50 mM). | ||
| Ligand | k 2/min−1 | K D/mM | n |
|---|---|---|---|
| Tiron | 0.091 | 1.01 | 1.1 |
| Bipy | 0.035 | 1.03 | 1.2 |
Similarly, reactions of native holo Hs Fd and Sp Fd with bipy are relatively slow and a plot of rate versus [Fd] shows a linear relationship (ESI†). Since kobs1 ≈ k2, and the rate ≈ k2[Fe–S], k2 is determined to be ∼4.6 × 10−3 min−1, which is 8-fold smaller than that determined for the reaction of C48S Sp Fd with bipy (Table 2).
Addition of urea, a denaturing agent that perturbs protein tertiary structure, facilitates the reaction of native Hs Fd with bipy. In the presence of 3 M urea, the measured rate constants (kobs1) for reaction of bipy with native Hs Fd were 0.035 min−1 (bipy, 10 mM) and 0.028 min−1 (bipy, 5 mM) (ESI†). The kobs1 value is dependent on the concentration of bipy and differs from the results obtained for the reactions of native Fd with bipy in the absence of urea. As noted earlier, when [ligand] ≫ KD, kobs1 ≈ k2, and the reaction rate d[Fe(ligand)3]/dt ≈ k2[Fe–S], then the reaction is seen to be linearly dependent on [Fd] (ESI†). However, in the absence of denaturing agents, penetration by bipy into the iron–sulfur cluster pocket is difficult and binding is weak. Consequently, the condition [ligand] ≫ KD is eliminated and kobs1 is observed to be dependent on [bipy].
In the presence of urea, the tertiary structure of Hs Fd is forced to change, and so bipy more readily penetrates the iron–sulfur cluster pocket, yielding a faster reaction. The importance of accessibility is also reflected by reactions of tiron and bipy with both native Hs ISU and a D37A mutant that has been shown to stabilize the bound cluster.12,24 Each of the kobs values determined for native Hs ISU with tiron (Table 1) are seen to be larger than those measured for D37A Hs ISU, consistent with reduced solvent access to the cluster.24
The reactions of iron–sulfur proteins with tiron and bipy indicate that iron chelators can remove iron and promote the degradation of protein-bound iron–sulfur clusters. The mechanism of iron removal from iron–sulfur proteins by iron chelators is similar to that from iron proteins, such as transferrin.17,23 First, iron chelators disturb the structure of the protein to allow access to the iron–sulfur cluster center, with formation of an intermediate complex (Scheme 1). Subsequent iron removal from the iron–sulfur cluster by the iron chelator results in degradation of the iron–sulfur cluster. Since there exist two types of iron ions (cluster-bound and solvent-promoted cluster degradation product8,9,13,22,25), the kinetic profile for production of the iron chelate complex is typically biphasic, while cluster disappearance is monophasic, as expected. The rate constants for iron removal from various iron–sulfur cluster proteins reflect solvent accessibility and non-cysteinyl ligation by the protein.
Solvent accessibility of protein-bound iron–sulfur clusters is also an important factor in physiological cluster transfer reactions. ISU is a scaffold protein that mediates the biosynthetic assembly of Fe–S clusters prior to transfer to target proteins.24 An ISU-bound cluster is solvent accessible and consequently is readily extracted by chelating ligands, with kobs values for cluster degradation that are larger than those observed for Fd. This is consistent with the prior observation that substitution of aspartate 37 with alanine in Hs ISU yields a smaller kobs for degradation,13,25 presumably reflecting the enhanced hydrophobicity of the cluster-binding pocket, with less solvent accessibility to the cluster pocket.§
This work was supported by the National Science Foundation CHE-0111161.
Notes and references
- H. Beinert, R. H. Holm and E. Munck, Science, 1997, 277, 653 CrossRef CAS.
- M. K. Johnson, Curr. Opin. Chem. Biol., 1998, 2, 173 CrossRef CAS.
- R. Lill, K. Diekert, A. Kaut, H. Lange, W. Pelzer, C. Prohl and G. Kispal, Biol. Chem., 1999, 380, 1157 CrossRef CAS.
- D.-H. Jo, Y.-M. Chiou and L. Que, Jr., Inorg. Chem., 2001, 40, 3181 CrossRef CAS.
- F. Hannemann, M. Rottmann, B. Schiffler, J. Zapp and R. Bernhardt, J. Biol. Chem., 2001, 276, 1369 CrossRef CAS.
- B. Xia, H. Cheng, V. Bandarian, G. H. Reed and J. L. Markley, Biochemistry, 1996, 35, 9488 CrossRef CAS.
- Y. Kakuta, T. Horio, Y. Takahashi and K. Fukuyama, Biochemistry, 2001, 40, 11007 CrossRef CAS.
- A. Agarwal, D. Li and J. A. Cowan, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 9440 CrossRef CAS.
- S. Bian, C. F. Hemann, R. Hille and J. A. Cowan, Biochemistry, 1996, 35, 14544 CrossRef CAS.
- M. W. Foster and J. A. Cowan, J. Am. Chem. Soc., 1999, 121, 4093 CrossRef CAS.
- J. N. Agar, C. Krebs, J. Frazzon, B. H. Huynh, D. R. Dean and M. K. Johnson, Biochemistry, 2000, 39, 7856 CrossRef CAS.
- M. W. Foster, S. S. Mansy, J. Hwang, J. E. Penner-Hahn, K. K. Surerus and J. A. Cowan, J. Am. Chem. Soc., 2000, 122, 6805 CrossRef CAS.
- G. Wu, S. S. Mansy, S. Wu, K. K. Surerus, M. W. Foster and J. A. Cowan, Biochemistry, 2002, 41, 5024 CrossRef CAS.
- G. Wu, S. S. Mansy, C. Hemann, R. Hille, K. K. Surerus and J. A. Cowan, JBIC, J. Biol. Inorg. Chem., 2002, 7, 526 CrossRef CAS.
- C. Krebs, J. N. Agar, A. D. Smith, J. Frazzon, D. R. Dean, B. Huynh and M. K. Johnson, Biochemistry, 2001, 40, 14069 CrossRef CAS.
- A. Stintzi and K. N. Raymond, JBIC, J. Biol. Inorg. Chem., 2000, 5, 57 CrossRef CAS.
- I. Turcot, A. Stintzi, J. Xu and K. N. Raymond, JBIC, J. Biol. Inorg. Chem., 2000, 5, 634 CrossRef CAS.
- E. Farkas and H. Csoka, J. Inorg. Biochem., 2002, 89, 219 CrossRef CAS.
- Z. Zhang and R. B. Jordan, Inorg. Chem., 1996, 35, 1571 CrossRef CAS.
- J. N. Agar, L. Zheng, V. L. Cash, D. R. Dean and M. K. Johnson, J. Am. Chem. Soc., 2000, 122, 2136 CrossRef CAS.
- A. M. Josceanu and P. Moore, J. Chem. Soc., Dalton Trans., 1998, 369 RSC.
- S. S. Mansy, Y. Xiong, C. Hemann, R. Hille, M. Sundaralingam and J. A. Cowan, Biochemistry, 2002, 41, 1195 CrossRef CAS.
- S. A. Nguyen, A. Craig and K. N. Raymond, J. Am. Chem. Soc., 1993, 115, 6758 CrossRef.
- S. Wu, G. Wu, K. K. Surerus and J. A. Cowan, Biochemistry, 2002, 41, 8876 CrossRef CAS.
- S. Wu and J. A. Cowan, Biochemistry, 2003, 42, 5784 CrossRef CAS.
- J. M. Moulis and J. Meyer, Biochemistry, 1982, 21, 4762 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Absorbance time profiles and data fits. See DOI: 10.1039/b610665j |
| ‡ Current address: Department of Applied Chemistry, National Chiao Tung University, Taiwan. |
| § General chemicals. Ascorbate, bathophenanthrolinedisulfonate, bipy, cytochrome c, Na2S, tiron, and urea were purchased from Sigma (St. Louis, MO). Tris-HCl was purchased from Acros (New Jersey). Iron–sulfur proteins. Purification of human ISU (Hs ISU) was performed as previously reported.12 An expression vector for human ferredoxin (Hs Fd) was kindly provided by J. L. Markley and the protein was purified as previously described.6,24Iron quantitation. Protein concentrations were quantitatively assessed from the measured extinction coefficient and confirmed using calculations based on the BioRad protein assay. Iron content was measured by the method of Moulis and Meyer.26 The iron ion was quantified by measuring the absorbance at 535 nm and the result was compared to a calibration curve obtained from solutions of known FeCl3 concentration within the range used. Reactions of Fe–S cluster proteins with tiron and bipy. Fe–S proteins (50 µM in 1 mL 100 mM Tris-HCl, pH 7.4) were mixed with tiron or bipy to the desired final concentrations (typically ≥10 mM) of ligands The reactions were monitored at the wavelengths of the relevant chromophores (480 nm, tiron; 520 nm, bipy), as well as the cluster absorbances, using 5 mm path-length cells at 25 °C, and the absorbance change was fit to eqn (1). Extinction coefficients of Fe(tiron)39− and Fe(bipy)32+ were 6200 M−1 cm−1 (at 480 nm)18 and 8400 M−1 cm−1 (at 520 nm),20 respectively. Extinction coefficients for Fd- and ISU-bound clusters are reported elsewhere.6,12,24,25 |
| This journal is © The Royal Society of Chemistry 2007 |
