Conformational and thermal behavior of a pH-sensitive bolaform hydrogelator
Karen
Köhler†
a,
Annette
Meister
a,
Günter
Förster
a,
Bodo
Dobner
b,
Simon
Drescher
b,
Friederike
Ziethe
b,
Walter
Richter
c,
Frank
Steiniger
c,
Markus
Drechsler‡
d,
Gerd
Hause
e and
Alfred
Blume
*a
aMLU Halle-Wittenberg, Institut für Physikalische Chemie, Mühlpforte 1, 06108 Halle, Germany
bMLU Halle-Wittenberg, Institut für Pharmazeutische Chemie, Wolfgang-Langenbeck-Str. 4, 06120 Halle, Germany
cKlinikum der FSU Jena, Elektronenmikroskopisches Zentrum, Ziegelmühlenweg 1, 07740 Jena, Germany
dFSU Jena, Institut für Pharmazie, Philosophenweg 14, 07743 Jena, Germany
eMLU Halle-Wittenberg, Biozentrum, Weinbergweg 22, 06120 Halle, Germany
First published on 29th November 2005
Abstract
The phase behavior of the symmetric long-chain bolaamphiphile dotriacontan-1,1′-diyl-bis[2-(dimethylammonio)ethyl phosphate] (Me2PE-C32-Me2PE) was investigated in aqueous suspensions of different pH by transmission electron microscopy (TEM), differential scanning calorimetry (DSC), and Fourier transform infrared spectroscopy (FT-IR). At pH 5 the compound exhibits excellent properties as a hydrogelator by forming a dense network of helically structured nanofibrils with a diameter of 3–4 nm. The phase behavior of Me2PE-C32-Me2PE is compared with the previously examined trimethylammonio analogue PC-C32-PC, whose self-assembly process seems to be exclusively driven by hydrophobic interactions. At pH 5, Me2PE-C32-Me2PE can form intermolecular hydrogen bonds between the headgroups, which causes a significantly higher stability of the nanofibrils up to at least 75 °C. In contrast, no pronounced gelling properties are observed at pH 10. Obviously, the negatively charged headgroups seem to prevent the suspension from gelling. Nevertheless, nanofibrils are formed, but at 75 °C a fragmentation into smaller aggregates occurs due to the lack of stabilizing effect of intermolecular hydrogen bonds and the destabilizing effect of the negatively charged headgroups.
Introduction
Gels, which are ubiquitous in our everyday life, attract considerable attention in very different fields of research due to their wide range of possible applications in photography, drug delivery, cosmetics, sensors, food processing, and the textile industry, to name just a few.1 These semirigid colloidal systems present an intermediate state of matter, containing both solid and liquid components. The more solid part comprises a three-dimensional network of interconnected molecules or aggregates, which immobilize the liquid continuous phase. According to the nature of the bonds involved in the network, gels can be classified as chemical or physical gels depending on whether the network is held together by strong covalent bonds or by intermolecular forces (hydrogen-bonding, aromatic π-stacking, electrostatic, van der Waals interactions). A wide variety of polymeric gelling agents have been described,2–5 but low-molecular weight gelators have the advantage that one can easily control the gel characteristics by changing conditions such as pH, temperature and the composition of the mixture.6–8 Besides low-molecular weight organogelators9–13, small molecules that self-assemble in water to form stable supramolecular hydrogels have attracted considerable interest.14–18 Certain gelators are even able to gel organic as well as aqueous media.19,20One class of substances exhibiting a manifold aggregation behavior21–23 in a wide variety of solvents is the class of bolaform amphiphiles.24,25 Among them are also some hydrogelators, which form an extended network in aqueous solutions.26–32 In most cases this self-assembly process is driven by the formation of strong hydrogen bonds.
We have recently reported the temperature dependent aggregation behavior of the symmetric long-chain bolaamphiphile dotriacontan-1,1′-diyl-bis[2-(trimethylammonio)ethyl phosphate] (PC-C32-PC).33,34 This compound gels water very efficiently at concentrations as low as 1 mg ml−1 by forming a dense network of nanofibers. A specific feature of this self-assembly process is that it seems to be solely driven by hydrophobic interactions of the long alkyl chains. Above a temperature of 49 °C, the breakdown of the fibers into smaller aggregates is observed, accompanied by the loss of the gel character.
Since hydrogels are of particular interest for pharmaceutical applications, for example as carrier for therapeutic agents, we are interested in utilizing the aggregation properties of PC-C32-PC but at the same time having a reactive site within the molecule for the possibility of a chemical modification.
In the present work we describe the temperature dependent aggregation behavior of dotriacontan-1,1′-diyl-bis[2-(dimethylammonio)ethyl phosphate] (Me2PE-C32-Me2PE) in aqueous suspension. This molecule has a similar chemical structure as PC-C32-PC but contains a dimethylammonio group instead of a trimethylammonio group (Fig. 1). This substitution hardly affects the size of the whole molecule, only the headgroup is slightly smaller. With the additional proton a second pH-sensitive group is present, which enables Me2PE-C32-Me2PE to form intermolecular hydrogen bonds between neighbouring headgroups when it is in its zwitterionic state at lower pH. We will show that this slight modification has a significant effect on its aggregation and thermotropic behavior, when it is in its zwitterionic state at low pH. In addition, we will show that the dissociation of the proton at high pH has a strong influence on the gelling and thermotropic properties of the system.
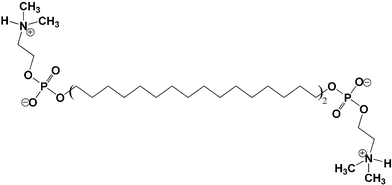 | ||
| Fig. 1 Chemical structure of the bolaamphiphile Me2PE-C32-Me2PE consisting of two bulky headgroups connected by a C32 alkyl chain. | ||
Materials and methods
Chemicals
Me2PE-C32-Me2PE was synthesized as described previously.35 Sodium acetate, acetic acid (100%), sodium carbonate and sodium hydrogencarbonate (p.a.) were purchased from Merck (Darmstadt, Germany), Riedel-de Haën (Seelze, Germany), Acros (New Jersey, USA) and Solvay Alkali GmbH (Rheinsberg, Germany), respectively.Sample preparation
The appropriate amount of Me2PE-C32-Me2PE was suspended in 10 mmol l−1 and for the IR measurements in 300 mmol l−1 acetate buffer at pH 5 to ensure that the bolaamphiphile was in its zwitterionic form. The deprotonated form of Me2PE-C32-Me2PE was obtained by applying a carbonate buffer at pH 10. A homogeneous dispersion of the bolaamphiphile in the aqueous phase was achieved by heating the mixture to 80 °C and vortexing.Determination of the pKa values
Between 1.3 and 2.7 mg of the bolaamphiphile were dissolved in 20 ml of a 0.15 mol l−1 KCl solution and adjusted to a pH of 2 with 0.5 mol l−1 HCl. Subsequently, the Me2PE-C32-Me2PE solutions were titrated with 0.5 mol l−1 NaOH using an automatic titrator PCA101 (Sirius Analytical Instruments Ltd., East Sussex, UK) till a pH of 12 was reached. For the determination of the pKa values four measurements were averaged.DSC
DSC measurements were performed using a MicroCal VP-DSC differential scanning calorimeter (MicroCal Inc., Northampton, MA, USA). Before the measurements, the sample solution and the buffer reference were degassed under vacuum while stirring. A heating rate of 1° min−1 was used and the measurements were performed in the temperature interval from 2 to 100 °C. To check the reproducibility, three consecutive scans of each sample were recorded. The reference thermogram (water–water baseline) was subtracted from the thermograms of the samples and the DSC scans were evaluated using the MicroCal ORIGIN 5.0 software.FT-IR
Infrared spectra were measured using a Bruker Vector 22 Fourier transform spectrometer with a DTGS-detector operating at 2 cm−1 resolution. The sample with a concentration of 50 mg ml−1 was placed between two BaF2 windows, which were separated by a 25 µm spacer for measurements in H2O and a 56 µm spacer for measurements in D2O, respectively. IR-spectra were measured every 2° in the temperature range from 5 to 95 °C. After an equilibration time of 8 min, 32 scans were recorded and accumulated. The corresponding spectra of the solvent were subtracted from the obtained sample spectra using the OPUS software supplied by Bruker.TEM
For cryo-TEM a volume of 4 µl of the dispersion was placed onto a grid covered by a perforated carbon support foil (Quantifoil Micro Tools Jena). The grid was blotted by a self-made auto-controlled blotting system equipped with a climatic chamber and then plunged rapidly into liquid ethane. The frozen sample was transferred with the Gatan-626 single tilt cryotransfer system into the cryo-electron microscope Philips-CM120. Samples were prepared by quenching solutions starting at room temperature and at 75 °C.The negatively stained samples were prepared by spreading 5 µl of the dispersion onto a Cu grid coated with a formvar-film. After 1 min of adsorption, excess liquid was blotted off with filter paper and 5 µl of 1% aqueous uranyl acetate were placed on the grid and drained off after 1 min. The dried specimens were examined with a Zeiss EM 900 transmission electron microscope.
Results
Temperature dependent aggregation at different pH values
Me2PE-C32-Me2PE (Fig. 1) has a dissociable proton at the dimethylammonium group. Therefore, at intermediate pH the molecule should be in its zwitterionic state, whereas at higher pH the dissociation of the proton should lead to a lipid with two negatively charged headgroups, and at very low pH the molecule should be doubly positively charged due to the protonation of the phosphate group. We determined the pKa values of the bolaamphiphile headgroup by titrating a suspension of the lipid in water. The measurements yielded an apparent pKa value for the phosphate group of 3.3, and one of 6.5 for the dimethylammonium group. To ensure that both functional groups were charged, the investigations were carried out in acetate buffered solutions at pH 5. | ||
| Fig. 2 Photo of an aqueous suspension of 1 mg ml−1 Me2PE-C32-Me2PE (pH 5) in a vial turned upside down. An almost clear hydrogel is formed. | ||
Cryo-electron microscopy images of an aqueous suspension containing 0.3 mg ml−1 Me2PE-C32-Me2PE show a dense network of fibrils at room temperature, which have a thickness of approximately 3–4 nm and a length of up to several micrometres (Fig. 3A). The fibers adopt mainly an extended conformation indicating a slight flexibility of these structures. However, some fibrils enclose lenticular areas, exhibiting an extreme curvature at their ends. Without further image recognition it is difficult to say something about the fine-structure of the fibers, but they seem to have a helical structuring. The arrow in Fig. 3B points to a region where a helical arrangement is apparent. Besides the fibrillar aggregates, darker, almost rectangular, areas can also be found in the electron micrographs. The origin of these structures and the underlying aggregation principle is completely unclear. The structures might be caused by a sheet-like aggregate, but how the molecules are arranged in these structures is a puzzle. Negative staining electron microscopy shows the same type of structures, but due to the perturbation of the system by the staining procedure the fibers are not as straight and long as in the cryo-electron micrographs (see Fig. 3C). At 75 °C the fibers are still intact but they are strongly bent and seem to be more flexible (Fig. 3D).
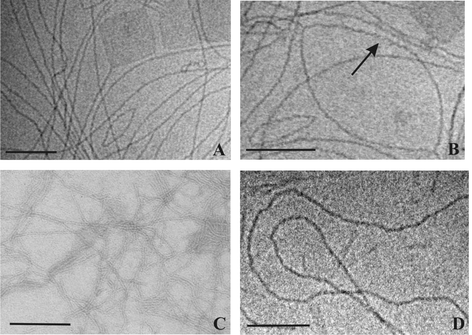 | ||
| Fig. 3 Cryo-electron micrographs of an aqueous suspension of 0.3 mg ml−1 Me2PE-C32-Me2PE (pH 5) quenched from room temperature (with two different magnifications, A and B) and from 75 °C (D). The arrow points to an apparent helical structuring of the fibers. (C) shows a TEM image of an aqueous uranyl acetate stained suspension of 0.3 mg ml−1 Me2PE-C32-Me2PE at room temperature. The bar corresponds to 100 nm. | ||
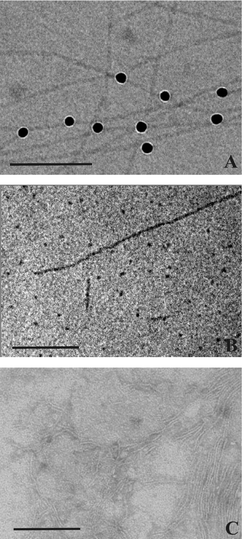 | ||
| Fig. 4 Cryo-electron micrographs of an aqueous suspension of 0.3 mg ml−1 Me2PE-C32-Me2PE (pH 10) quenched from room temperature (A) and from 75 °C (B). The black dots in (A) are 10 nm Au nanoparticles added for better focussing. The Au nanoparticles are attached to the fibers and show that their diameter is approximately 4 nm. In (B) short fiber segments and spherical aggregates are discernible. (C) shows a TEM image of an aqueous uranyl acetate stained suspension of 0.3 mg ml−1 Me2PE-C32-Me2PE at room temperature. The bar corresponds to 100 nm. | ||
DSC measurements
DSC measurements of different concentrated suspensions of Me2PE-C32-Me2PE were performed to characterize the temperature dependent behavior of this system and to quantify possible phase transitions. The DSC thermograms are shown in Fig. 5. From the heating curves it can be seen that for suspensions at pH 5 three endothermic phase transitions occur between 2 and 100 °C (see Fig. 5A). The first peak, which has the largest latent heat, appears at 45.5 °C and is macroscopically connected with a change in the visco-elastic behavior of the gel. A second transition is observed at 69.5 °C. Finally there is a third transition at approximately 85 °C, whose exact position depends on the concentration of the suspension. With increasing concentration the peak shifts slightly to lower temperatures. The exact transition temperatures and their corresponding enthalpy values are summarized in Table 1.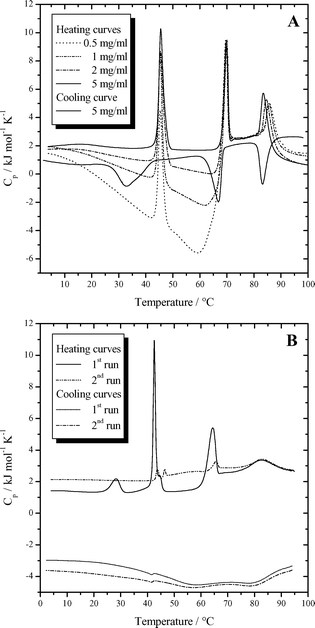 | ||
| Fig. 5 (A) DSC curves for different concentrations of Me2PE-C32-Me2PE in buffer solution at pH 5. (B) DSC curves (two scans) of 5 mg ml−1 Me2PE-C32-Me2PE in buffer solution at pH 10. | ||
| Phase transition | T m (pH 5)/°C | ΔH (pH 5)/kJ mol−1 | T m (pH 10)/°C | ΔH (pH 10)/kJ mol−1 |
|---|---|---|---|---|
| a Transition temperature increases with decreasing concentration. | ||||
| Pre-transition | — | — | 28.3 | 4.4 |
| 1st transition | 45.5 | 19.8 | 42.5 | 17.4 |
| 2nd transition | 69.5 | 12.8 | 64.3 | 12.5 |
| 3rd transitiona | 83.4 | 10.1 | 82.6 | 8.2 |
| Sum | — | 42.7 | — | 42.5 |
One characteristic feature of the DSC heating curves, which was already observed for the trimethylammonio analogue of Me2PE-C32-Me2PE,34 is the temperature and concentration dependent course of the apparent molar heat capacity. These heat capacities were calculated from the shift of the DSC curves of the sample relative to the water–water baseline, assuming a partial specific volume of Me2PE-C32-Me2PE of 1 mg ml−1. At 5 °C all curves of the different concentrations start at approximately the same apparent molar heat capacity of 1.8 kJ mol−1 K−1. Until the beginning of the second transition the heat capacity decreases and the slope diminishes with increasing concentration. For a concentrated suspension with 5 mg ml−1 bolalipid the heat capacity is nearly constant. The diluted samples even show negative apparent molar heat capacities. At the second transition a striking jump to values of around 2.5 kJ mol−1 K−1 can be observed for all concentrations. This jump is less pronounced for more highly concentrated samples. The transition at 85 °C is connected with a small jump of the heat capacity as well, which in this case leads to lower values of Cp. This jump is most pronounced for more highly concentrated samples.
A sample that was stored for eight hours at 2 °C before recording the DSC heating curve, shows no difference in thermal behavior to the up-scans presented here, which follow a tempering time of 10 min at 2 °C. This indicates that the thermodynamically stable state is reached within a short time.
Besides the heating curves, a cooling curve for a suspension of 5 mg ml−1 Me2PE-C32-Me2PE is also shown in Fig. 5A. Independent of the concentration of the bolaamphiphile, the cooling curves do not show such a complicated Cp-curve, but lie on top of each other. All three transitions are observed again during the temperature decrease. The peak at the highest temperature appears at the same temperature as in the heating curve, whereas the two others show a hysteresis and are observed at lower temperatures. The second peak is down-shifted by 2.6 °C to 66.9 °C and the first transition, which is observed at 32.2 °C is accompanied by the re-formation of the gel. The peak is broadened and shows the largest undercooling. All three transitions have approximately the same enthalpy compared with their values in the heating curves.
The DSC curves of samples at pH 10 are presented in Fig. 5B. The first heating curve shows three endothermic transitions and an additional pre-transition at 28.3 °C. The peak with the largest latent heat appears at 42.5 °C. Further transitions are observed at 64.3 °C and 83 °C. During the cooling scan three transitions can be observed, which have lower transition enthalpies compared with their values in the heating curves. All peaks show a hysteresis and are shifted to lower temperatures. The third peak is down-shifted by 3 °C, the second peak by 8 °C, and the first peak by 1 °C. The peaks are extremely broadened. The pre-transition peak could not be detected. The second heating curve started immediately after cooling and shows a completely different thermal behavior. Only very small peaks are observed at 42 °C and 64 °C, whereas the peak at 83 °C is more or less unchanged. This suggests that the first two peaks are connected to the re-formation of the nanofibrils and that this process was not complete during the time-span of the cooling process.
FT-IR measurements
Temperature dependent FT-IR measurements of a suspension with 50 mg ml−1 Me2PE-C32-Me2PE were performed in order to gain insight into the structure and the phase behavior of the bolaamphiphile, e.g. conformational changes in the hydrophobic chains. The CH2 stretching and deformation vibrational bands and the symmetric PO2− stretching band at low wavenumbers are the most characteristic features of the bolalipid spectra at different temperatures. Fig. 6 shows the temperature dependent course of these bands for a suspension at pH 5. The wavenumber of the symmetric and antisymmetric methylene stretching vibrations (ν(CH2)) provides a sensitive measure for the conformational order of the alkyl chains. Both methylene stretching vibrations exhibit a three-step behavior (see Fig. 6A,B). The transitions take place at 44.8 °C, 68.7 °C and 80.6 °C. These temperatures agree well with those determined by DSC except for the third transition. The lower temperature for the high temperature transition is due to the higher concentration of the bolalipid suspension in the IR-experiments, since the concentration dependent DSC measurements showed a shift of this transition to lower temperatures with increasing concentration. At these three phase transition temperatures the wavenumber and the half-width of the CH2 stretching bands increases sharply, indicating a drastic decrease of the trans–gauche ratio of the C–C bonds. At low temperatures the wavenumbers for the antisymmetric and symmetric CH2 stretching vibration are 2918.7 and 2849.7 cm−1, respectively, whereas at 96 °C they have values of 2923.8 and 2853.3 cm−1. It is quite obvious that the changes in frequency for the two vibrational modes do not run parallel to each other. Compared to the band of the antisymmetric methylene stretching vibration, the symmetric vibration shows a comparatively larger increase of the wavenumber at the first transition. It is the largest frequency jump for the symmetric band and the smallest one for the antisymmetric band. In the cooling curves the second and the third phase transition occur at the same temperature as during heating and the two curves lie on top of each other, but for the first transition a hysteresis is observed showing the jump of the wavenumbers at just 38.9 °C. The methylene scissoring vibration δ(CH2), indicative of the chain packing geometry, shows a pronounced temperature dependence as well (Fig. 6C). At low temperature the wavenumber of the narrow and intense band decreases continuously from 1472.5 to 1471.0 cm−1 at 44.8 °C and drops steeply to 1468.0 cm−1 at the first transition. This jump is accompanied by an intensity loss and a broadening of the band. At the second and third phase transition an abrupt but smaller decrease of the wavenumber can be observed as well. At 95 °C the methylene stretching vibration is observed at 1466.5 cm−1. As observed for the frequencies of the methylene stretching vibrations a delayed reconversion of the low temperature phase is seen in the cooling curve.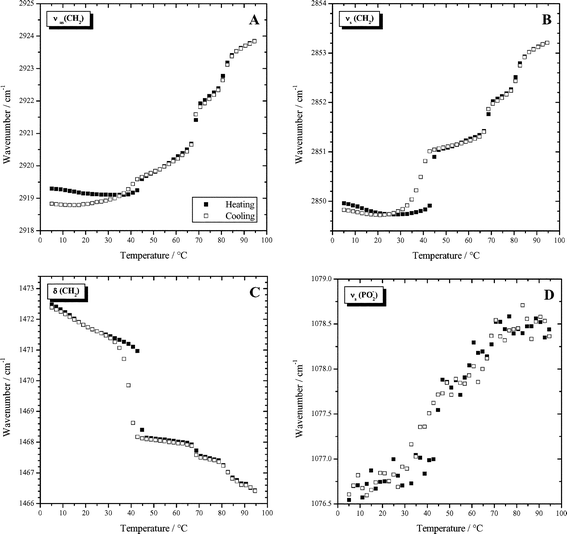 | ||
| Fig. 6 Wavenumbers of characteristic IR-bands as a function of temperature determined for a dispersion of 50 mg ml−1 Me2PE-C32-Me2PE in D2O at pH 5: (A) antisymmetric methylene stretching vibration; (B) symmetric methylene stretching vibration; (C) methylene scissoring vibration;. (D) symmetric PO2− stretching vibration. | ||
Whereas the CH2-bands indicate the change in conformations of the hydrophobic chains, the phosphate PO2− stretching modes probe the degree of hydration of the headgroup region. The broad band of the antisymmetric phosphate vibration shifts continuously from 1216 to 1218 cm−1 (not shown) within the measured temperature range. The wavenumber of the symmetric PO2− stretching vibration increases with increasing temperature, too, but at approximately 45 °C a sudden jump to higher frequencies can be observed (Fig. 6D). The wavenumbers of the symmetric PO2− stretching vibration in the cooling curve lie on top of the values of the heating trace, however a slight shift to lower temperatures can be observed for the first transition. The increase in wavenumber with temperature observed for both phosphate stretching vibrations indicate a decreasing number of hydrogen bonds with increasing temperature.
Fig. 7 shows the temperature dependence of the CH2 stretching vibrational bands and the symmetric PO2− stretching band for a suspension at pH 10 where the bolaamphiphile headgroup is now negatively charged and no intermolecular hydrogen bonds can be formed. Both methylene stretching vibrations exhibit a two-step behavior (see Fig. 7A,B). At the temperature of the pre-transition, determined by DSC, no significant wavenumber shift is observed. At the first phase transition temperature, the wavenumber and the half-width of the CH2 stretching bands increase sharply. The second and third transitions can not be separated. Instead a larger frequency shift as compared to the one observed for the first transition occurs. The symmetric phosphate stretching vibration also shifts to a higher wavenumber. This is surprising, because it indicates a decreasing number of hydrogen bonds with increasing temperature. At pH 10, hydrogen bonds cannot be formed with the deprotonated dimethylamino group, but only with water molecules. Usually, in phospholipid systems, an increase in hydration is observed with increasing temperature. In this case, the observation can only be interpreted in the sense that the phosphate groups are more shielded from water at high temperature.
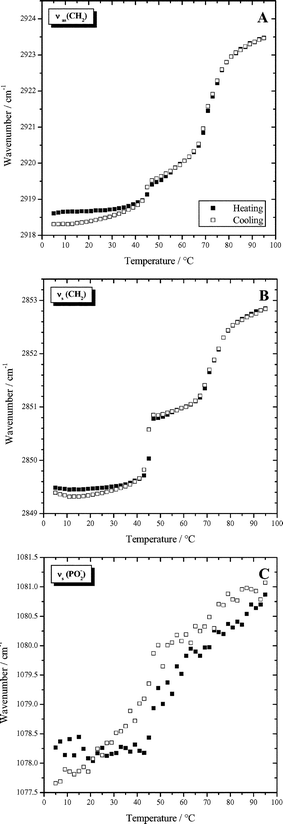 | ||
| Fig. 7 Wavenumbers of characteristic IR-bands as a function of temperature determined for a dispersion of 50 mg ml−1 Me2PE-C32-Me2PE in D2O at pH 10: (A) antisymmetric methylene stretching vibration; (B) symmetric methylene stretching vibration; (C) symmetric PO2− stretching vibration. | ||
Discussion
Aggregation behavior at room temperature
The substitution of the methyl group in PC-C32-PC by a hydrogen atom in Me2PE-C32-Me2PE does not affect the length of the fully extended molecule, both bolaamphiphiles have the same length of 5–6 nm. This corresponds approximately to the measured diameter of the fibrils, which amounts to 3–4 nm in the case of Me2PE-C32-Me2PE. The PC-C32-PC fibers have a slightly larger diameter of 5–6 nm. This difference might be attributed to a tilt of the molecules with respect to the helix axis in the case of Me2PE-C32-Me2PE. Furthermore, the headgroup of Me2PE-C32-Me2PE can, in contrast to that of PC-C32-PC, act as hydrogen bond donor at low pH and thus form intermolecular hydrogen bonds with neighboring phosphate groups. This can definitely have a pronounced influence on the molecular arrangement since hydrogen bonds are directed intermolecular interactions. The formation of sheet structures seen in the electron micrographs besides the fibers might be understood by these stronger intermolecular interactions as well. However, the fibrils seem to be energetically favored compared to the sheet structures so that these sheets are very rarely seen and are totally absent in electron micrographs with negative staining. At present, we have no suggestions for the structural arrangement of the molecules within the sheets.
There are some reports on bolaamphiphiles containing a chiral center that self-assemble into helical structures.31,38–40 Because Me2PE-C32-Me2PE like PC-C32-PC does not have a chiral center, equal amounts of right- and left-handed helices must be formed. The first aggregating molecules should determine the overall handedness of the growing helix. We explained this phenomenon before by spontaneous symmetry breaking caused by packing restrictions.34
Temperature dependent behavior
A sharp transition with a transition enthalpy of 20 kJ mol−1 is observed at 45 °C. The transition enthalpy value is too low to be caused by a complete fluidization of the chains, as the FT-IR data show. The DSC curves show that after the first transition, a curve with negative slope still persists until the second transition at 70 °C occurs. For this transition an enthalpy change of 13 kJ mol−1 was observed. The final high temperature transition at 83 °C has a transition enthalpy of 10 kJ mol−1. The sum of the enthalpy values of 43 kJ mol−1 shows that at high temperature the chain is in a state comparable to the chains of phospholipids in the liquid-crystalline lamellar phase, because it corresponds approximately to the total enthalpy changes observed for phospholipids between lamellar gel and liquid-crystalline phases.41
The differences between the thermal behavior of the previously studied PC-C32-PC, where no intermolecular interactions between headgroups are possible, and the compound Me2PE-C32-Me2PE at pH 5 is obvious. Both bolaamphiphiles show three phase transitions, but they look quite different. The first transition, in the case of PC-C32-PC connected with the breakdown of the gel behavior, is very small, but it is much larger for Me2PE-C32-Me2PE at pH 5. In the latter case a definite partial fluidization of the chain occurs as seen in the FT-IR-spectra. For PC-C32-PC the major change in fluidity of the chain occurs around 50 °C, whereas in Me2PE-C32-Me2PE at pH 5 a further increase in chain fluidity occurs at much higher temperature, namely 70 °C. The high temperature transitions have in both cases relatively low transition enthalpies. For Me2PE-C32-Me2PE at pH 5 this transition is more cooperative. The electron micrographs indicate that for PC-C32-PC only small aggregates remain at this temperature. For Me2PE-C32-Me2PE at pH 5 fiber structures can still be seen when the sample is quenched from 75 °C. We presume that above 83 °C for Me2PE-C32-Me2PE at pH 5 only small aggregates with fluid chains remain as well. However, due to the high Tm of this transition, we were not able to quench the sample in a controlled way from this high temperature.
There are also distinct differences in the cooling behavior of PC-C32-PC and Me2PE-C32-Me2PE at pH 5. The reformation of the fibrils is much faster for Me2PE-C32-Me2PE. For PC-C32-PC the complete reformation of the fibrils needs more than eight hours at low temperatures. For this compound the cooling curve in the DSC measurements showed no hysteresis for the upper transition but a large hysteresis for the middle transition and a low transition enthalpy. For Me2PE-C32-Me2PE at pH 5 the down-scans in the DSC curves show that the two upper peaks have no or only a moderate hysteresis, and the lowest transition is only slightly shifted to lower temperature. This indicates that during the cooling process the reformation of the fiber network is already occurring.
The FT-IR-measurements support the conclusions stated above. At room temperature, the wavenumber for the methylene stretching vibrations indicates an almost completely extended hydrocarbon chain in the all-trans conformation, and the low wavenumber of the two PO2− bands suggests strong hydrogen bonds to the phosphate groups coming from intermolecular headgroup hydrogen bonding.
For the gel at pH 5, the first transition at 45.5 °C exhibits the largest latent heat. It is macroscopically connected with a decrease of the complex viscosity by a factor of ten. This can be interpreted as a change from a strongly cross-linked to a more weakly cross-linked gel.42 At this temperature, the trans–gauche ratio of the C–C bonds decreases only slightly, as judged from the frequency of the antisymmetric CH2-stretching vibration. Surprisingly, the wavenumber for the antisymmetric CH2 stretching vibration increases only by ca. 0.5 cm−1, whereas the frequency for the symmetric stretching vibration increases by more than one wavenumber. Usually, the frequency of the antisymmetric CH2-vibration is more sensitive to the formation of gauche conformers than the symmetric one. Changes in chain packing and the conformational order are also evident from the CH2-bending vibrational band.
At the second and third phase transition further rearrangements within the chain region take place and the trans–gauche ratio of the C–C bonds decreases. At the second phase transition at 70 °C, the increase in frequency of the antisymmetric CH2-vibration is now larger than for the symmetric one. Above 85 °C the wavenumbers of the methylene stretching vibrations reach values as found in the lamellar liquid-crystalline phases of phospholipids such as DPPC,43 indicating a high degree of disorder. The wavelength range in which the frequencies of the methylene stretching vibrations change, is exactly the same as that observed for PC-C32-PC.34
Because of the possibility of building up intermolecular hydrogen bonds, the temperature dependent behavior of the phosphate stretching vibrations of Me2PE-C32-Me2PE is different from that of PC-C32-PC. Whereas the antisymmetric PO2− stretching vibration lies in the same range for both bolalipids and shows an increasing wavenumber with increasing temperature, the symmetric band behaves completely contradictorily. For PC-C32-PC the wavenumber decreases with increasing temperature, which can be understood supposing that the head groups are surrounded with an increasing number of water molecules. The formation of smaller aggregates at 60 °C should promote this higher accessibility of the head groups for hydrogen bonding with water. In the case of Me2PE-C32-Me2PE the wavenumbers are much lower, which may be due to the formation of strong intermolecular hydrogen bonds between the head groups. With increasing temperature the wavenumber shifts to higher values. One possible interpretation for this observation might be that the hydrogen bonds between the bolaamphiphile molecules are replaced by weaker hydrogen bonds to the solvent molecules.
Conclusions
The symmetric bolaamphiphile Me2PE-C32-Me2PE proved to be an effective hydrogelator in its zwitterionic state even at concentrations below 1 mg ml−1. Cryo-electron microscopy showed that gel formation is caused by the formation of long intertwined helical fibrils with a width of 3–4 nm and a length of several micrometres. This aggregation behavior can be explained by the mismatch between the large cross-sectional areas of the headgroup compared to the smaller one of the long hydrocarbon chain, which is very unfavorable for an arrangement in a lamellar phase. A possible model with increased contact between the hydrophobic alkyl chains might be that the molecules arrange next to each other but slightly twisted, resulting in helical fibers with a diameter equal to the molecular length. IR measurements at room temperature confirm an extended conformation of the bolaform molecules. For Me2PE-C32-Me2PE aqueous solutions a first phase transition is observed at 45.5 °C at which the visco-elastic gel behavior changes. Surprisingly the fibers do not break into smaller aggregates but they show a slightly increased disorder of the alkyl chains. In the DSC curves additional phase transitions at 69.5 °C and around 85 °C are seen, where the latter is shifted to higher temperatures with decreasing concentration of the suspension. At both high temperature transitions IR measurements reveal an increasing amount of gauche conformers in the chain. Due to the possibility of hydrogen bonding between the Me2PE-C32-Me2PE molecules in comparison to the formerly investigated PC-C32-PC the phase transition behavior is changed. Investigations of Me2PE-C32-Me2PE at pH 10 show the sensitivity of the system to the pH. An increase in pH results in the absence of the gel character despite the formation of fibrils, due to the dissociation of the proton from the dimethylammonium headgroup. This leads to a negatively charged headgroup with repulsive electrostatic interactions. At the same time the ability to form intermolecular hydrogen bonds is lost. This bolaamphiphile system can therefore be used as a temperature and pH-sensitive hydrogelator.Further experiments with bolaamphiphiles modified in the alkyl chain length are under way and will show the influence of this variable on the self-assembly process and the thermal phase behavior.
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (A.B., A.M., G.F., and B.D.).References
- J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263–2266 CrossRef.
- G. Chen and A. S. Hoffman, Nature, 1995, 373, 49–52 CrossRef CAS.
- R. Yoshida, K. Uchida, Y. Kaneko, K. Sakai, A. Kikuchi, Y. Sakurai and T. Okano, Nature, 1995, 374, 240–242 CAS.
- C. Wang, R. J. Stewart and J. Kopeček, Nature, 1999, 397, 417–420 CrossRef CAS.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef.
- K. Hanabusa, K. Shimura, K. Hirose, M. Kimura and H. Shirai, Chem. Lett., 1996, 885–886 CAS.
- R. J. H. Hafkamp, B. P. A. Kokke, I. M. Danke, H. P. M. Geurts, A. E. Rowan, M. C. Feiters and R. J. M. Nolte, Chem. Commun., 1997, 545–546 RSC.
- K. L. Niece, J. D. Hartgerink, J. J. J. M. Donners and S. I. Stupp, J. Am. Chem. Soc., 2003, 125, 7146–7147 CrossRef CAS.
- S. Murdan, G. Gregoriadis and A. T. Florence, J. Pharm. Sci., 1999, 88, 608–614 CrossRef CAS.
- P. Terech, D. Pasquier, V. Bordas and C. Rossat, Langmuir, 2000, 16, 4485–4495 CrossRef CAS.
- U. Beginn and B. Tartsch, Chem. Commun., 2001, 19, 1924–1925 RSC.
- F. Placin, J.-P. Desvergne, C. Belin, T. Buffeteau, B. Desbat, L. Ducasse and J.-C. Lassègues, Langmuir, 2003, 19, 4563–4572 CrossRef CAS.
- N. Mohmeyer and H.-W. Schmidt, Chem.–Eur. J., 2005, 11, 863–872 CrossRef CAS.
- S. Bhattacharya and S. N. G. Acharya, Chem. Mater., 1999, 11, 3121–3132 CrossRef CAS.
- F. M. Menger and K. L. Caran, J. Am. Chem. Soc., 2000, 122, 11679–11691 CrossRef.
- C. Marmillon, F. Gauffre, T. Gulik-Krzywicki, C. Loup, A.-M. Caminade, J.-P. Majoral, J.-P. Vors and E. Rump, Angew. Chem., Int. Ed., 2001, 40, 2626–2629 CrossRef CAS.
- S. Kiyonaka, K. Sugiyasu, S. Shinkai and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 10954–10955 CrossRef CAS.
- S. Kiyonaka, K. Sada, I. Yoshimura, S. Shinkai, N. Kato and I. Hamachi, Nat. Mater., 2004, 3, 58–64 CrossRef CAS.
- R. Oda, I. Huc and S. J. Candau, Angew. Chem., Int. Ed., 1998, 37, 2689–2691 CrossRef CAS.
- S. Kiyonaka, S. Shinkai and I. Hamachi, Chem.–Eur. J., 2003, 9, 976–983 CrossRef CAS.
- J. Sirieix, N. Lauth-de Viguerie, M. Riviere and A. Lattes, New J. Chem., 2000, 24, 1043–1048 RSC.
- J. Sirieix, N. Lauth-de Viguerie, M. Riviere and A. Lattes, Langmuir, 2000, 16, 9221–9224 CrossRef CAS.
- C. Di Meglio, S. B. Rananavare, S. Svenson and D. H. Thompson, Langmuir, 2000, 16, 128–133 CrossRef CAS.
- H. Morawetz and A. Y. Kandanian, J. Phys. Chem., 1966, 70, 2995–3000 CrossRef CAS.
- J.-H. Fuhrhop, H.-H. David, J. Mathieu, U. Liman, H.-J. Winter and E. Boekema, J. Am. Chem. Soc., 1986, 108, 1785–1791 CrossRef CAS.
- G. R. Newkome, G. R. Baker, S. Arai, M. J. Saunders, P. S. Russo, K. J. Theriot, C. N. Moorefield, L. E. Rogers, J. E. Miller, T. R. Lieux, M. E. Murray, B. Phillips and L. Pascal, J. Am. Chem. Soc., 1990, 112, 8458–8465 CrossRef CAS.
- T.-P. Engelhardt, L. Belkoura and D. Woermann, Ber. Bunsen-Ges. Phys. Chem., 1996, 100, 1064–1072 CAS.
- R. Iwaura, K. Yoshida, M. Masuda, K. Yase and T. Shimizu, Chem. Mater., 2002, 14, 3047–3053 CrossRef CAS.
- L. A. Estroff and A. D. Hamilton, Angew. Chem., Int. Ed., 2000, 39, 3447–3450 CrossRef CAS.
- L. A. Estroff, L. Leiserowitz, L. Addadi, S. Weiner and A. D. Hamilton, Adv. Mater., 2003, 15, 38–42 CAS.
- H. J. Jung, S. Shinkai and T. Shimizu, Chem.–Eur. J., 2002, 8, 2684–2690 CrossRef CAS.
- C. Zhan, P. Gao and M. Liu, Chem. Commun., 2005, 462–464 RSC.
- K. Köhler, G. Förster, A. Hauser, B. Dobner, U. F. Heiser, F. Ziethe, W. Richter, F. Steiniger, M. Drechsler, H. Stettin and A. Blume, Angew. Chem., Int. Ed., 2004, 43, 245–247 CrossRef.
- K. Köhler, G. Förster, A. Hauser, B. Dobner, U. F. Heiser, F. Ziethe, W. Richter, F. Steiniger, M. Drechsler, H. Stettin and A. Blume, J. Am. Chem. Soc., 2004, 126, 16804–16813 CrossRef.
- F. Ziethe, PhD Thesis, MLU Halle-Wittenberg, 2003.
- J.-H. Fuhrhop, D. Spiroski and C. Boettcher, J. Am. Chem. Soc., 1993, 115, 1600–1601 CrossRef CAS.
- H. Yanagawa, Y. Ogawa, H. Furuta and K. Tsuno, J. Am. Chem. Soc., 1989, 111, 4567–4570 CrossRef CAS.
- I. Nakazawa, M. Masuda, Y. Okada, T. Hanada, K. Yase, M. Asai and T. Shimizu, Langmuir, 1999, 15, 4757–4764 CrossRef CAS.
- T. Shimizu, R. Iwaura, M. Masuda, T. Hanada and K. Yase, J. Am. Chem. Soc., 2001, 123, 5947–5955 CrossRef CAS.
- J. Song, S. Kopta and R. C. Stevens, J. Am. Chem. Soc., 2001, 123, 3205–3213 CrossRef CAS.
- A. Blume, Biochemistry, 1983, 22, 5436–5442 CrossRef CAS.
- A. Meister, S. Koschoreck, W. Richtering and A. Blume, unpublished results.
- H. H. Mantsch and R. N. McElhaney, Chem. Phys. Lipids, 1991, 57, 213–226 CrossRef CAS.
Footnotes |
| † Current address: Max-Planck Institute of Colloids and Interfaces, 14424 Golm/Potsdam, Germany |
| ‡ Current address: University of Bayreuth, Macromolecular Chemistry II, 95440 Bayreuth, Germany |
| This journal is © The Royal Society of Chemistry 2006 |
