Aromatic annulation strategy for naphthalenes fused at 1,2- and 3,4-positions with two heterocycles†
Min
Zhang
,
Hui-Ying
An
,
Bao-Guo
Zhao
and
Jian-Hua
Xu
*
Department of Chemistry, Nanjing University, Nanjing, P. R. China. E-mail: xujh@nju.edu.cn; Fax: (+86)25 8331 7761; Tel: (+86)25 8359 2709
First published on 18th November 2005
Abstract
An efficient regioselective naphthoannulation strategy able to fuse a newly formed naphthalene ring at its 1,2- and 3,4- positions to two different heterocycles has been developed.
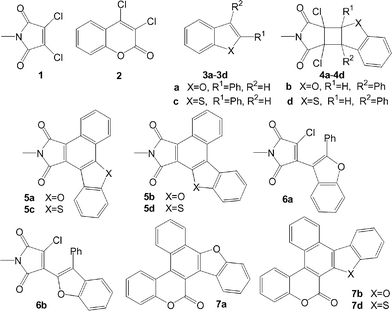
Selective synthesis of functionalized polycyclic heteroaromatic compounds by annulation methods1 has been an important task in organic synthesis because polycyclic heterocycles are closely associated with a variety of natural products and unnatural synthetic targets of biological importance, and play an important role in developing new organic electro-optical materials.2 In this respect, radical cyclization3 and electrocyclic reaction4 are among the most powerful and versatile tools. We report here a simple and efficient naphthoannulation strategy5 starting from photoreactions of N-methyl-3,4-dichloromaleimide (1) and 3,4-dichlorocoumarin (2) with phenyl-substituted heterocycles such as 2- and 3-phenylbenzofurans and 2 and 3-phenylbenzothiophenes 3a–3d which involves radical cyclization and tandem electrocyclic reactions.
Irradiation of 1 with 3a in benzene gave 4a (73%) and 5a (24%). While 4a is a normal [2 + 2] cycloadduct between triplet excited 1 (31*) and 3a, 5a can be viewed as derived from a formal Diels–Alder adduct I (Scheme 1) between 1 and 3a by eliminating two HCl molecules. However, a control experiment showed that 5a could not be formed by thermal reactions of 1 with 3a in the dark either at ambient temperature or at elevated temperature by prolonged refluxing in toluene. Similar irradiation of 2 with 3a afforded the annulation product 7a in 58% yield. A cyclobutane product was not found in this case (Table 1). We further found that 4a can be transformed to 5a in high yield by first heating on silica gel at 100 °C to give the cyclobutane ring-opening product 6a (94%) and 5a (1%), and then irradiating 6a in acetone solution to give 5a (96%). As a result, the total yield of 5a in the photoreaction and subsequent conversion of 4a is 94%. The mechanism for the formation of 5a in the photoreaction of 1 with 3a and by the conversion of 4a is proposed in Scheme 1.
 | ||
| Scheme 1 | ||
| Photoreaction | Conversion of 4 | Total yield of 5 and 7 (%)a | |||
|---|---|---|---|---|---|
| Reactants | Irradiation time/h | Conversion (%) | Products and yield (%) | Products and yield (%) | |
| a Total yield of isolated products in the photoreaction and conversion of 4. b Not further investigated. | |||||
| 1, 3a | 21 | 100 | 4a (73), 5a (24) | 5a (92) | 5a (94) |
| 1, 3b | 48 | 63 | 4b (87), 5b (6) | 5b (95) | 5b (89) |
| 1, 3c | 27 | 79 | 4c (84), 5c (7) | 5c (94) | 5c (87) |
| 1, 3d | 44 | 95 | 4d (75), 5d (8) | 5d (86) | 5d (72) |
| 2, 3a | 48 | 43 | 7a (58) | 7a (58) | |
| 2, 3b | 48 | 36 | 7a (71) | 7b (71) | |
| 2, 3c | Slow reactionb | ||||
| 2, 3d | 72 | 36 | 7d (67) | 7d (67) | |
Addition of 31* with 3a leads to the diradical intermediate A in which the unpaired electron at C4 is delocalized to the o- and p-carbon atoms in the phenyl ring. Then 1,4- and 1,6-diradical recombination after intersystem crossing (ISC) to singlet manifold gave 4a and 5a, respectively. Heating 4a absorbed on silica gel results in the elimination of hydrogen chloride to afford the thermally labile cyclobutene B which underwent electrocyclic ring-opening to give 6a.6 Subsequent 6π electrocyclization7 of 6a can be achieved thermally or photochemically. As mentioned above, thermal transformation of 6a to 5a is very sluggish and by heating 4a on silica gel for 10 h, only 1% yield of 5a is formed. On the other hand, irradiating 6a in acetone led to smooth transformation to 5a in high yield (96%). These results are attributed to the fact that thermal disrotatory ring-closure in 6a gave product C where H and Cl atoms are in a cis-configuration, making their elimination difficult and driving the reversible eletrocyclization backward to the ring-opening form 6a. At the same time, photoinduced conrotatory ring-closure gave D with the H and Cl atoms trans- to each other for facile elimination to give product 5a. A key point to the success of the conversion of 4a to 5a is the feasibility of 4a to eliminate a hydrogen chloride when heating on silica gel. This is because 4a has a sterically less hindered anti-configuration for the pyrroledione and the benzofuran moieties so that the H and Cl atoms are trans- to each other, favoring the elimination. This is substantiated by an X-ray crystallographic analysis of 4a‡ (Fig. 1).
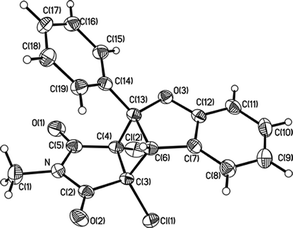 | ||
| Fig. 1 X-Ray structure of 4a. | ||
The competition of 1,4- and 1,6-diradical recombination pathways in the diradical intermediates A and E (Scheme 1) are likely decided by spin distribution and steric hindrance in the two ring-closure modes. Density functional theory (DFT) calculations at the UB3LYP 6-31G level8 of A and E reveal that both in the optimized geometry and in conformations suitable for rapid ISC and bond formation,9 not only do the two radical center C atoms (C1 and C4) have a large spin density, but the o- and p-carbon atoms in the phenyl ring also share significant spin density to allow direct 1,6-cyclization to the annulation products 5a and 7a.
Irradiation of 1 with 3b furnished 4b (87%) and 5b (6%). Heating 4b on silica gel at 90 °C for 12 h resulted in complete conversion of 4b and gave 6b in 96% yield. Photolysis of 6b gave 5b in 99% yield. Therefore, the yield of 5b covering photoreaction and further conversion of 4b is 89%. The structure of 5b was also confirmed by an X-ray crystallographic analysis§ (Fig. 2). Similar to the photoreactions of 2 with 3a, irradiation of 2 with 3b gave the annulation product 7b as the only isolable product in 71% yield.
 | ||
| Fig. 2 X-Ray structure of 5b. | ||
This annulation protocol was also performed for 2- and 3-phenylbenzothiophenes 3c and 3d. The results are in Table 1.
Therefore, this naphthoannulation strategy is highly regioselective and high yielding, leading to the simple (sometimes stratightforward) synthesis of polycyclic heterocycles in which coumarin or maleimide (at their 3,4-position) and benzofuran or benzothiophene (at their 2,3-position) are fused at the 1,2- and 3,4-positions of a newly formed naphthalene ring, respectively. Regiochemistry in the annulation product can be controlled simply by changing the position of the phenyl group in the benzofuran and benzothiophene rings.
Aromatic annulated furans,10 polycyclic thiophene derivatives,11 3,4-disubstituted and annulated maleimides12 and angularly fused polycyclic coumarin derivatives13 are of current research interest in view of their biological, optical and electrochemical properties, and their syntheses have drawn much attention. However, more thorough investigation of these compounds is still hampered by the lack of general and efficient synthetic methods. The naphthoannulation strategy reported here provides a convenient methodology for the synthesis of these polycyclic systems, and these previously unknown polycyclic systems 5 and 7 would be interesting target compounds for the screening of biological activity and optoelectric properties. All of these compounds are bright yellow or yellow-green colored and are strongly fluorescent in the blue region. The light absorption and emitting properties are shown in Table 2.
| Compound |
λ
abmax/nm![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
ε max (×104)/L mol−1 cm−1 |
λ
fmax/nm![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
Φ f |
|---|---|---|---|---|
| a The longest wavelength absorption peak in the electronic spectrum. b The emission maximum in the fluorescence spectrum. | ||||
| 5a | 397 | 0.70 | 450 | 0.41 |
| 5b | 403 | 1.15 | 455 | 0.63 |
| 5c | 405 | 0.55 | 465 | 0.33 |
| 5d | 401 | 1.00 | 463 | 0.38 |
| 7a | 403 | 2.31 | 441 | 0.20 |
| 7b | 410 | 1.59 | 448 | 0.73 |
| 7d | 418 | 0.95 | 457 | 0.42 |
Research on further use of this annulation strategy for phenyl-substituted heterocycles other than phenylbenzofurans and phenylbenzothiophenes is in progress.
We thank the NSFC for financial support (20272024).
Notes and references
- M. E. Jung, Tetrahedron, 1976, 32, 3 CrossRef CAS; G. H. Posner, Chem. Rev., 1986, 86, 831 CrossRef CAS; G. A. Molander, Acc. Chem. Res., 1998, 31, 603 CrossRef CAS.
- R. G. Harvey, Polycyclic Aromatic Hydrocarbons, Wiley-VCH, New York, 1996 Search PubMed; R. G. Harvey, in Handbook of Environmental Chemistry, D. Hutzinger and A. Neilson, eds., Springer, Berlin, Heidelberg, 1997, vol. 3, part 1, ch. 1 Search PubMed; M. D. Watson, A. Fechtenkotter and K. Mullen, Chem. Rev., 2001, 101, 1267 Search PubMed; K. Y. Law, Chem. Rev., 1993, 93, 449 Search PubMed.
- L. Yet, Tetrahedron, 1999, 55, 9349 CrossRef CAS; D. P. Curran, in Comprehensive Organic Synthesis, B. M. Trost and I. Fleming, eds., Pergamon, New York, 1991, vol. 4, ch. 4, p. 770 Search PubMed; M. Malacria, Chem. Rev., 1996, 96, 289 Search PubMed; T. R. Rheault and M. P. Sibi, Synthesis, 2003, 803 CrossRef CAS.
- G. Resimoni, G. Tacconi, A. Barco and G. P. Pollini, Natural Product Synthesis Through Pericyclic Reactions, ACS monograph 180, M. C. Caserio, ed., American Chemical Society, Washington DC, 1983 Search PubMed.
- For recent naphthoannulation reactions, see: P. M. Donovan and L. T. Scott, J. Am. Chem. Soc., 2004, 126, 3108 Search PubMed; J. D. Tovar, A. Rose and T. M. Swager, J. Am. Chem. Soc., 2002, 124, 7762 CrossRef CAS.
- W. R. Dolbier, Jr., H. Koroniak, K. N. Houk and C. Sheu, Acc. Chem. Res., 1996, 29, 471 CrossRef.
- For stilbene-phenanthrene transformation, see: F. B. Mallory, Org. React. (N. Y.), 1984, 30, 1 Search PubMed ; for similar transformation of heterocyclic analogs of stilbene, see: M. M. Krayushkin, Chem. Heterocycl. Compd., 2001, 37, 15 CAS; M. Irie, Chem. Rev., 2000, 100, 1685 Search PubMed.
- Gaussian 03, Revision B.04, Gaussian, Inc., Pittsburgh, PA, 2003 Search PubMed.
- A. G. Griesbeck, H. Mauder and S. Stadtmueller, Acc. Chem. Res., 1994, 27, 70 CrossRef CAS.
- Y. Matsuya, K. Sasaki, M. Nagaoka, H. Kakuda, N. Toyooka, N. Imanishi, H. Ochiai and H. Nemoto, J. Org. Chem., 2004, 69, 7989 CrossRef CAS and refs. cited therein.
- J. Roncali, Chem. Rev., 1992, 92, 711 CrossRef CAS; W. D. Wilson, Y. H. Wang, S. Kusuma, S. Chandrasekaran, N. C. Yang and D. W. Boykin, J. Am. Chem. Soc., 1985, 107, 4989 CrossRef CAS; S.-M. Yang, J.-J. Shie, J.-M. Fang, S. K. Nandy, H.-Y. Chang, S.-H. Lu and G. Wang, J. Org. Chem., 2002, 67, 5208 CrossRef CAS and refs. cited therein Q. T. Zhang and J. M. Tour, J. Am. Chem. Soc., 1997, 119, 5065 Search PubMed.
- Y. Yamamoto, H. Hayashi, T. Saigoku and H. Nishiyama, J. Am. Chem. Soc., 2005, 127, 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 804 CrossRef CAS and refs. cited therein N. Ono, H. Hironaga, K. Simizu, K. Ono, K. Kuwano and T. Ogawa, J. Chem. Soc., Chem. Commun., 1994, 1019 Search PubMed.
804 CrossRef CAS and refs. cited therein N. Ono, H. Hironaga, K. Simizu, K. Ono, K. Kuwano and T. Ogawa, J. Chem. Soc., Chem. Commun., 1994, 1019 Search PubMed. - R. G. Harvey, C. Cortez, T. P. Ananthanarayan and S. Schmolka, J. Org. Chem., 1988, 53, 3936 CrossRef CAS and refs. cited therein I. A. Kham, M. V. Kulkarni, M. Gopal, M. S. Shahabuddin and C.-M. Sun, Bioorg. Med. Chem. Lett., 2005, 15, 3584 Search PubMed and refs. cited therein.
Footnotes |
| † Electronic supplementary information (ESI) available: general experimental procedures and analytical data for all products. See DOI: 10.1039/b514496e |
| ‡ Crystal data for 4a. C19H13Cl2NO3, M = 374.20, colorless block, 0.40 × 0.30 × 0.28 mm, monoclinic P21/c, a = 12.895(3), b = 9.910(2), c = 13.175(3) Å, β = 90.54(3)°, V = 1683.6(6) Å3, Z = 4, μ = 0.404 mm−1, 2θmax = 50°. A total of 3110 measured reflections and 2971 independent reflections were measured at T = 293(2) K. Rint and final R are 0.0426 and 0.0536. CCDC reference number 280979. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b514496e. |
| § Crystal data for 5b. C19H11NO3, M = 301.29, yellow needles, 0.40 × 0.30 × 0.24 mm, monoclinic P21/c, a = 9.5260(19), b = 18.319(4), c = 7.9420(16) Å, β = 96.25(3)°, V = 1377.7(5) Å3, Z = 4, μ = 0.099 mm−1, 2θmax = 50.14°. A total of 2629 measured reflections and 2439 independent reflections were measured at T = 293(2) K. Rint and final R are 0.0433 and 0.0551. CCDC reference numbers 280980. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b514496e. |
| This journal is © The Royal Society of Chemistry 2006 |