One-pot chemo-enzymatic synthesis of reporter-modified proteins
Andrew S.
Worthington
and
Michael D.
Burkart
*
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0358, USA. E-mail: mburkart@ucsd.edu; Fax: +1 858 822 2182; Tel: +1 858 534 5673
First published on 24th November 2005
Abstract
To meet recent advancements in the covalent reporter labeling of proteins, we propose a flexible synthesis for reporter analogs. Here we demonstrate a one-pot chemo-enzymatic synthesis of reporter-labeled proteins that allows the covalent tethering of any amine-terminal fluorescent or affinity label to a carrier protein or fusion construct. This two-reaction sequence consists of activated panthothenate coupling, biosynthetic conversion to the coenzyme A (CoA) analog, and enzymatic carrier protein modification via phosphopantetheinyltransferase (PPTase). We also probe substrate specificity for CoAA, the first enzyme in the pathway. With this approach CoA analogs may be rapidly prepared, thus permitting the regiospecific attachment of reporter moieties from a variety of molecular species.
Introduction
The post-translational modification of carrier protein domains from polyketide, non-ribosomal peptide, and fatty acid biosyntheses has recently attracted the attention of the biological chemistry community for the selective covalent modification of proteins with reporter molecules. Since introduction of this technology,1 it has been used as a multiplex functional assay,2 as a fusion protein in vitro and in vivo,3 and as a phage library reporter.3b Each of these studies utilized reporters attached to coenzyme A through selective reactivity of the CoA thiol to a maleimide functionality incorporated into each reporter molecule.4 While convenient, this coupling method shows limited flexibility for diverse reporter identity, as many desirable reporters require multi-step syntheses to introduce the maleimide functionality. Additionally, excess maleimide can lead to non-specific protein labeling if not rigorously purified from unreacted starting materials after coupling to CoA. The resulting sulfanylsuccinimide linkage may also not be compatible with selected downstream applications, where a less-bulky or more natural linkage is preferred. We have therefore developed the following one-pot sequence for carrier protein labeling that may be used with any amine-terminal reporter and allows great flexibility in reporter and linkage choice.In order to produce modified CoA analogs, we sought a linear sequence via synthetic modification of CoA precursors. Along with the introduction of a modular approach to panthetheine and phosphopantetheine synthesis,5 CoA biosynthetic enzymes came to our attention with the potential of demonstrating promiscuous substrate specificity akin to phosphopantetheinyltransferases (PPTases).1 CoA biosynthesis has been a topic of research for decades, yet only recently have prokaryotic and eukaryotic pathways been fully elucidated.6 CoA is biosynthesized from pantothenate (vitamin B5) through a series of five enzymes, CoAA–E. CoA synthesis has also been shown to be possible via an abbreviated approach beginning with pantetheine.7,8 We found that CoAA, which is understood to accept pantothenate as a metabolic substrate, will also catalyze the phosphorylation of pantetheine and pantetheine derivatives at the 4′-hydroxyl group.9 Subsequently, CoAD (Phosphopantetheine adenylyltransferase, PPAT) and CoAE (dephosphocoenzyme A kinase, DPCK) can be used to complete the CoA molecule from 4′-phosphopantetheine. The human version of these enzymes exists as a bifunctional fusion protein (PPAT–DPCK)6b, 10 and is utilized in our studies. Here we introduce a synthetic scheme that allows any amine-terminal reporter for covalent protein attachment. A one-pot, two-reaction sequence, this scheme uses only two recombinant proteins to make reporter-modified CoA analogs (Scheme 1), and a third enzyme carries out carrier protein attachment (Scheme 2).
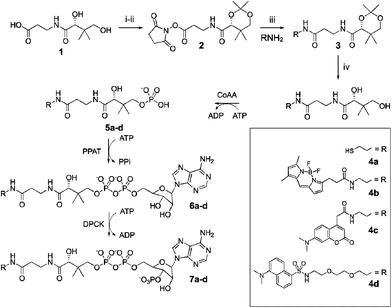 | ||
| Scheme 1 Pantothenate 1 activation and coupling to amine-terminal reporters 4a–c, followed by deprotection of 3 and in vitro enzymatic synthesis of CoA analogs 7a–c by E. coli CoAA, H. sapiens PPAT–DPCK in one pot. Reagents: (i) acetone, p-toluenesulfonic acid; (ii) EDC, N-hydroxysuccinimide, iPr2NEt, DMF; (iii) Et3N, THF; (iv) 1 M HCl, THF. | ||
 | ||
| Scheme 2 In vitro labeling of a carrier protein (CP) by a PPTase with 7a–d to yield labeled protein 8a–d. | ||
Results and discussion
The chemo-enzymatic synthesis begins with the chemical synthesis of pantetheine analogs via activated panthothenate coupling with an N-terminal reporter. Subsequent enzymatic incubation with two enzymes, recombinant E. coli CoAA and human PPAT–DPCK, completes the synthesis of labeled CoA analogs. PPAT–DPCK was chosen as a convenient natural fusion protein, thus abbreviating recombinant enzyme production. Human DPCK has also been shown to demonstrate more rapid kinetic turnover for the natural substrate 4′-phosphopantetheine than the E. coli variant.8,6bReaction of 2 with an amine-terminal reporter (1.5 eq.), followed by acetonide deprotection (1 M HCl), led to the formation of reporter-coupled pantothenate 3 (Scheme 1). Subsequent buffering and addition of recombinant E. coli CoAA (7.5 µg), human PPAT–DPCK (50 µg), pyruvate kinase (5 units), and inorganic pyrophosphatase (1 unit), as well as necessary cofactors (8 mM ATP, 0.5 mM PEP), generated labeled CoA 7a–c. In the same pot, recombinant Vibrio cholerae carrier protein VibB (30 µg) was phosphopantetheinylated with a catalytic amount of the Bacillus subtilis PPTase Sfp (Scheme 2). Samples were run on SDS-Page, and reporter-labeled VibB was visualized by UV fluorescence and Western blotting (Fig. 1).†
 | ||
| Fig. 1 One-pot synthesis of reporter-labeled VibB 8b–d separated by SDS-PAGE and detected by fluorescence (λ = 254 nm, lanes 2–4) and Western blot (BCIP–NBT, lane 5). Lane 1 shows Coomassie-stained one-pot reaction, with VibB (33 kDa) and PPAT/DPCK (62 kDa) indicated. Carrier protein species is indicated below each lane. | ||
Using this scheme, fluorescent reporters BODIPY, coumarin, and dansyl (4b–d, respectively) were coupled to pantothenate and subsequently modified by the CoA-generating enzymes CoAA and PPAT–DPCK to form labeled CoA derivatives. The phosphopantetheine moiety of these derivatives was then covalently bound to the V. cholerae carrier protein VibB via recombinant Sfp, a PPTase from B. subtilis. Each reporter fluoresced when illuminated with ultraviolet radiation (Fig. 1, lanes 2–4). In addition, the dansyl reporter was detected using Western blot techniques with anti-dansyl antibody (lane 5). Western blot methods have a picogram detection limit for reporter-modified carrier proteins versus the microgram limits of fluorescence. Therefore, the Western blotting technique in conjunction with this one-pot methodology is useful for the identification of small quantities of protein.
Importantly, this coupled enzyme system showed remarkable permissiveness to substrate identity. While 4a and 4b contain short ethylenediamine linkers between pantothenic acid and reporter, 4d incorporates a PEG tether distancing dansyl from pantothenate. This was chosen to enable the antibody to bind the dansyl moiety under native conditions. From studies by Rock, we presume that the greatest selectivity in this enzyme system is borne by CoAA, which acts as a gatekeeper for the downstream pathway.11 To investigate the selectivity of CoAA, we analyzed the substrate selectivity of E. coli CoAA on our synthetic pantetheine derivatives (Table 1). Our kinetic values were in agreement with previously reported values for CoAA activity on 1, the natural substrate, although our enzyme fractions appear to have slightly lower specific activity.8 Interestingly, CoAA displayed little preference for the natural substrate (1) over the synthetic substrates 4c and 4d. However, we observed a slight difference in the substrate specificity of pantothenate compared with pantetheine. More significantly, the difference in specificity between pantothenate and the fluorescent analog 4b suggests the enzyme discriminates only modestly against more bulky substrates. This is not surprising, as CoA precursors may exist in disulfide form in vivo. Nevertheless, these values indicate that extended pantetheine analogs are excellent substrates of E. coli CoAA, which appears to function with broad promiscuity. Future studies will be aimed at further determining the substrate specificity of CoAA from E. coli and other organisms.
| Substrate | k cat/s−1 | K m/µM | k cat/Km/s−1 mM−1 |
|---|---|---|---|
| 1 Pantothenate | 0.551 ± 0.021 | 26.7 ± 5.1 | 20.6 ± 4.0 |
| 4a Pantetheine | 0.320 ± 0.016 | 91 ± 10 | 3.53 ± 0.44 |
| 4b | 0.101 ± 0.012 | 583 ± 131 | 0.174 ± 0.044 |
| 4c | 0.395 ± 0.019 | 24.9 ± 3.7 | 15.9 ± 2.5 |
| 4d | 0.413 ± 0.021 | 36.1 ± 6.2 | 11.4 ± 2.0 |
In conclusion, broad enzymatic protein modification with any amine-terminated reporter molecule can be obtained from the proposed one-pot reaction. The use of this methodology for native and engineered carrier proteins is currently under investigation.
Acknowledgements
The authors would like to thank Andrei Osterman for the generous gift of PPAT–DPCK clone and helpful discussions. Funding was provided by the University of California, San Diego, Department of Chemistry and Biochemistry, NSF CAREER award under Grant No. 0347681.Notes and references
- J. J. La Clair, T. L. Foley, T. R. Schegg, C. M. Regan and M. D. Burkart, Chem. Biol., 2004, 11, 195 CrossRef CAS
.
- A. C. Mercer, J. J. La Clair and M. D. Burkart, ChemBioChem, 2005, 6, 1335 CrossRef CAS
.
-
(a) N. George, H. Pcik, H. Vogel, N. Johnsson and K. Johnsson, J. Am. Chem. Soc., 2004, 126, 8896 CrossRef CAS
; (b) J. Yin, F. Liu, X. Li and C. T. Walsh, J. Am. Chem. Soc., 2004, 126, 7754 CrossRef CAS
.
- K. Shimada and K. Mitamura, J. Chromatogr. B, Biomed. Appl. , 1994, 659, 227 CrossRef CAS
.
- A. L. Mandel, J. J. La Clair and M. D. Burkart, Org. Lett., 2004, 6, 4801 CrossRef CAS
.
-
(a) P. K. Mishra and D. G. Drueckhammer, Chem. Rev., 2000, 100, 3283 CrossRef CAS
; (b) M. Daugherty, B. Polanuyer, M. Farrell, M. Scholle, A. Lykidis, V. de Crecy-Lagard and A. Osterman, J. Biol. Chem., 2002, 277, 21431 CrossRef CAS
; (c) R. Leonardi, Y. Zhang, C. O. Rock and S. Jackowski, Prog. Lipid Res., 2005, 44, 125 CrossRef CAS
.
- I. Nazi, K. P. Koteva and G. D. Wright, Anal. Biochem., 2004, 324, 100 CrossRef CAS
.
- E. Strauss and T. P. Begley, J. Biol. Chem., 2002, 277, 48205 CrossRef CAS
.
- K. M. Clarke, A. C. Mercer, J. J. La Clair and M. D. Burkart, J. Am. Chem. Soc., 2005, 127, 11234 CrossRef CAS
.
- S. Aghajanian and D. M. Worrall, Biochem. J., 2002, 365, 13 CAS
.
- S. Jackowski and C. O. Rock, J. Bacteriol., 1981, 148, 926 CAS
.
Footnote |
| † General procedure for the one pot synthesis of reporter-labeled CoA and enzymatic carrier protein modification: To N-hydroxysuccinimide ester of pantothenic acid 2 dissolved in dry THF is added a THF solution of amine-terminal reporter (1.5 eq.). The reaction is stirred at room temperature for 2 h, followed by evaporation to dryness. The acetonide protecting group is subsequently hydrolyzed by the addition of a minimum volume of 1 M HCl and stirring at rt for 10 min, leading to the in-situ formation of reporter-coupled pantothenate. Subsequent addition of 1 M MES buffer (pH 8.0) to a pH of 6.0 and 75 mM MES, 12.5 mM MgCl2 is followed by addition of recombinant E. coli CoAA (7.5 µg), recombinant PPAT–DPCK (50 µg), pyruvate kinase (5 units), and inorganic pyrophosphatase (1 unit), 8 mM ATP, and 0.5 mM PEP, recombinant Vibrio cholerae VibB (30 µg), and recombinant Bacillus subtilis Sfp. The mixture is incubated at room temperature for a minimum of 1 hour. For verification, samples are run on 4–12% SDS-Page, where reporter-labeled VibB is visualized by UV fluorescence or Western blotting. |
| This journal is © The Royal Society of Chemistry 2006 |
