Hot off the press
Robert A. Hill and Andrew Sutherland
Department of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bobh@chem.gla.ac.uk; andrews@chem.gla.ac.uk
First published on 18th January 2006
Abstract
A personal selection of 38 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products, such as the first naturally occurring cyclonucleoside, N3,5′-cycloxanthosine, from an Eryus species.
 Bob Hill | Bob Hill graduated in 1972 from the University of Cambridge where he also obtained his PhD degree in 1975 by studying the biosynthesis of polyketide fungal metabolites with Jim Staunton. He then moved to the University of Glasgow, where he worked on the biosynthesis of bacterial metabolites with Gordon Kirby before joining the academic staff at Glasgow in 1977. Bob has always had an interest in the structure and biosynthesis of natural products and has been writing regular reviews of Triterpenoids in this journal with Joe Connolly since 1985. In 1996, together with Andrew Pitt, he wrote the first Hot off the Press article, which has been continued in each issue since that time. His co-author changed to Marie Claire Parker in 2000, and he was joined by Andrew Sutherland at the end of 2003. |
 Andrew Sutherland | Andrew Sutherland graduated from University of Edinburgh in 1994 and was awarded a PhD in 1997 from the University of Bristol where he worked on the chemoenzymatic synthesis of α-hydroxy and α-amino acids for natural product synthesis with Christine Willis. He then joined John Vederas at the University of Alberta where he studied the diaminopimelate metabolism for the design of novel antibiotics, as well as the characterisation of the LNKS protein, the first purified Diels–Alderase. He returned to the University of Bristol to take up a junior research fellowship with Timothy Gallagher on the design and synthesis of neuronal nicotinic receptors. In 2003 he was appointed to the academic staff at the University of Glasgow. He currently runs a research group of 7 PhD students whose work focuses on the use of new synthetic methodology for the synthesis of chiral, biologically active and medicinally important molecules. |
Omriolide A 1, a rearranged spongiane diterpenoid from the marine sponge Dictyodendrilla aff. retiara, has a concave ring system (Y. Kashman and co-workers, Tetrahedron Lett., 2005, 46, 8613). The authors propose a biosynthetic pathway to omriolide A. The dimeric cyclolaurane sesquiterpenoid 2 has been isolated from the red alga Laurencia microcladia (V. Roussis and co-workers, Tetrahedron, 2006, 62, 182). The structure of 2 is incorrectly drawn as the meso-isomer in the reference. The gorgonian coral Pseudopterogorgia bipinnata is the source of a group of diterpenoids including caucanolide B 3, which has an unusual formamidine moiety (A. D. Rodriguez and co-workers, J. Nat. Prod., 2005, 68, 1519).
The trachylobane diterpenoid mitrephorone A 4, with an oxetane ring system, has been found in Mitrephora glabra (N. H. Oberlies and co-workers, Org. Lett., 2005, 7, 5709). A similar ring system is found in petatrichol B 5, a triterpenoid from Petasites tricholobus (Z. J. Jia and co-workers, Chin. Chem. Lett., 2005, 16, 1351). X-Ray analysis was used to establish the structure of the rearranged tetranortriterpenoid cipadesin A 6, from Cipadessa cinerascens, which has a C10/C11 linkage joining rings A and C (G.-L. Zhang and co-workers, Org. Lett., 2005, 7, 5051). Kadlongilactone A 7, from Kadsura longipedunculata, has an intriguing rearranged triterpenoid skeleton (H.-D. Sun and co-workers, Org. Lett., 2005, 7, 5079). The structure of kadlongilactone A 7 was also confirmed by X-ray analysis.
The Taiwanese medicinal plant Kadsura philippinensis is the source of the lignan taiwankadsurin A 8 (Y.-C. Shen et al., Org. Lett., 2005, 7, 5297). The authors propose a biosynthetic pathway to taiwankadsurin A 8 involving C–C bond formation to a methyl ether and a ring cleavage. The red alga Callophycus serratus produces three diterpenoid benzoate macrolides including the cytotoxin bromophycolide A 9 (J. Kubanek et al., Org. Lett., 2005, 7, 5261). The structure of bromophycolide A 9 was confirmed by X-ray analysis, as was the structure of the acetylenic spiroketal 10 from buds of Chrysanthemum indicum (T. P. You and co-workers, Chin. Chem. Lett., 2005, 16, 1341). Synthetic studies have established that the stereochemistry of the lactone lethaloxin 11, a metabolite of Mycosphaerella lethasis, is the same as the previously known pinolidoxin (J. A. Marco and co-workers, J. Org. Chem., 2005, 70, 9822).
Quantum mechanical 13C NMR chemical shift calculations were used to establish the regiochemistry of aplidinone A 12 from the Mediterranean ascidian Aplidium conicum (E. Fattorusso and co-workers, Eur. J. Org. Chem., 2005, 5024). Edulisone A 13 is a benzo[b]oxepine derivative with a bisamide side chain from the bark of Aglaia edulis (A. D. Kinghorn and co-workers, Tetrahedron Lett., 2005, 46, 9021).
Brefelamide 14 has been isolated from the fruiting bodies of the cellular slime moulds Dictostelium brefeldianum and Dictostelium giganteum (Y. Oshima and co-workers, J. Org. Chem., 2005, 70, 8854). The structure of brefelamide 14 was confirmed by synthesis. The hanasanagitake mushroom Isaria japonica is the source of hanasanagin 15, which is 3,4-diguanidinobutanoyl-DOPA (K. Imai and co-workers, Tetrahedron Lett., 2005, 46, 9057).
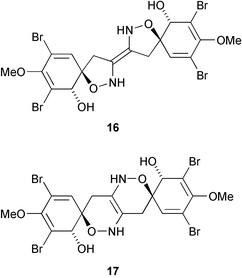
The structure of the bromotyrosine derivative zamamistatin, from the Okinawan sponge Pseudoceratina sp., has been revised from 16 to 17 on the basis of comparison of the 13C NMR data of zamamistatin with synthetic model compounds (H. Kigoshi and co-workers, Tetrahedron Lett., 2006, 47, 155).
The yeast Malassezia furfur is thought to be one of the causative agents of the skin mycosis pityriasis versicolor. Three indole derivatives, including pityriarubin A 18, have been isolated from cultures of the yeast, and they show inhibition of respiratory burst in human neutrophils activated by various agents (H.-J. Krämer et al., ChemBioChem, 2005, 6, 2290). The first naturally occurring cyclonucleoside, N3,5′-cycloxanthosine 19, has been isolated from an Eryus species of Australian marine sponge (R. J. Capon et al., J. Nat. Prod., 2005, 68, 1689).
A classification of the different natural product classes in a tree-like manner has been proposed that provides a tool for the design of natural-product-derived compound collections (M. A. Koch et al., Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17272). Over 800 alkaloids have been isolated from amphibian skin. J. W. Daly et al. have surveyed these alkaloids and note that many of them are derived from dietary sources (J. Nat. Prod., 2005, 68, 1556). Insect pathogenic fungi are a rich source of structurally novel biologically active compounds. Y. Thebtaranonth and co-workers have reviewed current knowledge of these fungal metabolites (Acc. Chem. Res., 2005, 38, 813).
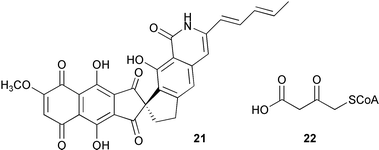
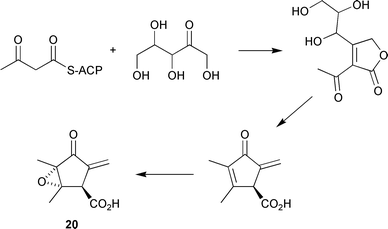
The biosynthesis of methylenomycin 20 from Streptomyces coelicolor has been proposed on the basis of labelling studies and examination of the mmy gene cluster (C. Corre and G. L. Challis, ChemBioChem, 2005, 6, 2166). The authors propose that methylenomycin 20 is formed from acetoacetyl ACP with a pentulose (Scheme 1). The biosynthetic gene cluster which produces the antitumour agent fredericamycin 21 from Streptomyces griseus has been cloned, sequenced and analysed (B. Shen and co-workers, J. Am. Chem. Soc., 2005, 127, 16442). The fdm cluster consists of 28 open reading frames encoding a type II polyketide synthase as well as tailoring, regulatory and resistance proteins. J. B. Spencer and co-workers have developed a method for trapping the intermediates of polyketide biosynthesis (Angew. Chem., Int. Ed., 2005, 44, 7079). This approach involves the use of a nonhydrolysable malonyl-coenzyme A analogue 22 which reacts with the growing polyketide chain but cannot undergo transesterification back onto the PKS, resulting in the accrual of intermediates.
 | ||
| Scheme 1 | ||
D. Hilvert and co-workers have used a combination of experimental and computational evidence to show that isochorismate pyruvate lyase (IPL) catalyses the conversion of isochorismate to salicylate and pyruvate by a one-step pericyclic mechanism (Scheme 2) (J. Am. Chem. Soc., 2005, 127, 15002). S. F. Brady and J. Clardy have identified the biosynthetic origin of the isocyanide carbon atom of the antibiotic 23 by systematic investigation of the E. coli metabolome (Angew. Chem., Int. Ed., 2005, 44, 7045). By controlling E. coli metabolism using strains carrying mutations in primary metabolic pathways, they identified the C2 atom of ribulose-5-phosphate as the source of the isocyanide carbon.
 | ||
| Scheme 2 | ||
C. T. Walsh and co-workers have reviewed the many post-translational modifications of proteins in eukaryotic cells, highlighting areas such as controlled proteolysis, autocleavage and peptide-bond rearrangement (Angew. Chem., Int. Ed., 2005, 44, 7342). L.-J. Ming and co-workers have demonstrated the catechol oxidase activity of di-Cu2+-substituted aminopeptidase from Streptomyces griseus (J. Am. Chem. Soc., 2005, 127, 16380). The authors believe this work is another example of an enzyme which challenges the predominant theory concerning the evolution of enzymes to perform specific chemical transformations.
In seeking selective inhibitors of NO-mediated processes, S. Caddick and co-workers have shown that pentafluorophenyl (PFP) sulfonates can reversibly inhibit dimethylarginine dimethylaminohydrolase (DDAH) and arginine deiminase (ADI) (Chem. Commun., 2005, 5563). These compounds, which are the most potent inhibitors of bacterial DDAH known, are easily prepared using a 1,3-dipolar cycloaddition reaction, resulting in the efficient preparation of diverse arrays of PFP derivatives. J. C. Vederas and co-workers have synthesised aziridine analogues of diaminopimelic acid (DAP) and shown these to be irreversible inhibitors of DAP epimerase, an important enzyme in bacterial cell wall biosynthesis (Org. Biomol. Chem., 2005, 3, 4402). MALDI-TOF mass spectrometry analysis of the digested, inactivated enzyme showed that L,L-azi-DAP 24 inactives DAP epimerase by reaction with Cys-73 while D,L-azi-DAP 25 reacts with Cys-217.
M. Volk and co-workers have shown that substitution of individual amino acids in an alanine-based peptide affects both the helical stability of the peptide as well as the kinetics of helical-coil relaxation (Chem. Commun., 2005, 5985). To help understand the process of amyloidogenesis thought to cause Alzheimer's disease, J. W. Kelly and co-workers have prepared an E-olefin dipeptide isostere 26 (J. Am. Chem. Soc., 2005, 127, 15366). Incorporation of this isostere into the 40-residue Alzheimer's amyloid peptide allowed examination of specific intermolecular H-bonding. K. S. Gates and co-workers have demonstrated that fasicularin 27, from the ascidian Nephriteis fasicularis, alkylates DNA at the N7-position of guanine residues (J. Am. Chem. Soc., 2005, 127, 15004). The results are consistent with the generation of the aziridinium ion 28 analogous to the clinically used anticancer drugs such as chlorambucil.
The use of engineered phenylalanine dehydrogenase N145A supported on Celite for the reductive amination of phenylpyruvate in organic solvents has been successfully demonstrated (G. Cainelli and co-workers, Org. Biomol. Chem., 2005, 3, 4316). This reaction was carried out in the presence of solvents such as acetone, methanol, n-hexane, toluene and dichloromethane, and used for the reductive amination of the water-insoluble p-nitrophenylpyruvic acid (Scheme 3). A new thermophilic NAD-dependent alcohol dehydrogenase (TADH) from Thermus sp. has been identified for the stereoselective reduction of prochiral ketones (A. Schmid and co-workers, Tetrahedron: Asymmetry, 2005, 16, 3512). The enzyme has been utilised on a multigram scale with the cofactor, NADH, being regenerated using catalytic amounts of the organometallic complex [Cp*Rh(bpy)(H2O)]2+ and formate as the reducing agent.
 | ||
| Scheme 3 | ||
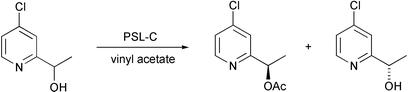
A number of chiral 4-(N,N-dimethylamino)pyridine (DMAP) derivatives have been prepared using enzymatic kinetic resolution of a series of 4-chloro-2-(1-hydroxyalkyl)pyridines as the key step (V. Gotor and co-workers, Tetrahedron: Asymmetry, 2005, 16, 3427). Pseudomonas cepacia lipase (PSL) catalysed the acylation of these compounds, producing enantiomerically pure substrates and products in excellent yields (Scheme 4). A similar lipase-catalysed desymmetrisation process has been used for the generation of P-chirogenic compounds (D. Wiktelius and co-workers, Org. Lett., 2005, 7, 4991). Stereoselective acylation of prochiral phosphine–boranes using vinyl acetate in the presence of Candida antarctica lipase B gave the R-enantiomer in 92% ee. (Scheme 5).
 | ||
| Scheme 4 | ||
 | ||
| Scheme 5 | ||
H. F. Olivo and co-workers have developed a racemic synthesis of the psychostimulant agent modafinil using a biocatalytic one-pot oxidation/amidation process (Tetrahedron: Asymmetry, 2005, 16, 3507). Fermentation of benzyhydrylsulfanyl acetic acid with the fungus Amycolatopsis orientalis produced modafinil in 65% yield (Scheme 6). A stepwise approach also gave the S-enantiomer in high ee.
 | ||
| Scheme 6 | ||
| This journal is © The Royal Society of Chemistry 2006 |