Research Highlights
First published on 8th December 2005
Dirty chips
The fundamental benefits associated with miniaturising analytical systems are well-understood and recognised. In particular, chip-based microfluidic systems have been shown to have many advantages over their conventional (larger) analogues. These include improved efficiency with regard to sample size, response times, cost, analytical performance, process control, integration, throughput, and automation. Of these, the inherent improvements in analytical efficiency that accompany system downsizing are perhaps the most interesting to the research scientist. An obvious example of this behaviour occurs during the miniaturisation of capillary electrophoresis systems. Theory and experiment demonstrate that by simply reducing the cross-sectional dimensions of the channel or capillary analysis times are significantly reduced, whilst separation efficiencies are improved through the ability to apply higher electric fields. These gains have proved key in applying chip-based CE systems to areas such as DNA analysis and proteomics.The high separation efficiencies achievable in microfabricated CE devices are in large part due to the increased surface-to-volume ratios which allow efficient heat dissipation even when high electric fields are used. This is clearly advantageous for improving resolution, but in other applications the increased influence of the surface is nothing but problematic. Very simply molecules in such environments encounter the surface more frequently than in macroscale systems and thus are more likely to interact. This means that fouling and blocking of microchannel systems is a relatively common problem faced by microfluidic researchers, and may ultimately define the utility and application of a particular microdevice. A recent article by Rajendrani Mukhopadhyay in Analytical Chemistry provides an interesting discussion of the problems of fouling within microfluidic systems.1
Interviews with various researchers highlight the problems of dealing with ‘real’ samples such as blood within microfluidic systems. In addition to fouling through conventional molecular adsorption, other phenomena such as surface-activated triggering of reactions and inefficient microchannel design can contribute to the general problem and generate time-dependent flow characteristics. Not surprisingly, the article discusses various approaches to surface passivation but highlights problems associated with the characterisation and uniformity of surface coatings, stability of modified surfaces and the lack of generic approaches for the prevention of non-specific adsorption. Although no firm conclusions about how fouling problems can be addressed in a systematic manner are forthcoming, the article highlights a key issue that all microfluidic researchers have battled with over the past decade and provides an interesting summary of current opinion in the field.
Air breathing microfluidic fuel cell
Current developments in micro fuel cell technology have been driven by a need for high energy density power sources for portable applications. Interestingly, recent studies by various researchers have shown that the use of laminar flow within microfluidic devices can be highly efficient at compartmentalising fuel and oxidant streams within a single flow channel without the need of a physical barrier (most commonly a membrane). Such developments are significant since they allow definition of flexible fuel cell structures having high performance characteristics whilst reducing system complexity.The basic structure of a laminar flow-based fuel cell is relatively simple and typically involves an aqueous stream containing a fuel such as methanol or hydrogen and an oxidant stream containing for example hydrogen peroxide or dissolved oxygen co-flowing along a microfluidic channel whose sidewalls act to define the anode and cathode. Although satisfactory current densities can be obtained using such structures, performance using dissolved oxygen (the oxidant of choice) tends to be mass transfer limited due to the reduced diffusivity of oxygen in aqueous media. In addition, low oxygen concentrations make replenishment of cathodic depletion zones inefficient. To address these limitations Paul Kenis and co-workers at the University of Illinois at Urbana-Champagne and INI Power Systems, North Carolina have recently reported the integration of an air-exposed gas diffusion electrode within a laminar flow-based fuel cell.2 The fuel cell (Fig. 1) consists of a PMMA microfluidic device with a graphite plate coated with Pt nanoparticles acting as the anode. The gas diffusion cathode is fabricated from carbon paper also coated with Pt black nanoparticles. Two inlets feed the microchannel system, with a formic acid/sulfuric acid stream entering on the anode side and a sulfuric acid electrolyte stream separating the formic acid/sulfuric acid stream from the cathodic side.
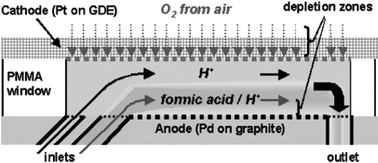 | ||
| Fig. 1 Schematic design of a laminar flow-based fuel cell with a porous, air-breathing gas diffusion electrode. (Adapted with permission. Copyright 2005, American Chemical Society.) | ||
When operated at high formic acid concentrations the mass transfer limitations characteristic of conventional laminar flow-based fuel cells are removed and the device exhibits a maximum current density of 130 mA cm−2 and a maximum power density of 26 mW cm−2. These values are significantly better than those reported in previous laminar flow-based fuel cells operating with an oxygen saturated aqueous stream. Further studies also demonstrate that the integration of the gas diffusion electrode in the laminar flow-based fuel cell allows efficient oxygen replenishment and thus high achievable current densities.
Interestingly, the authors also describe how such technology can be developed to provide for acceptable energy conversion efficiencies. It is expected that operation of multiple fuel cell units in parallel will be the most direct route. Furthermore, the issue of splitting and recirculating the fuel and electrolyte streams, whilst replenishing the fuel stream is currently being addressed using separator plate technology. Overall, the ability of gas diffusion electrodes to significantly enhance oxygen reduction rates is a major advancement for laminar flow-based fuel cell technology and it is likely that such an approach will be successfully used in conjunction with a variety of fuels and oxidant media.
Microfluidic sensing of liquids
Testing the freshness or quality of beverages such as milk and fruit juices has traditionally been best performed through human sensory tests. Although successful in many applications such tests are subjective at best. In recent years surface acoustic wave (SAW) microsensors have begun to provide a potential solution to the creation of electronic noses and tongues. In basic terms acoustic wave sensors are so named because their detection mechanism is based on an acoustic wave. As the acoustic wave propagates through or on the surface of the sensor, any changes to the characteristics of the propagation path affect the velocity and/or amplitude of the wave. Consequently, interaction of the acoustic wave with mass, density and dielectric properties in the propagating medium generates a sensing response. SAW sensors possess a number of attractive characteristics including small footprints, real time readout, robustness and relatively low fabrication costs.SAW sensors are made using standard photolithographic techniques. Typically, a piezoelectric substrate is first cleaned and polished. Next, a metal layer is deposited onto the substrate and lithographically patterned to create an interdigital transducer. Changes in the length, width, position, and thickness of the interdigital transducer are subsequently used to optimise the performance of the sensor. A problem with SAW devices of this kind arises from changes in the impedance of the interdigital transducer due to the presence of liquid on top of it. This in turn leads to unwanted sensitivity with respect to the electrical properties of the liquid. To address this issue Julian Gardner and associates at the University of Warwick and the University of Arkansas have reported the design, fabrication and testing of a smart tongue system for the analysis of liquids.3 Their sensor system incorporates a microfluidic cell positioned above an acoustic sensor on a LiTaO3 substrate, to prevent liquid spreading over the interdigital transducer surface.
The SAW device is fabricated on a 36° -rotated Y-cut X-propagating LiTAO3 substrate with Au/Cr metallization. It consists of an electrically shorted side that acts as a reference and an open side used for sensing. The microfluidic cell is fabricated using a microstereolithography system and involves the photo-polymerisation of a hexanediol diacrylate monomer using UV radiation. The cell has an internal volume of 10 ml and is positioned over the sensing area that lies between the interdigital transducers. This configuration prevents liquid spreading over the interdigital transducers and therefore prevents variations in input impedance. The authors subsequently use this set-up to analyse a range of fruit juices and beverages, through measurement of the amplitude and phase of the shorted and free surfaces. Using principle component analysis of the data, the authors are able to classify and discriminate between a wide range of fluids in real time. In conclusion, the studies demonstrate that the SAW microsensor can be used to detect chemical identity and other parameters such as pH, and due to the low unit cost of individual devices the technology should find use in the analysis of biological fluids and homeland security applications.
Microfluidic biomaterials
The fields of tissue engineering, drug delivery and implantable materials have all developed at increasingly rapid rates over the past decade. Key to developments in all these areas has been and continues to be the design and creation of new materials possessing appropriate chemical and mechanical properties.An important issue facing biomaterial scientists today is the ability to control and vary the concentration and loading of soluble species (such as metabolites and therapeutic agents) within synthetic materials. In living tissues such control is facilitated by the vascular system, and therefore the ability to mimic and create similar control systems within synthetic materials is an important and timely challenge. In an attempt to address this issue Abraham Strook and colleagues at Cornell University have recently developed a strategy for creating ‘microfluidic biomaterials’.4 These materials can be defined as biomaterials possessing embedded and defined microfluidic networks which are also permeable enough to allow diffusion of both small and large solutes.
In initial experiments the researchers fabricate a microfluidic system entirely within a calcium alginate hydrogel (a material commonly used in medical dressings). This is done using soft lithographic techniques. Briefly, gel slabs are structured by moulding against lithographically defined masters. Slabs are then bonded together by dissolving slab surfaces with sodium citrate, contacting the melted surfaces of different slabs and then applying calcium chloride to re-gel the interface. Fig. 2 shows an example of a sealed device. Using this approach the authors are able to create fluidic channels with dimensional cross sections as small as 25 × 25 microns. To characterise the physical characteristics of the fabricated structures, they are submerged in aqueous solutions containing a fluorescent solute (fluorescein or fluorescently labelled dextran). Pressure driven flow through microchannels can then be used to deliver or extract the solute via the fluidic network. Results of these experiments clearly demonstrate that microfluidic alginate biomaterial is adequately robust and impermeable to define distinct fluidic networks, but is permeable to the diffusion of both large (dextran) and small (fluorescein) solutes. Soluble species are able to diffuse between microchannels and the bulk, and since pressure-driven flow through the channels enhances mass transfer through the device, delivery and extraction operations can be performed in a controllable manner.
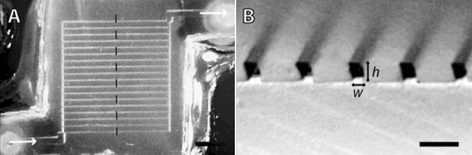 | ||
| Fig. 2 Optical micrographs showing top (a) and cross-sectional (b) views of a calcium alginate microfluidic biomaterial. The scale bars are 2.5 mm (a) and 500 µm (b). (Adapted with permission. Copyright 2005, American Chemical Society.) | ||
The conclusions of this proof of principle study are exciting since they demonstrate that the concentration of soluble species within a bulk material can be controlled with precision. Moreover, the authors see direct application of these ideas in the development of microfluidic scaffolds for tissue engineering.
Andrew J. de Mello
References
- R. Mukhopadhyay, When Microfluidic Devices go Bad, Anal. Chem., 2005, 77, 429A CAS.
- R. S. Jayashree, L. Gancs, E. R. Choban, A. Primak, D. Natarajan, L. J. Markoski and P. J. A. Kenis, Air-Breathing Laminar Flow-Based Microfluidic Fuel Cell, J. Am. Chem. Soc., 2005 DOI:10.1021/ja054599k.
- S. Jacesko, J. K. Abraham, T. Ji, V. K. Varadan, M. Cole and J. W. Gardner, Investigations on an Electronic Tongue with Polymer Microfluidic Cell for Liquid Sensing and Identification, Smart Mater. Struct., 2005, 14, 1010 CrossRef.
- M. Cabodi, N. W. Choi, J. P. Gleghorn, C. S. D. Lee, L. J. Bonassar and A. D. Stroock, A Microfluidic Biomaterial, J. Am. Chem. Soc., 2005, 127, 13788 CrossRef.
| This journal is © The Royal Society of Chemistry 2006 |
