A microfabricated electrical SPLITT system
Nithin
Narayanan
*a,
Avinash
Saldanha
b and
Bruce K.
Gale
a
aUtah State Center of Excellence for Biomedical Microfluidics, 50 South Central Campus Drive, Room 2240, Department of Mechanical Engineering, University of Utah, Salt Lake City, UT 84116, USA. E-mail: nithinn@gmail.com; Fax: +1 801-585-9826; Tel: +1 801-585-3176
bKLA-Tencor, San Jose, CA 95134, USA
First published on 5th December 2005
Abstract
A growing need for methods to analyze and prepare monodisperse nanoparticles on an industrial scale exists and may be solved by the application of split flow thin fractionation (SPLITT) at the microscale. Microfluidic systems of this type have the ability to separate nanoparticles with high precision in a continuous manner. A miniaturized SPLITT system can be fabricated using standard microfabrication technologies, works in a continuous mode, and can be used as a sample preparation instrument in a micro-total-analysis-system (μ-TAS). In this paper, a miniaturized electrical SPLITT system, which separates particles continuously based on electrophoretic mobility, has been characterized. The advantages of miniaturization have been elucidated. The various aspects of the micro SPLITT system discussed in this paper can be broadly classified into: micro SPLITT system design, fluidics modeling to refine the splitter arrangements, and experimental characterization of the SPLITT system. The design of the micro SPLITT system has been elucidated focusing on the two designs that were implemented. Fluid modeling, used to arrive at a new SPLITT design, was done using a commercially available CFD package to investigate behavior of the fluid in the microchannel with various splitter arrangements. Testing was done with nanoparticles of varying diameter and electrophoretic mobilities to verify the modeling results and demonstrate functionality of the SPLITT system. Particles eluted from both outlets of the SPLITT system were characterized using AFM and SEM to verify the function of the system.
Introduction
Nanoparticle fabrication techniques have been developing at a rapid rate with the recent worldwide interest in nanotechnology. The applications for these nanoparticles are highly diverse, ranging from lubricants to drug delivery systems. A major limitation in many nanoparticle manufacturing techniques is that the particles produced1–3 are highly polydisperse with regard to size or some other physical property. Most nanotechnology applications, though, require nanoparticles with optimal and consistent properties. A number of research teams are actively seeking methods to produce monodisperse nanoparticles directly or by applying a separation or filtration process to polydisperse particles. Size exclusion chromatography,4–6 molecular sieves,7 filters,8 flow cytometry,9,10 coulter counters,11 capillary electrophoresis12 and sedimentation13 are separation methods where monodisperse nanoparticles are separated from a polydisperse sample. Direct laser based ablation14 and electro spray techniques15 are methods where monodisperse nanoparticles are produced directly. While all of these techniques have uses in particular applications, all of these techniques have significant drawbacks in general applications that may include: low throughput, batch processing, clogging or fouling, complexity, expense, and material incompatibility.A family of techniques developed over the past 30 years may provide an answer to the challenge of producing monodisperse nanoparticles in a general way. Split flow lateral transport thin separation (SPLITT), developed by Giddings,16 is capable of high-resolution separation of colloids and nanoparticles suspended in solution. SPLITT systems operate by applying a field across a long, thin channel and forcing particles in the system to migrate across the channel, as shown in Fig. 1. This system has been demonstrated for a variety of particles ranging in size from 5 nm to several microns.17–29SPLITT does not require filters or a “stationary phase” to accomplish these separations. The SPLITT system, because of its ability to operate continuously, could potentially be used to separate and prepare nanoparticles with a variety of applications including: biological labelling,30 DNA delivery,31,32 cosmetics,33 drug delivery vehicles,34,35 carbon nanotubes,36,37 composite materials,38 and others. Other applications of SPLITT include: sorting and preparation of agglomerated proteins and starches for use in the food industry;39 sorting and analysis of clays in ground and surface water;40,41 sorting and preparation of monodisperse polymers; and sorting and sample preparation of viruses, spores, bacteria and biowarfare agents.42
 | ||
| Fig. 1 (left) The principle of electrical SPLITT, where separation occurs based on electrophoretic mobility of the nanoparticles across the thin dimension of the separation channel. The width of the channel is 1 mm. (right) Photograph of the top view of the microfabricated SPLITT system. | ||
SPLITT systems may also prove ideal in micro-total-analysis-systems (μ-TAS) as a continuous sample preparation system with high resolution and selectivity.42 Sample preparation is integral to a successful μ-TAS. Current macroscale analysis systems require sample preparation steps such as centrifugation that do not scale well to the microscale. Therefore, other techniques for sample preparation, which can be implemented on the microscale, need to be developed to realize an efficient μ-TAS system. SPLITT is one of the prospective techniques, because of its potential for continuous, selective, and high speed, binary separations in a microscale configuration. Accordingly, in this work we seek to implement a microscale SPLITT system that can be used as part of a μ-TAS or as part of a continuous nanoparticle manufacturing process.
As shown in Fig. 1, SPLITT channels are comprised of a long, thin flow channel with flow splitters at the outlet or at both inlet and outlet. The inlet splitter allows smooth merging of two inlet flows and the outlet splitter allows smooth collection of fractionated samples into different outlets without remixing. A field is applied along the channel thickness, perpendicular to the channel bulk flow direction to separate in a binary fashion the sample based on its susceptibility to the field. The SPLITT system can be operated either in a continuous mode or batch mode. Microfabricated SPLITT systems are expected to not only claim all of the normal micromanufacturing advantages such as reduced fabrication costs, smaller size (portability), lower power consumption, and reduced sample size, but they may also claim performance advantages such as reduced analysis, equilibration, and relaxation times, improved resolution, and higher field strength separations when compared to macroscale SPLITT systems.43,44 Miniaturization also allows for multiple separation channels for both parallel and serial processing, and the possibility of on-chip detection and signal processing, which would allow development of so-called “lab-on-a-chip” systems that would be able to analyze multiple samples accurately.45,46 Scaling advantages such as those associated with miniaturizing field flow fractionation systems, which have similar geometries, are also expected.47,48
Of particular interest to us are electrical SPLITT systems due to their ease of manufacture and operation, their flexibility in applying fields, their ease of integration with other components, and our experience with related electrical field flow fractionation (ElFFF) systems.47,48 Accordingly, we report here on our efforts to develop just such a system based on a miniaturized electrical SPLITT system, which separates colloids based on their electrophoretic mobility.
SPLITT theory
Driving forces for SPLITT include gravitational, centrifugal, thermal, and magnetic, but the focus of this work will be electrical. SPLITT fractionation can be performed in either transport or equilibrium mode,25 but all work presented here involves transport mode, which is presented in Fig. 1. To clearly understand SPLITT fractionation, it is helpful to define an imaginary plane between the two inlet flows called the inlet splitting plane (ISP) and another imaginary plane between the two outlet flows, called the outlet splitting plane (OSP). ISP and OSP positions are determined by the relative flowrates of the inlet and outlet streams. In transport mode, nanoparticles in liquid carriers are introduced into one inlet stream, while only a carrier solution is introduced into the other inlet at flowrates higher than the sample inlet stream. This procedure initially confines the samples in a small zone near the wall, which produces a consistent starting point for particles in the channel and generates better resolution (but reduces throughput). A field is applied along the channel thickness, perpendicular to the channel bulk flow direction, to separate the sample in a binary fashion based on its susceptibility to the field. Samples driven at relatively high transport rates (open circles in Fig. 1) move across the transport region and exit at outlet a′ of the system. Samples with lower transport rates (filled circles in Fig. 1) that fail to cross the transport region move along the upper wall and exit through outlet b′ of the SPLITT system. Accordingly, for electrical SPLITT, nanoparticles are separated based on their electrophoretic mobility. The electrophoretic mobility of a nanoparticle is related to the rate at which a particle moves when acted upon by an applied electric field. It is defined as the observed velocity (v) of a particle divided by electric field strength (E) in a given medium.25 Nanoparticles with an electrophoretic mobility greater than a certain critical value will exit through outlet a′ and the rest of the particles in the channel will exit through outlet b′. To determine this critical value, and other operating parameters for a microscale SPLITT system, a brief review of SPLITT theory will be completed.The mathematics that describe the operation of SPLITT systems have been published several times and are not expected to be significantly different for microscale systems other than diffusion, which will likely play a greater role. When the volumetric flowrate at inlet a, ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) (a), exceeds that of inlet b,
(a), exceeds that of inlet b, ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) (b), then the ISP moves upward from the inlet splitter, compressing the incoming sample against the upper wall into a thin band. Similarly, the OSP divides the flow into two separate streams that emerge at a′ and b′ and are controlled by the ratio of the outlet volumetric flow rates,
(b), then the ISP moves upward from the inlet splitter, compressing the incoming sample against the upper wall into a thin band. Similarly, the OSP divides the flow into two separate streams that emerge at a′ and b′ and are controlled by the ratio of the outlet volumetric flow rates, ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) (a′) and
(a′) and ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) (b′) respectively.25
(b′) respectively.25
The basic equation25 used to determine the percentage of samples exiting through the a′ outlet is
 | (1) |
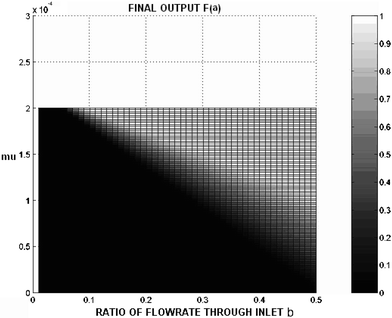 | ||
| Fig. 2 Surface plot of the predicted ratio of particles exiting through outlet a′ compared to the total number of particles in the system. The x-axis is the ratio of the flow rate through inlet b and the total flow rate entering the channel. For this simulation, the flow rate through a′ is the same as through inlet b. The total inlet and outlet flowrate is 10 mL h−1. The data is generated using eqn (1), an applied voltage of 1270 mV, and channel dimensions of 2 cm × 1 mm × 40 µm. | ||
Using eqn (1) and published experimental data,25 an electrical SPLITT channel was modeled to predict the output of the single SPLITT system for various combinations of flow rates, electric field and channel dimensions. The results of these simulations were used to prepare a design for a microscale system and to optimize its function.
When considering design of an electrical SPLITT channel, the reader should note that the effective field in an electrical SPLITT channel is much less than the generically calculated applied field due to double layer effects.47 Some concern may be expressed over whether such low values would be adequate for effective separation. It has been demonstrated that even at such low values48 effective separation does occur in microscale systems. The reduced field strength has been taken into consideration during modeling of the device and the effective field was considered to be limited to 1% of the applied field in all simulations.
Methodology
The various design constraints for a microsystem were taken into consideration while developing the design of the micro SPLITT system, and a bottom up approach was used in that the individual component requirements were determined and then the components were all combined into a complete system. The design constraints we considered most important included: material function, material compatibility, available manufacturing methods, existing interfaces, geometry and its effects on fluid flow, and cost. This section elucidates the methodology for building and testing the electrical SPLITT system.Microfluidic modeling
Since the geometry of the SPLITT system plays a significant role in both the fluid dynamics in the system, which determines the limits on operating performance, and on the manufacturing method for the system, which determines the ultimate robustness and cost of the device, a significant effort was devoted to determining the most simple geometry that could be implemented and still produce a practical SPLITT system. Accordingly, fluidic modeling was performed to compare the fluid dynamics of several simple (from a micromanufacturing standpoint) geometries to the ideal SPLITT setup. Two new geometries were examined, which included a SPLITT system with no splitter and one using offset channels as shown in Fig. 3. | ||
| Fig. 3 Various configurations of SPLITT channels examined in this work and compared to standard SPLITT systems with a splitting layer. | ||
The basic objective of the microfluidic analysis was to investigate the behavior of the fluid along the cross-section of the channel for the different splitter arrangements. A two dimensional model was used. The 2-D model was constructed and meshed using Gambit software. The meshed structures were then analyzed using Fluent 2D, which is a commercial CFD package. The material specified in Fluent was water. The flow was specified in terms of velocity at the respective inlets and outlets. The solver used was first order upwind. Upwind schemes are very stable and accurate.49 The streamline distribution was analyzed for the different splitter arrangements and comparisons between the geometries regarding the location and stability of the OSP and ISP were made.
The microfluidic simulations were performed using dimensions and flow velocities that were determined using the analytical models, such as those presented in Fig. 2. The CFD simulations were used to investigate the flow patterns in the SPLITT channel with different splitter arrangements. Since the offset splitter was more robust and practical to build, the simulation was used to compare the flow pattern of the offset splitter with the conventional SPLITT channel and determine if the offset SPLITT design was rational. A primary concern was mixing at the inlets. Microfabricated SPLITT systems with SU-8 splitters, in an offset splitter arrangement, and without splitters, were all built, simulated, and tested. From the CFD modeling and the tests with the different splitter arrangements, it was concluded that the offset splitter was the best solution for the microfabricated SPLITT system because of both the ease of manufacture and the results achieved in simulation and experiments, and this splitter arrangement was implemented in all the experimental results presented in this work.
Geometric design
To determine appropriate dimensions for a micromanufactured electrical SPLITT system, various combinations of flow rate, voltage and channel dimensions were modeled and the values for maximizing output (percentage of particles exiting through outlet a′) were derived using eqn (1). These optimal values of flow rates, voltage, channel dimensions and mobilities were used to design and experimentally test the microfabricated electrical SPLITT system. The channel dimensions chosen based on this analysis were: channel length of 2 cm, width of 40 µm and breadth of 1 mm. The voltage used in the analytical model was 1.2 V, though a variety of voltages and flow rates will be used in the experimental systems. Based on the simulation results using eqn (1), particles with mobilities in the range of 1.3 × 10−4 (cm2 V−1 s−1) to 4.5 × 10−4 (cm2 V−1 s−1) should be able to be characterized with the proposed SPLITT configuration, assuming the flow rates are in the few mL h−1 range.SPLITT system fabrication
Once the analysis of the fluidic models suggested that an offset splitter arrangement would be sufficient (as reported in the results), a design of the microscale SPLITT system was implemented using the offset splitter and the results of the geometric design efforts. Initially, the SPLITT system was based on silicon substrates,42 but subsequent systems were based on glass substrates to improve robustness. Sputtered gold electrodes on top of a 10 nm titanium layer were deposited on the glass. SU-8 was used to define the walls of the micro-channels and two identical substrates were bonded together with a slight offset to create the splitter. Some of the key fabrication steps are summarized in Fig. 4. | ||
| Fig. 4 Fabrication flow chart for the SPLITT channel using with glass substrates. | ||
SPLITT system setup
For the fluidic connections, the inlets and outlets of the SPLITT system were connected to two syringe pumps. The fluidic connections were all purchased from Scivex (Oak Harbor, WA) and included fluidics from the syringe pump to the microsystem and from the microsystem, through the detectors to the syringe pumps. Nanoport reservoir assemblies (N-131) were used at the inlet and outlet ports. 1/16″ diameter tubes with ferrule (P-200x) attachments connected the reservoir and the detectors. Finger tight peek nuts (F-130X) were used at the detector inlets. The injection ports (P-296) were used for sample injections using a T (P-633) attachment at the inlet. The tubing was connected to the syringes using a luer lock attachment. The fluidic connections were specifically designed to minimize dead volumes, provide leak proof joints, and fluidic interconnects which could be dismantled easily. A Hamilton µL syringe was used to inject the 0.4 µL sample into the SPLITT system. The electrical connections were made through a multimeter (Agilent 34401A) to the electrical leads on the microsystem. The multimeter was used for constant monitoring of the current in the system and was in series with the power supply. A standard DC power supply (Agilent E3642A) was used to apply the voltage to the SPLITT system. The detectors used were ESA (Model 520) absorbance detectors monitoring at 224 nm for polystyrene samples. A photograph of the test setup with UV/VIS detectors and syringe pumps is shown in Fig. 5. | ||
| Fig. 5 Test setup for the SPLITT system: (a) electrical input, pumps, and detectors; (b) close up of SPLITT channel showing electrical and fluidic connections. | ||
Characterization of the microscale SPLITT channel
The miniature SPLITT system was tested to characterize the operation of SPLITT at the microscale. The tests conducted can be subdivided into four phases. The first phase was done using acetone as the injected sample, to characterize the detectors. The second phase of testing was done to show control of particle elution at the outlets and the shifting of peaks with a change in polarity. These tests were done at the same flowrates at both the inlets. The third phase was done at varied flowrates and without a voltage. This was done to check the effect of varying flowrate on particle trajectories in the channel as well as diffusion effects. The fourth phase included testing the SPLITT system with varying flowrates and voltage. This final phase was done to characterize miniature SPLITT as a nanoparticle separation method. The particles tested were polystyrene with diameters ranging from 100 nm to 400 nm. The carrier fluid used was DI water. The voltages were varied in steps of 0.2 and a minimum of three runs were performed at each voltage step. The sequence of the voltages tested was varied to avoid time dependent effects on the results. When higher voltages were used, the system was allowed to discharge before recharging it with a lower voltage. The absorbance peaks from the detectors connected to outlet a′ and b′ were recorded. The area under the curve was determined for each of the absorbance peaks using the trapezoid rule. This area was used to determine the percentage of sample exiting through each of the outlets. The test setup of the SPLITT system for the peak height control experiments is similar to that shown in Fig. 5 , with the negative electrode on the b side of the system. The total spacing between electrodes was 40 µm.Detector and system characterization with acetone
Acetone samples were used to determine the basic detector response for both detectors, to characterize the elution peaks on both the detectors, and to calibrate the detectors relative to each other. The detectors were characterized for the same flow rates. The peak heights on both the detectors were determined for the same sample size and flow rate. The peak heights were compared and the results were recorded.Characterization of polarity effects
To test the effects of changing the polarity of the system, the same flowrates were used at both the inlets and the outlets, and the voltages or field strengths were varied. Polystyrene particles (290 nm) at 1% concentration by volume were injected into the channel. The polarities of the electrodes were reversed and the voltages applied ranged between ±1.4 V. Varying the voltage should control the number of particles exiting through two outlets. The flow rates on both the inlets were the same and were equal to 1 mL h−1.Channel characterization using monodisperse standards
Four different varieties of nanoparticles (130 nm, 190 nm, 290 nm, and 400 nm) were injected in the SPLITT system to characterize its capabilities. Some of these nanoparticles also had different chemical modifications such as carboxyl and amino terminations. The motivation for testing particles with different diameters and end groups was to establish a cut-off voltage for separation and determine a functional mixture that can be used to characterize the separation process with a micro SPLITT systemFor monodisperse sample characterization, the voltages as well as the flowrates through each input and output of the system were varied. All the varieties of nanoparticles were tested. After injection of a 0.4 µL sample, the percentage of particles exiting through each outlet was measured from the ratio of peak areas through each detector. The peak area was estimated numerically using the trapezoid rule. The ratio of the percentage of sample exiting through both the outlets were plotted to establish a cut off voltage for each particle size. The cut-off voltage is defined as the voltage at which the majority of the particles switch outlets and elute from the opposite outlet. Experimentally the cut-off voltage is determined to be the voltage where the ratio of the percentage of particles exiting through outlets b′ and a′ crosses unity.
Channel characterization using nanoparticle mixtures
A mixture of 108 nm and 220 nm amino coated particles was processed by the microscale SPLITT system, once the cut-off voltage for both particles was found. The particles were mixed in a 50 ∶ 50 ratio by volume and the concentration of the mixture used was 1% by volume. The 220 nm diameter amino-terminated polystyrene beads had a mobility of 4.48 × 10−4 (cm2 V−1 s−1) and the 108 nm diameter amino-terminated polystyrene beads had a mobility of 2.47 × 10−4 ( cm2 V−1 s−1). These mobilities were measured using a zeta potential analyzer and five trials were conducted for each particle type. The mobilites of the particles measured during the five trials differed by less than 1%, indicating that the mobility is reasonably stable. A 0.4 µL volume of the mixture was injected into the SPLITT channel and a voltage load of 1.2 V was applied. The polarity of the electrodes was not changed during this phase of testing. The measured absorbance over time at each outlet for the particle mixture was compared to the results for the individual particles to verify separation. The samples exiting at the outlets b′ and a′ were collected for scanning electron microscope (SEM) and atomic force microscope (AFM) analysis. After processing by the SPLITT system, the samples from the outlet were dried on to glass slides before they were imaged using an SEM (Hitachi S3000-N) and AFM. The SEM slide was coated with a thin layer of gold before imaging was attempted. The sample imaged was a collection of 10 runs performed at the same voltage.Results
The results from the microfluidic modeling are shown in Fig. 6. From the pathline plots for various SPLITT models; the behavior of the fluid in the SPLITT system with the offset splitter was found to be similar to the traditional setup, so an offset splitter was used in all of these experiments. Without a splitter, the ISP appeared somewhat unstable, which could lead to mixing and poor resolution in the system. The unstable streamlines could be an artifact of the simulation, but experiments with systems with no splitter or offset showed significant mixing, possibly related to the 3-D structure of the experimental system and the impossibility of building a perfectly symmetric channel with a sufficiently high aspect ratio at the inlets. Thus, the experiments confirmed mixing in the microscale systems without any splitter, so the design without any splitting mechanism was rejected. Thus, only data obtained using the offset SPLITT system is presented.![Fluidic modelling results for SPLITT system with various splitter configurations, SPLITT system with a splitter [a] SPLITT system without a splitter [b] and SPLITT system with an offset splitter [c] respectively. The locations of the ISP (inlet splitting plane) and OSP (outlet splitting plane) are labelled accordingly.](/image/article/2006/LC/b504936a/b504936a-f6.gif) | ||
| Fig. 6 Fluidic modelling results for SPLITT system with various splitter configurations, SPLITT system with a splitter [a] SPLITT system without a splitter [b] and SPLITT system with an offset splitter [c] respectively. The locations of the ISP (inlet splitting plane) and OSP (outlet splitting plane) are labelled accordingly. | ||
Characterization of polarity effects
The effect of varying the electric field strength and polarity in the channel was examined to establish a range of fields that can be used for separation of particles and to understand the effect of increased voltage on the percentage of particles leaving a specific exit. Note that the flowrates for all inlets and outlets are identical in this experiment.Fig. 7 shows the ratio of measured absorbance from outlet b to the absorbance from outlet a for 290 nm polystyrene particles. At −1.2 V, the ratio is 3.4, which shows that most of the particles are exiting through outlet b′. For a voltage of 1.1 V, the ratio is 0.45, which indicates that most of the particles are exiting through outlet a′. As expected, by reversing the polarity of the electrodes, the ratio of particles exiting from the two exits was reversed. The diffusion of particles at voltages less than 0.8 V for both the polarities was significant and swamped any apparent voltage effects. Therefore, the curve in Fig. 7 is not smooth between −0.8 V and 0.8 V but stays close to 1 i.e. the amount of sample exiting at both the outlets is almost equal. This result points out the need for developing a “transport region” (see Fig. 1) by providing different flow rates to the various inlets and outlets. Without the transport region, diffusion dominates the analysis and useful results can only be obtained at the extremes of voltage. Note that the tests at all voltages were repeated five times and the results plotted are averages from all the tests performed.
 | ||
| Fig. 7 Ratio of absorbance measured by the detectors sampling each outlet as a function of the applied voltage showing the effect of changes in the applied electric field on the elution of 290 nm PS nanoparticles. All inlet and outlet flow rates are identical. | ||
Channel characterization using monodisperse standards
Various polystyrene particles in the size range of 100 nm to 400 nm in diameter were characterized to determine a mixture that could be used for separation. These experiments were performed after generating a transport region by providing unequal flow rates as indicated in Fig. 1. For example, in Fig. 8, the cut-off voltage for 290 nm particles is 0.8 V. The cut-off voltage for the 190 nm –COOH coated particles from the data is 0.6 V as there is a moderate decrease in the percentage of particles eluting through the opposite outlet between 0.6 V and 1.1 V. For 470 nm particles, the ratio between exit percentages decreases as the voltage is increased from 0.5 V to 1.1 V. The cut off voltage from the data is 0.9 V, which is similar to the cut-off voltage for the 290 nm particles.![Ratio of percentage of particles eluting through outlet b′ to percentage eluting through outlet a′, with flowrate set at 1 mL h−1 for inlet a and 0.5 mL h−1 for inlet b for [a] 290 nm particles, [b] 190 nm COOH particles and [c] 470 nm particles.](/image/article/2006/LC/b504936a/b504936a-f8.gif) | ||
| Fig. 8 Ratio of percentage of particles eluting through outlet b′ to percentage eluting through outlet a′, with flowrate set at 1 mL h−1 for inlet a and 0.5 mL h−1 for inlet b for [a] 290 nm particles, [b] 190 nm COOH particles and [c] 470 nm particles. | ||
Channel characterization using nanoparticle mixtures
To demonstrate a separation, 220 nm amino particles and 108 nm amino coated particles were chosen due to their difference in cutoff voltage, as shown in Fig. 9. As indicated by the data, the cutoff voltage for the 220 nm particles is somewhat lower than for the 108 nm particles. From analysis of the experimental data, a voltage of about 1.2 V should provide for primary exit of 108 nm particles at exit b′, while primarily 220 nm particles should exit from a′. Accordingly, the voltage for the separation of the mixture was set at 1.2 V. From the experimental data, the voltage required to move the 220 nm amino coated particles was less than that required for the 108 nm amino coated particles, thus indicating that the 220 nm particles had a higher electrophoretic mobility when compared to the 108 nm particles. The electrophoretic mobility of the 220 nm particles was twice that of the 108 nm particles as measured using a zeta potential analyzer. | ||
| Fig. 9 Percentage of (a) 108 nm and (b) 220 nm particles eluting through the outlets vs. voltage. | ||
The detector responses after injection of 108 nm and 220 nm particles separately as well as the detector response for a mixture of the particles with an applied field of 1.2 V are shown in Fig. 10. By comparing the detector responses for 108 nm amino and 220 nm amino particles separately with the detector responses for a mixture, we clearly notice that a separation has potentially taken place, but to further confirm the extent of separation, some other form of verification was required. To verify that separation has occurred, we turned to SEM and AFM imaging of the eluted particles.
![[a] Absorbance peaks for 108 nm amino coated particles at 1.2 V; [b] absorbance peaks for 220 nm amino coated particles at 1.2 V; [c] absorbance peaks for the mixture of 108 nm and 220 nm amino coated particles.](/image/article/2006/LC/b504936a/b504936a-f10.gif) | ||
| Fig. 10 [a] Absorbance peaks for 108 nm amino coated particles at 1.2 V; [b] absorbance peaks for 220 nm amino coated particles at 1.2 V; [c] absorbance peaks for the mixture of 108 nm and 220 nm amino coated particles. | ||
SEM characterization
Fig. 11a is the image of samples collected from outlet a′ after the experiment using 108 nm and 220 nm in a mixture. There are a large number of 220 nm particles when compared to 108 nm particles, which shows that at an applied field of 1.2 V, more 220 nm particles moved towards the opposite outlet than did 108 nm particles, as expected. Analysis of Fig. 11b showing the sample collected from outlet b′, shows more 108 nm particles (including a large coagulation of small particles) than 220 nm particles, which, when combined with the image presented in Fig. 11a, indicates that a separation has taken place. Note that the magnification used for Fig. 11a was lower than in image b as it was difficult to locate a small region with an abundance of particles. | ||
| Fig. 11 SEM images from outlets a′ (a) and b′ (b) respectively. | ||
To quantify the level of separation, particle counts based on these and other images were made. The SEM pictures included in the paper are from one set of samples that were analyzed. For the total particle counts and the separation analysis, a total of five samples were analyzed. Based on visual particle counts from the SEM images, the total number of 220 nm particles exiting through outlet a′ was 185 and the total number of 108 nm particles exiting through outlet a′ was 84. For Outlet b′, the total number of 220 nm particles exiting through this outlet was 52 and the total number of 108 nm particles exiting through this outlet was 296. Looking specifically at Fig. 11a the number of 220 nm particles visible in the image from a scan is approximately 36 and the number of 108 nm particles visible is about 18. The percentage of 220 nm particles is 67%. After a visual particle count from Fig. 11b, the percentage of 220 nm particles is about 5%. From Fig. 11b we can conclude that the majority of the particles which eluted through outlet b′ were 108 nm particles, which reinforces the fact that the majority of the 220 nm particles eluted through outlet a′. The approximate total particle counts for both the outlets also indicate that the majority of the 220 nm particles eluted through outlet a′. Note that because the initial mixture was prepared by volume, and not by number, there should be about 4 times as many 108 nm particles in the sample as 220 nm particles. Using a weight analysis instead of a number analysis, the percentage of particles exiting for outlet a changes to 94% 220 nm particles and 6% 108 nm particles. For outlet b, the numbers become 30% for the 220 nm particles and 70% for the 108 nm particles. The resolution of separation for 220 nm particles was reduced due to diffusion of 108 nm particles towards the opposite outlet. The dispersive effects of diffusion are more prominent for particles with a lower diameter, as the diffusivity is inversely proportional to the diameter of the particle. The number of 220 nm particles at outlet b′ is comparatively less as the diffusion of 220 nm particles is comparatively low, but since they are the particles being moved across the transport region, some of the particles exit at b′ since the conditions are not ideal for collecting 100% of the 220 nm particles, and the voltage should not be increased since it will also increase the number of 108 nm particles that cross the transport region. Thus, a balance must be struck to optimize the separations.
From all the SEM images for outlet b′, the number selectivity of the system for 108 nm particles is approximately 90% of all particles. The number selectivity for outlet a′ is approximately 75% for 220 nm particles as there is a significantly large population of 108 nm particles exiting from this outlet. Similar data were obtained when the collected samples were characterized using an atomic force microscope (AFM).
Conclusion and future work
In this work, a microscale electrical SPLITT system was designed, fabricated, and characterized. The design and fabrication of the single SPLITT system was improved using the results from both a mathematical and a CFD model. An offset splitter channel design was determined to be the best for the microfabricated SPLITT system after exploring several design and fabrication options. The microscale SPLITT system was characterized with various sizes of polystyrene particles ranging from 100 nm to 470 nm and shown to generally operate as predicted by theory. Separation results were shown for a mixture of 108 nm and 220 nm amino coated particles. Scanning electron microscope and atomic force microscope images were used to verify the separation. The number selectivity for one particle compared to another was shown to be up to 90% for the SPLITT system using data from the SEM and AFM images of outputs from the SPLITT systemThe microfabricated electrical SPLITT system can be used to analyze and separate nanoparticles and may find applications in any environment requiring continuous separation or sample preparation. Microfabrication may allow for future serial and parallel devices which should augment the application of SPLITT on an industrial scale. By combining two SPLITT systems in series, the sample from outlet a′ can be sent through a second SPLITT system, which should achieve close to 100% purification.
Significant potential opportunities for improvement remain for microscale SPLITT systems. The SPLITT system was not tested with particles greater than 470 nm diameter. Examining larger particles may be a useful effort in the future as the diffusion effects decrease with the increase in particle diameter, meaning the SPLITT system should work better with larger particles, and the effect of diffusion on particle separation can be characterized. Instrumentation may be improved as well. Large dead volumes are not a problem with continuous separations, but they can be problematic in analytical situations where a sample is injected into the SPLITT system as a bolus. On–chip detection can be integrated with the SPLITT system, which should considerably reduce dead volumes and improve detection properties. Material changes, such as using graphite substrates instead of gold coated glass substrates may prove more robust. Higher fields can be applied with graphite substrates which will result in improved selectivity and efficiency of separation.50 PDMS substrates are ideal for microfluidic applications and various techniques can be used to make PDMS conductive, possibly making PDMS molding a viable technique for the manufacture of SPLITT systems. Overall, we expect microscale SPLITT systems to find a niche for sample preparation and processing of nanoparticles in the future, especially in lab-on-a-chip applications.
References
- Jun Yang, Jim Yang Lee, T. C. Deivaraj and Heng-Phon Too, Preparation and characterization of positively charged ruthenium nanoparticles, J. Colloid Interface Sci., 2004, 271, 308–312 CrossRef CAS.
- Zili Zhan, Wenhui Song and Denggao Jiang, Preparation of nanometer-sized In2O3 particles by a reverse micro emulsion method, J. Colloid Interface Sci., 2004, 271, 366–371 CrossRef CAS.
- T. Reihs, M. Müller and K. Lunkwitz, Preparation and adsorption of refined polyelectrolyte complex nanoparticles, J. Colloid Interface Sci., 2004, 271, 69–79 CrossRef CAS.
- Guor-Tzo Wei and Fu-Ken Liu, Separation of nanometer gold particles by size exclusion chromatography, J. Chromatogr., 1999, 836, 253–260 Search PubMed.
- Ch.-H. Fischer, H. Weller, L. Katsikas and A. Hengle, HPLC investigation of small CdS particles, Langmuir, 1989, 5(2), 429–432 CrossRef.
- Chi-Han Chiou, Gwo-Bin Lee, Hui-Ting Hsu, Pang-Wei Chen and Pao-Chi Liao, Micro devices integrated with microchannels and electrospray nozzles using PDMS casting techniques, Sens. Actuators, B, 2002, 86, 280–286 CrossRef.
- R. D. Stramel, T. Nakamura and J. K. Thomas, Photophysical and photochemical properties of CdS with limited dimensions, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 1287 RSC.
- Peter K. Kang and Dinesh O. Shah, Filtration of nanoparticles with dimethyldioctadecylammonium bromide treated microporous polypropylene filters, Langmuir, 1997, 13, 1820–1826 CrossRef.
- Ling Maa, Werner Scheersa and Peter Vandenberghe, A flow cytometric method for determination of absolute counts of myeloid precursor dendritic cells in peripheral blood, J. Immunol. Methods, 2004, 285, 215–221 CrossRef.
- D. A. Veal, D. Deere, B. Ferrari, J. Piper and P. V. Attfield, Fluorescence staining and flow cytometry for monitoring microbial cells, J. Immunol. Methods, 2000, 243, 191–210 CrossRef CAS.
- J. H. Nieuwenhuis, F. Kohlb, J. Bastemeijer, P. M. Sarro and M. J. Vellekoop, Coulter counter based on 2-dimensional liquid aperture control, Sens. Actuators, B, 2004, 102, 44–50 CrossRef.
- Won-Mi Hwang, Chang-Youl Lee, Doo Wan Boo and Joong-Gill Choi, Separation of nanoparticles in different sizes and compositions by capillary electrophoresis”, Bull. Korean Chem. Soc., 2003, 24.
- W. Peukert and C. Wadenpohl, Industrial separation of fine particles with difficult dust properties, Powder Technol., 2001, 118, 136–148 CrossRef CAS.
- J. Bosbach, D. Martin, F. Stietz, T. Wenzel and F. Träger Fachbereich Physik, Laser-based method for fabricating monodisperse metallic nanoparticles, Appl. Phys. Lett., 1999, 74, 2605–2607 CrossRef CAS.
- D. Gomez, S. Bingham, L. de Juan and K. Tangt, Production of protein nanoparticles by electrospray drying, J. Aerosol Sci., 1998, 29(5/6), 561–574 CrossRef.
- J. C. Giddings, A system based on split-flow lateral-transport thin (SPLITT) separation cells for rapid and continuous particle fractionation, Sep. Sci. Technol., 1985, 20, 749–768 CrossRef CAS.
- P. S. Williams et al., Continuous SPLITT fractionation based on a diffusion mechanism, Ind. Eng. Chem. Res., 1992, 31(9), 2172–2179 CrossRef CAS.
- J. Zhang et al., Separation of cells and cell-sized particles by continuous SPLITT fractionation using hydrodynamic lift forces, Sep. Sci. Technol., 1994, 29(18), 2493–2522 CrossRef.
- C. Contado et al., Separation of particulate environmental samples by SPLITT fractionation using different operating modes, Anal. Chim. Acta, 1997, 34, 99–110 CrossRef.
- S. Levin and J. C. Giddings, Continuous separation of particles from macromolecules in SPLITT cells, J. Chem. Technol. Biotechnol., 1990, 43–55.
- J. C. Giddings, Marcus Myers and C. B. Fuh, Analytical SPLITT fractionation: rapid particle size analysis and measurement of oversized particles, Anal. Chem., 1992, 64(24), 3125–3132 CrossRef CAS.
- S. Gupta, P. M. Ligrani and J. C. Giddings, Investigations of performance characteristics including limitations due to flow instabilities in continuous SPLITT fractionation, Sep. Sci. Technol., 1997, 32(10), 1629–1655 CrossRef CAS.
- P. S. Williams, Particle trajectories in field flow fractionation and SPLITT fractionation channels, Sep. Sci. Technol., 1994, 29(1), 11–45 CrossRef CAS.
- J. C. Giddings, Optimization of transport driven continous SPLITT fractionation, Sep. Sci. Technol., 1992, 27(11), 1489–1504 CrossRef CAS.
- C. B. Fuh, Split flow thin fractionation, Anal. Chem., 2000, 266–271.
- Y. Zhang, D. R. Emerson and J. M. Reese, General theory for flow optimisation of split-flow thin fractionation, J. Chromatogr. A, 2003, 1010, 87–94 Search PubMed.
- P. S. Williams, L. R. Moore, J. J. Chalmers and M. Zborowsk, Splitter imperfections in annular split-flow thin separation channels: effect on nonspecific crossover, Anal. Chem., 2003, 75, 1365–1373 CrossRef CAS.
- M. H. Moon, D. J. Kang, H. B. Lim, J. Eunohand and Y. Seokchang, Continuous fractionation of fly ash particles by SPLITT for the investigation of PCDD/Fs levels in different sizes of insoluble particles, Environ. Sci. Technol., 2002, 36, 4416–4423 CrossRef CAS.
- Y. Jiang, M. E. Miller, M. E. Hansen, M. N. Myers and P. S. Williams, Fractionation and size analysis of magnetic particles using FFF and SPLITT technologies, J. Magn. Magn. Mater., 1999, 94, 53–61 CrossRef.
- J. L. West and N. J. Halas, Applications of nanotechnology to biotechnology, Curr. Opin. Biotechnol., 2000, 11, 215–217 CrossRef CAS.
- D. Luo, E. Han, N. Belcheva and W. M. Saltzman, A self-assembled, modular DNA delivery system mediated by silica nanoparticles, J. Controlled Release, 2004 Search PubMed.
- J. Wang, Nanoparticle-based electrochemical DNA detection, Anal. Chim. Acta, 2003, 500, 247–257 CrossRef CAS.
- S. A. Wissing and R. H. Müller, Cosmetic applications for solid lipid nanoparticles (SLN), Int. J. Pharm., 2003, 254, 65–68 CrossRef CAS.
- Y. Dong and S.-S. Feng, Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs, Biomaterials, 2004, 25(14), 2843–2849 CrossRef CAS.
- A. K. Gupta and S. Wells, Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies, IEEE Trans. Nanobiosci., 2004, 3(1), 66–73 Search PubMed.
- S. Sato, A. Kawabata, M. Nihei and Y. Awano, Growth of diameter-controlled carbon nanotubes using monodisperse nickel nanoparticles obtained with a differential mobility analyzer, Chem. Phys. Lett., 2003, 382, 361–366 CrossRef CAS.
- Jitianu, T. Cacciaguerra, R. Benoit, S. Delpeux, F. Beguin and S. Bonnamy, Synthesis and characterization of carbon nanotubes–TiO2 nanocomposites, Carbon, 2004, 1147–1151 CrossRef.
- Q. L. R. Ji, M. Z. Zhang, M. Qiu and F. Klaus, Graft polymerization of vinyl monomers onto nanosized silicon carbide particles, Polym. Polym. Compos., 2002, 10(7), 531–539 Search PubMed.
- S. Levin, M. N. Myers and J. C. Giddings, Continuous separation of proteins in electrical split-flow thin (SPLITT) cells with equilibrium operation, Sep. Sci. Technol., 1989, 24, 1245–1258 CrossRef CAS.
- S. George, S. M. Steinberg and V. Hodge, The concentration, apparent molecular weight and chemical reactivity of silica from groundwater in Southern Nevada, Chemosphere, 2000, 40, 57–63 CrossRef CAS.
- C. Contado, F. Dondi, R. Beckett and J. C. Giddings, Separation of particulate environmental samples by SPLITT fractionation using different operating modes, Anal. Chim. Acta, 1997, 345, pp 99–110.
- A. Saldanha and B. K. Gale, Microfabricated electrical SPLITT system, in Proc. of the 10th International Symposium on Field Flow Fractionation, Amsterdam, Netherlands, July 2–5, 2002 Search PubMed.
- J. Khandurina and A. Guttman, Microscale separation and analysis, Curr. Opin. Chem. Biol., 2003, 7, 595–602 CrossRef CAS.
- S. Le Gac, S. Arscott, C. Druon, P. Tabourier and C. Rolando, Microfluidic system for high-throughput proteomics, Technical Proceedings of the Nanotechnology Conference and Trade Show, 2003, 1, 70–73 Search PubMed.
- S. Merugu, N. Narayanan and B. K. Gale, High throughput separations using a microfabricated serial electric SPLITT system, in Proc. of MicroTAS 2003, Lake Tahoe, CA, Oct 5–9, 2003 Search PubMed.
- S. Meregu, N. Narayanan and B. K. Gale, Serial electric SPLITT microsystem, in Proc. of the 11th International Symposium on Field Flow Fractionation, Cleveland, Ohio, Oct 7–10, 2003 Search PubMed.
- B. K. Gale, K. D. Caldwell and A. B. Frazier, A micromachined electrical field-flow fractionation system, IEEE Trans. Biomed. Eng., 1998, 45(12), 1459–1469 CrossRef CAS.
- B. K. Gale, K. D. Caldwell and A. B. Frazier, Scaling effects in a micromachined electrical field—flow fractionation system, in Proc. of 1999 First Joint BMES/EMBS Conference, Atlanta, GA, October 13–16, 1999, p. 842 Search PubMed.
- J. H. Ferziger and M. Peric, Computational methods for fluid dynamics, Springer Verlag, 1999.
- N. Tri, K. Caldwell and R. Beckett, Development of electrical field-flow fractionation, Anal. Chem., 2000, 72, 1823–1829 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
