Atmospheric pressure chemical vapor deposition of WSe2 thin films on glass—highly hydrophobic sticky surfaces
Nicolas D.
Boscher
,
Claire J.
Carmalt
and
Ivan P.
Parkin
*
Department of Chemistry, University College London, 20 Gordon Street, London, UK. E-mail: i.p.parkin@ucl.ac.uk
First published on 25th November 2005
Abstract
Atmospheric pressure chemical vapour deposition (APCVD) of tungsten selenide films on glass substrates was achieved by reaction of diethyl selenide with WCl6 at 500–650 °C. X-Ray diffraction showed that the WSe2 films were crystalline with cell constants close to those expected—some preferred orientation was noted at higher deposition temperature. Energy-dispersive analysis by X-rays (EDAX) gave a W ∶ Se ratio close to 1 ∶ 2 for all the films formed at 550 °C. The films were matt black in appearance, were adhesive, passed the Scotch tape test but could be scratched with a steel scalpel. SEM showed that the films were composed of either plate or needle like crystals which become longer and thicker with increasing deposition temperature. The films were highly hydrophobic with contact angles for water droplets in the range of 135–145°. Furthermore these surfaces were highly adherent for water droplets—that did not roll or slide even at a tilt angle of 90°.
Introduction
Tungsten diselenide has the hexagonal MoS2-structure type and consists of single Se and W layers sandwiched with a thickness of about 3.3 Å. There is strong covalent bonding within the Se–W–Se layers but only fairly weak van der Waals interactions between neighbouring sandwich layers.1 Only one polytype of tungsten diselenide has been reported: 2H-WSe2. Apart from WSe2, the only other reported tungsten selenide is the X-ray amorphous WSe3.2 Tungsten diselenide is a semiconductor with a band gap in the range 1.2–2 eV—dependent in part on variations in non-stoichiometry from the ideal W ∶ Se 1 ∶ 2. This black or grey odourless material has good thermal stability and a high melting point. Tungsten diselenide shows significant anisotropy in its physical properties.3Due to its high optical absorption, the layered arrangement between the cations, high resistance against photo-corrosion and the magnitude of its band gap, tungsten diselenide is an important material in photo-electrochemical conversion and photovoltaic solar energy conversion. Tungsten diselenide also plays an important role in a number of technologies like high temperature solid lubrication and rechargeable batteries.3–5
Single crystals of tungsten diselenide have been grown via a vapour transport technique, employing SeCl4, chlorine or iodine as a transport agent. Nanoparticles of WSe2 can be synthesised by a chemical reaction between tungsten carbonyl and selenium dissolved in para-xylene solution. Tungsten diselenide thin films can be obtained by many processes for example reaction of WO3 thin films in a H2Se atmosphere, solid state reaction between the constituents sequentially deposited in thin film form, electrodeposition, rf-sputtering, electrodeposition and van der Waals rheotaxy.5–9
There are very few papers that report the chemical vapour deposition (CVD) of WSe2 and these use difficult to handle WF6 and H2Se as precursor agents that produce HF as a byproduct of the reaction.10–12 The films were grown on different substrates between 300–700 °C and were reported to be stable and crystalline with a preferential orientation. A platelet microstructure with crystallites of 1 µm was observed. The optical absorption of CVD prepared thin films closely matched that of single-crystal WSe2.10–12
Contact angles for water droplets have been measured for a number of CVD prepared coatings—especially for metal oxides, sulfides and selenides.13,14 Titanium dioxide films show hydrophilic behaviour with an individual droplet smearing out over the surface, on exposure to UV radiation these films can become super-hydrophilic with contact angles between 0–5°. Metal selenide and sulfide surfaces tend to have water contact angles between 30–70°. It is highly unusual for a CVD prepared film to show contact angles above 90° on flat surfaces. Aligned vertical carbon nanotubes and nano-forests have water contact angles of 90–100°. These contact angles can be increased by coating with various low energy organic surfaces such as PTFE or polystyrene. Commercial spectacle lens manufacturers often create very hydrophobic surfaces by coating the lens with various polymers—these create hydrophobic surfaces with typical values of 80–100°. The advantage here is that the lens are somewhat water repellent and easier to clean. Very high contact angles for water droplets have been achieved on surfaces by two means; increasing surface roughness and by coating with a very low surface energy material—such as a fluorinated polymer.15 Typically, for the best polymer coated flat surface a contact angle of 120° can be achieved whilst for micro-engineered or patterned surfaces in combination with a low surface energy polymer contact angles in excess of 150° have been achieved. These films have been called super-hydrophobic and mimic the effect found in nature for lotus plants and butterfly wings where the patterned microstructure in combination with a waxy surface makes the underlying material water repellent.15 Indeed these surfaces have unique properties: water droplets both elastically bounce off and self-clean the surface. These surfaces are extremely slippery for water droplets and very low tip angles—sometimes 1° or less—are sufficient for the water droplets to roll off the surface. Very sticky super-hydrophobic surfaces are extremely rare—the first such surface was claimed by Jiang et al. in 2005.16 That work used a template assisted route—an anodised alumina membranes was used as a template for the growth of polystyrene nanorods, after dissolving away the alumina with concentrated base the polystyrene forest gave a contact angle in excess of 150°, furthermore the droplets remained adhesive to the surface even at a 90° tip angle and with relatively large droplet sizes of water (8 mg).
Here we present the CVD of WSe2 thin films from reaction of WCl6 and diethyl diselenide. The motivation for this work was to produce tungsten selenide films on glass and to use precursors without the high toxicity and with better handling ability than H2Se. We also looked into the potential functional properties of the materials—in particular the water-wetting ability of the coating and its extreme resistance to water run-off.
Experimental
Caution
It should be noted that the CVD of WSe2 could produce H2Se, which is highly toxic and malodorous. All the experiments should be conducted in a fume cupboard and the gas from the CVD process treated with bleach to destroy the possible presence of H2Se (the exhaust gas could also be absorbed on a commercial graphite filter, with an attendant copper sulfate bubbler to check for efficacy).Chemical vapour deposition studies
Deposition experiments were carried out under a dinitrogen atmosphere (99.99%) on glass substrates using a horizontal-bed cold-wall atmospheric-pressure CVD reactor. The glass substrate was SiO2 coated (ca. 50 nm thick SiO2 barrier layer—prevents diffusion of ions from the glass into the film) standard float glass (Pilkington, UK) 15 cm × 4 cm × 0.3 cm. The substrate was heated by a graphite block and the nitrogen carrier gas was preheated to 110 °C by being passed along 2 m lengths of coiled stainless steel tubing inside a tube furnace. The gases in the reaction were made to pass over the heated substrate and confined in position by a top plate that was ca. 4 mm above the substrate. After passage through the reaction chamber the gas stream was scrubbed of selenium containing compounds and vented inside a fume cupboard.Tungsten hexachloride was obtained form Aldrich Chemical Co. and diethyl selenide was supplied by Strem. They were both used without further purification. The diethyl selenide and WCl6 were placed into two different stainless steel bubblers, which were both heated by an external jacket.13 The WCl6, and diethyl selenide bubblers were heated to 260 °C and 70 °C respectively. They were both introduced into gas streams by passing hot N2 through the bubblers. Flow rates of nitrogen through the WCl6 bubbler and the diethyl selenide bubbler were kept within 1.5–4.0 L min−1 and 0.2–0.3 L min−1 (Table 1) and the flow rate through the mixing chamber was between 1.9 and 4.6 L min−1 for all depositions.
| Deposition Temp. | XRD, lattice constants/Å | EDAX |
|---|---|---|
| Flow rates through the plain line, the diethylselenide bubbler and the WCl6 bubbler:a 0.2 L min−1, 0.2 L min−1, 1.5 L min−1;b 0.3 L min−1, 0.3 L min−1, 4.0 L min−1. | ||
| 450 °Ca | — | No film |
| 500 °Ca | 2H-WSe2; a = 3.26, c = 12.84 | WSe1.6 |
| 550 °Ca | 2H-WSe2; a = 3.26, c = 12.84 | WSe1.6 |
| 650 °Ca | 2H-WSe2; a = 3.27, c = 12.82 | WSe1.7 |
| 450 °Cb | — | No film |
| 500 °Cb | 2H-WSe2; a = 3.26, c = 12.84 | WSe1.8 |
| 550 °Cb | 2H-WSe2; a = 3.27, c = 12.82 | WSe1.9 |
| 600 °Cb | 2H-WSe2; a = 3.26, c = 12.84 | WSe2.4 |
| 650 °Cb | 2H-WSe2; a = 3.26, c = 12.86 | WSe2.6 |
The reaction of diethyl selenide was studied for the range 250–600 °C. However depositions were only noted at temperatures in excess of 450 °C. Deposition time for all experiments was one minute. At the end of the deposition, the bubbler line was closed and the substrate was cooled under nitrogen in the reactor. Then the substrates were handled briefly in air before storage in a dry oxygen-free nitrogen atmosphere in a glove box. It was subsequently found that the samples were air and water stable, so this procedure was not necessary. Analyses were performed for each sample on a 2 cm × 4 cm band cut 2 cm from the front of the substrate.
Film analysis
X-Ray diffraction patterns were measured on a Siemens D5000 diffractometer using monochromated Cu Kα1 radiation (Kα1 = 1.5406 Å). The diffractometer used glancing incident radiation (1.5°). The samples were indexed using Unit cell and compared to database standards. EDAX was obtained on a Philips XL30ESEM instrument and SEM was obtained on a JEOL 6301 instrument. UV-Vis-NIR spectra were recorded in the range 190–1100 nm using a Helios double beam instrument. Reflectance and transmission spectra were recorded between 300 and 1200 nm by a Zeiss miniature spectrometer. Reflectance measurements were standardised relative to a rhodium mirror and transmission relative to air. Raman spectra were acquired on a Renishaw Raman System 1000 using a helium–neon laser of wavelength 632.8 nm. The Raman system was calibrated against the emission lines of neon. X-Ray photoelectron spectroscopy was undertaken using a VG ESCALAB 220I XL instrument with focussed (300 µm spot) monochromatic Al-Kα radiation at a pass energy of 20 eV. Scans were acquired with steps of 50 meV. A flood gun was used to control charging. Binding energies were referenced to an adventitious C 1 s peak at 284.6 eV (this peak is due to residual pump oil used in the XPS high vacuum system, but is removed on the first argon ion etching). Argon sputtering was used for approximately 1 min in a rastering mode in order to remove surface contamination. Contact angle measurements were determined by measuring the spread of a known volume of water and by goniometer measurements. Photographs were obtained of the water droplets using a high resolution digital Pentax camera; methylene blue was added to the water to aid visualisation prior to taking photographs.Results and discussion
Preparation and characterisation
Atmospheric pressure CVD reactions of WCl6 with diethyl selenide were studied on glass substrates at 250–650 °C. Deposition was observed on both the top plate and the substrate–all of the analysis presented in this paper is for the adherent film that formed on the substrate.At a substrate temperature below 450 °C, no films were formed. At a substrate temperature of 500 °C and above a matt black film was formed that passed the Scotch tape test, however the films were easily scratched with a steel scalpel. The films were insoluble in common organic solvents but were quickly decomposed in dilute nitric acid and bleach, yielding H2Se. The films were air stable and water stable. The extent of film coverage was dependent on the deposition temperature. For a deposition temperature of 500 °C, uniform thickness films were grown across the entire length of the substrate. With higher deposition temperatures, the growth profile was concentrated closer towards the leading edge of the substrate such that at a deposition temperature of 650 °C only the first 6 cm of the substrate was coated. EDAX showed that all the films contained tungsten and selenium with a W ∶ Se ratio that depended on the deposition rate and flow conditions, Table 1. At higher temperatures films with a stoichiometry WSe2.5 were formed whilst at 500 °C films with stoichiometry WSe1.5 were formed with no chlorine or carbon detected. Furthermore oxygen levels within the films were virtually undetectable (typically 1 atom%). The films showed uniformity in composition along the length of the substrate and across its width for any one film by both wide area (ca. 10 × 10 microns) and point EDAX analysis.
Scanning electron microscopy images of the films produced at 650 °C shows grains, which exhibit many very thin needles with lengths of a few hundred nanometers; the SEM images of the films deposited at 550 °C and 600 °C show slightly fewer, thicker needles with lengths of approximately a hundred nanometers. The SEM images of all the films shows either plate-like or needle-like crystallites, which seem to be largely orientated perpendicularly or radially away from the substrate, Fig. 1.
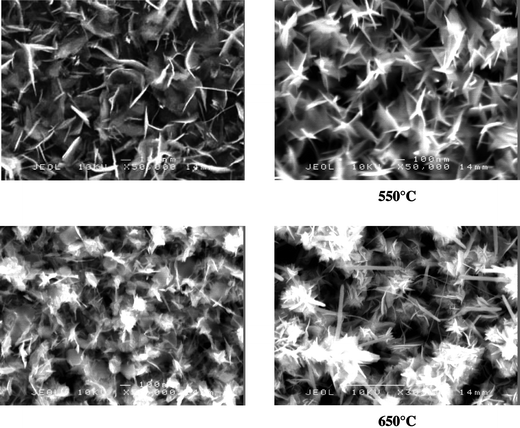 | ||
| Fig. 1 Scanning electron micrographs of the films produced from the APCVD of WCl6 and diethyl diselenide at 500–650 °C. | ||
The XPS of a film deposited from WCl6 and diethyl selenide at 650 °C shows that there are two tungsten environments present in the film, Fig. 2. The stronger peaks correspond to W 4f7/2 = 31.9 eV and W 4f5/2 = 34.0 eV of the tungsten diselenide.17 The second tungsten environment is due to oxide contaminations, which were both detected only at the surface of the sample (at ca. 10%). The reported peaks for the tungsten in WO3 are W 4f7/2 = 35.7 eV and W 4f5/2 = 37.8 eV.17 The selenium peak for the same film shows that there is only one selenium environment present in the films. The presence of the two peaks at 54.0 eV and 54.9 eV is due to Se 3d, Fig. 3.
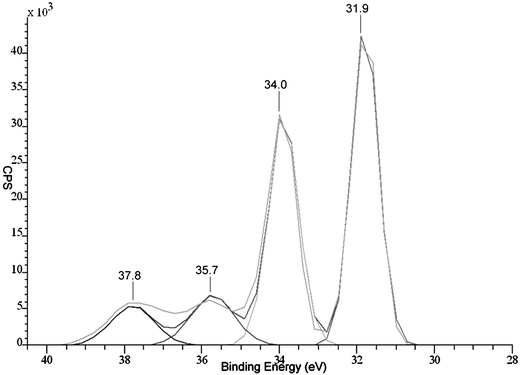 | ||
| Fig. 2 X-Ray photoelectron spectrum of the W 4f peaks from the surface of a film deposited from the reaction of WCl6 and diethyl selenide at 650 °C. | ||
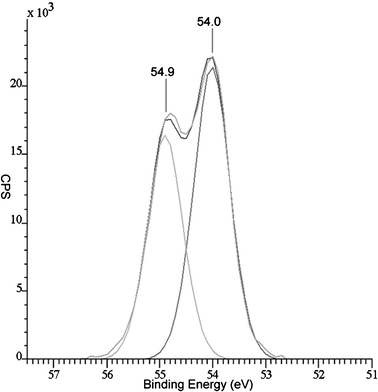 | ||
| Fig. 3 X-Ray photoelectron spectrum of the Se 3d peaks from the surface of a film deposited from the reaction of WCl6 and diethyl selenide at 650 °C. | ||
The XRD data show that all the films were crystalline and show a good match with the reported pattern for 2H-WSe2. The lattice parameters are calculated as a = 3.32 Å and c = 12.80 Å, which are in good agreement with the reported values for WSe2 (JCPDS File No. 006-0080), Fig. 4a. The films produced at temperatures above 550 °C showed no preferred growth orientation, whereas the film produced at 500 °C was strongly orientated along the (002) axis, Fig. 4d. A small reflection present in all the XRD patterns of the WSe2 films at 2θ = 24.9° is attributed to WO3.18 Notably this peak did not increase on remeasuring the films after storage in air for six months—this coupled with no change in the UV-vis spectra over the same time period indicates that the films are stable to atmospheric oxidation at room temperature.
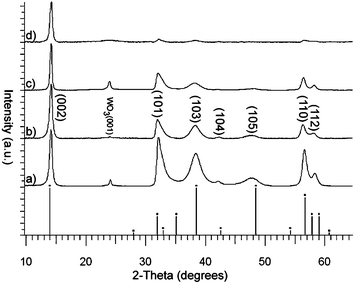 | ||
| Fig. 4 The XRD patterns obtained for the film formed on the glass from the APCVD of WCl6 and diethyl selenide at 650 °C (a), 600 °C (b), 550 °C (c) and 500 °C (d). | ||
Raman analysis of all films, irrespective of temperature or flow rates, showed the same pattern.13,19 The principle bands were at 180 cm−1 and a double peak around 250–256 cm−1; these bands correspond to the frequencies of the Raman active A1g, E2g1 and, E1g modes of the 2H-WSe2 polytype.
The Raman, XRD and XPS analytical analyses of the films are consistent with the formation of the 2H polytype of WSe2. The EDAX measurements show some variation in tungsten to selenium stoichiometry depending on deposition temperature, however they also showed negligible oxygen. The films do contain a small amount of tungsten oxide, as a small peak was seen in the XRD and XPS indicated ca. 10% oxide at the surface. The oxygen content found was not sufficient to give the sub-stoichiometric films a metal to non-metal ratio of 2 ∶ 1. It is known that tungsten diselenide can tolerate non-stoichiometry in the 2H form.
Contact angle measurements
The water contact angle measurements for the CVD prepared tungsten selenide films show that the films were highly hydrophobic for all the deposition temperatures with values in the range 135–145°, Fig. 5. The contact angle was lowest, 135°, for the films formed at 500 °C and increased with substrate deposition temperature to 145° for the film deposited at 650 °C. The contact angle measurements were consistent across the whole coated glass plate (10 independent measurements), and did not change on weekly inspection over a period in excess of two months. These contact angle measurements indicate that the films are unlikely to have a predominantly oxide-terminated surface such as WO3 because such a film would be expected to be hydrophilic (indeed XPS indicated that the surface layers had ca. 10% oxide). It is well known that the roughness or texture of a film can influence the contact angle of a water drop at its surface. To our knowledge, an unpatterned material has never been reported before this work with a contact angle higher than 125°. It should be noted that the contact angle of a micro-textured material (typically mechanically constructed groves or pillars) can jump to above 150°. The exceptionally high contact angles of the films prepared here suggest extreme roughness, which is confirmed by the SEM pictures of the films which show needle like points radiating away from the surface. Tilting experiments show that the water droplets cling to the surface of the film despite the highly hydrophobic character of the films. Indeed small droplets of water (4 mg) remain rounded with a tipping angle of 90° and do not roll or slide even at this acute angle. In fact the droplets evaporate instead of moving across the surface. This is exceptional—very hydrophobic films typically have extremely low tipping angles of a few degrees before the droplet slides off of the surface.15 Furthermore large water droplets of 50 mg and above in size remain stationary on the surface at the 90° tilt angle. The surface can even be tipped upside down and the water droplets remain adhered to the surface, Fig. 5.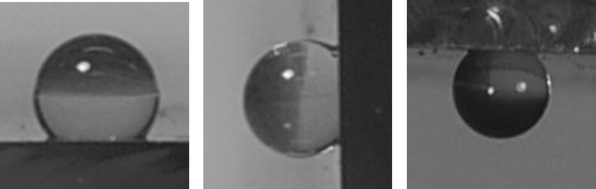 | ||
| Fig. 5 Water droplets (1 mg) on the tungsten selenide thin films. Left: water droplet on a film at 0° tipping angle; middle: water droplet at a 90° tilt angle; right: water droplet suspended upside down on the film. The water droplets contained a small amount of methylene blue dye to aid visualisation, this was not found to alter the contact angles on the droplet sizes used. | ||
The sticky super-hydrophobic state, as referred to by Quéré, has been described by Wenzel’s theory.20 In the Wenzel model, the liquid droplet retains contact at all points with the hydrophobic solid surface increasing the adhesion of the droplet. To minimise the surface contact with the hydrophobic film, the droplet will increase its contact-angle, enhancing the hydrophobic property of the film. The Wenzel model is in contrast to the Cassie–Baxter model21 where the water droplets do not penetrate the surface porosities. In this case, drops rest partially on air contained in the porosity and the peaks of the film protrusions, which lead to a more hydrophobic state and a slippery surface, typically with low tip angles before the droplets move across the surface. Hence the films produced here are thus best described by Wenzel theory and suggest that it is the surface microstructure that is dominating the materials behaviour—as contact angles greater than 125° have not been reported due to chemically modified surfaces (fluoro-terminated surfaces). Furthermore we have investigated other layered metal diselenide and disulfide materials such as ME2 (M = Ti, Nb, Sn) prepared by CVD methods and these materials typically have water contact angles between 5–100°.13 These materials however all have a sheet like microstructure—often orientated perpendicular to the substrate—but with relatively long crystal plate axis in contact with the water droplet. The tungsten diselenide films prepared here are different in that they have a different shape to the microstructure with needle like or point like protrusions from the surface. This kind of surface architecture has been described as an ideal theoretical surface for making highly rough hydrophobic surfaces. In effect the water droplets have very minimal contact points with the surface. Furthermore this extreme roughness accounts for why the water droplets do not slide down the surface even when exposed to very large tipping angles. Nature has produced many such hydrophobic surfaces—for example lotus leaves have numerous small spiky protrusions on the leaf surface which are covered in a wax like substance and are examples of slippery hydrophobic surfaces. It is possible that the layer structure exhibited by tungsten selenide enhances the hydrophobic character. However in over 50 different APCVD systems that we have investigated for a range of oxide, sulfide and selenide materials we have never seen a microstructure that resembles the one that we have produced here for WSe2 and indeed the rough spiky surface produced here is probably the primary reason for the high hydrophobicity.
Classically very hydrophobic surfaces have been of use for self-cleaning. This is not the case for the tungsten selenide films reported here because the water droplets do not move across the surface even at the maximum tilt angles, unless they are very large in size. However very sticky hydrophobic surfaces could readily be used as surfaces for biological micropipetting and assay where recovery of material from the surface is enhanced from a hydrophobic plate.16 However the use of a metal selenide surface for such a purpose, with a potential associated toxicity issue, may limit application. Compared to previously reported adhesive hydrophobic films by Jiang et al.16- the films made here were made in a single processing step and do not have a low energy polymer surface. Furthermore the surfaces are extremely resistant to water sliding and very large water droplets of 50 mg or more remain stationary on the surface even with a tip angle of 90°, whilst for Jiang's work droplets in excess of 8 mg move across the surface. The tungsten diselenide films made here are not quite in the class of material that would be described as super-hydrophobic—this requires a contact angle in excess of 150°, however they are significantly more hydrophobic than a wide range of commercially made polymer coatings. The fact that these can be made by CVD opens up many possibilities for patterned substrates, simply by using a masking technique.
Conclusion
Tungsten diselenide films have been deposited on glass substrate between 500 and 650 °C from the APCVD reaction of WCl6 and diethyl selenide. The films did not contain any chlorine contamination, were stable in air and were insoluble in common organic solvent. Films grown above 550 °C show grains which exhibit many very thin needles that matched the reported pattern for 2H-WSe2. The films grown at 500 °C show plate crystallites orientated perpendicular to the substrate. This perpendicular orientation is confirmed by the XRD pattern, which shows preferred growth orientated along the (002) axis. The films show Wenzel type hydrophobic character—water forms beads on the surface that do not roll or slide even at very high tipping angles.Acknowledgements
Dr G. Reid, Professor W. Levason and Dr A. Hector are thanked for useful discussions on related projects. The EPSRC is thanked for financial support.References
- D. Voss, P. Kruger, A. Mazur and J. Pollmann, Phys. Rev. B, 1999, 60, 14311 CrossRef CAS.
- H. Haraldsen, Angew. Chem., Int. Ed., 1966, 5, 51.
- T. Tsirlina, S. Cohen, H. Cohen, L. Sapir, M. Peisach, R. Tenne, A. Matthaeus, S. Tiefenbacher, W. Jaegermann, E. A. Ponomarev and C. Levy-Clement, Sol. Energy Mater. Sol. Cells, 1996, 44, 457 CrossRef CAS.
- A. H. Reshak and S. Auluck, Phys. Rev. B, 2003, 68, 195107 CrossRef.
- A. Khelil, H. Essaidi, J. C. Bernede, A. Bouacheria and J. Pouzet, J. Phys.: Condens. Matter, 1994, 6, 8527 CrossRef CAS.
- S. M. Delphine, M. Jayachandran and C. Sanjeeviraja, Mater. Chem. Phys., 2003, 81, 78 CrossRef CAS.
- J. Jebaraj Devadasan, C. Sanjeeviraja and M. Jayachandran, Mater. Chem. Phys., 2002, 77, 397.
- R. Tenne, E. Galun, A. Ennaoui, S. Fiechter, K. Ellmer, M. Kunst, C. Koelzow, C. Pettenkofer, S. Tiefenbacher, R. Scheer, H. Jungblut and W. Jaegermann, Thin Solid Films, 1996, 272, 38 CrossRef CAS.
- N. Guettari, J. Ouerfelli, J. C. Bernede, A. Khelil, J. Pouzet and A. Conan, Mater. Chem. Phys., 1998, 52, 83 CrossRef CAS.
- D. E. Miller, Energy Res. Abstr., 1986, 11 Search PubMed.
- R. Bourezg, G. Couturier and J. Salardenne, J. Chim. Phys. Phys.-Chim. Biol., 1991, 88, 2021 CAS.
- W. Sienicki, Pol. J. Chem., 1992, 66, 1139 CAS.
- C. J. Carmalt, E. S. Peters, I. P. Parkin, T. D. Manning and A. L. Hector, Eur. J. Inorg. Chem., 2004, 4470 CrossRef CAS.
- A. Mills, N. Elliott, I. P. Parkin, S. A. O'Neill and R. J. H. Clark, J. Photochem. Photobiol., A, 2002, 151, 171 CrossRef CAS.
- A. Nakajima, K. Hashimoto and T. Watanabe, Monatsh. Chem., 2001, 132, 31 CrossRef CAS.
- M. Jin, X. Feng, L. Feng, T. Sun, J. Zhai, T. Li and L. Jiang, Adv. Mater., 2005, 17, 1977 CrossRef CAS.
- Practical Surface Analysis-Auger and X-ray photoelectron spectroscopy, ed. D. Briggs and M. P. Seah, Wiley, Chichester, 1999 Search PubMed.
- ICDD powder diffraction file 20-1324.
- D. G. Mead and J. C. Irwin, Can. J. Phys., 1977, 55, 379 CAS.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
| This journal is © The Royal Society of Chemistry 2006 |
