Capabilities of laser ablation—inductively coupled plasma mass spectrometry for (trace) element analysis of car paints for forensic purposes
Isolde
Deconinck
a,
Christopher
Latkoczy
b,
Detlef
Günther
b,
Filip
Govaert
c and
Frank
Vanhaecke
*a
aLaboratory of Analytical Chemistry, Ghent University, Institute for Nuclear Sciences, Proeftuinstraat 86, B-9000 Ghent, Belgium. E-mail: Frank.Vanhaecke@UGent.be
bLaboratory for Inorganic Chemistry, ETH Hönggerberg, HCI, CH-8093 Zürich, Switzerland
cNational Institute for Criminology and Criminalistics NICC, Vilvoordse Steenweg 98/100, B-1120 Brussels, Belgium
First published on 18th January 2006
Abstract
The capabilities of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for depth profiling analysis of car paints were explored and the use of the signal profiles thus obtained for forensic purposes—identification of the vehicle involved in a hit-and-run accident—evaluated. Depth profiling analysis of car paint samples provided detailed information on the elemental composition of the individual layers (clear coat, base coat, primer surfacer and first primer). It was, however, established that when using a quadrupole-based ICP-MS instrument, the complex matrix composition of some of the layers—organic components and high concentrations of inorganic compounds, such as kaolin (Al2O3.2SiO2.2H2O), talc (3MgO.4SiO2.H2O), barite (BaSO4) and/or Fe-containing pigments—resulted in spectral interferences, significantly affecting the signal profiles obtained especially for the transition metals. Deviation of experimentally determined isotope ratios from the corresponding true values was assessed as a very useful indication of spectral overlap. Application of an inductively coupled plasma sector field-based mass spectrometer (ICP-SF-MS), operated at medium mass resolution (R = 4000), permitted most interfering ions to be identified and documented, primarily on the basis of their exact mass-to-charge ratio. Car paint fragments found at crime scenes are usually very small, such that the multi-element capabilities of LA-ICP-MS have to be exploited to the largest possible extent, allowing the measurement of as many analytes as possible, facilitating clear differentiation of the various paint sources. High mass resolution was therefore assessed as the best possible way to overcome the aforementioned spectral overlap. The signal profiles obtained via LA-ICP-MS permitted car paints of the same colour to be discriminated from one another on the basis of the elemental composition of the individual layers. Using the ratio of the signal intensity for the target elements to that of Ti—present at high concentration in the primer surfacer and the first primer in all of the car paint samples investigated—is suggested as a first step towards quantitative results, at least for these two layers.
Introduction
In Belgium, which has a surface area of 32![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 545 km2 and a population of 10
545 km2 and a population of 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 309
309![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 725 (01/01/01),1 there were over 13
725 (01/01/01),1 there were over 13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 hit-and-run accidents in 2002, almost 2000 of which resulted in serious injuries and 16 had death as consequence.2 The most important material evidence sometimes found at these crime scenes consists of small fragments of paint of the car(s) involved in the accident. Careful investigation of these fragments and the identification of their specific characteristics play an important role in the forensic investigation. Both physical and chemical characteristics of these multilayered materials should be considered. The chemical characteristics of car paints include the type of pigments, polymers and additives present, while the physical features comprise colour, layer thickness and layer sequence. Car paints typically consist of a first primer, a primer surfacer and a top coat, which—according to the type of paint—sometimes can be divided into a clear coat and a base coat. The composition of car paints shows differences according to manufacturer, colour, model and year of manufacture, although the overall composition is roughly similar for different car paints.
000 hit-and-run accidents in 2002, almost 2000 of which resulted in serious injuries and 16 had death as consequence.2 The most important material evidence sometimes found at these crime scenes consists of small fragments of paint of the car(s) involved in the accident. Careful investigation of these fragments and the identification of their specific characteristics play an important role in the forensic investigation. Both physical and chemical characteristics of these multilayered materials should be considered. The chemical characteristics of car paints include the type of pigments, polymers and additives present, while the physical features comprise colour, layer thickness and layer sequence. Car paints typically consist of a first primer, a primer surfacer and a top coat, which—according to the type of paint—sometimes can be divided into a clear coat and a base coat. The composition of car paints shows differences according to manufacturer, colour, model and year of manufacture, although the overall composition is roughly similar for different car paints.
Information on the possible makes, models and years of manufacture of the vehicle(s) involved in the accident can sometimes be deduced from comparison of these characteristics with existing databases.3,4 Comparison of a car paint found at the crime scene with a piece of car paint from a suspect vehicle, on the other hand, can also provide important evidence. It is important to note, however, that, although originating from one and the same vehicle, automotive paint samples might show differences in their appearance and/or composition due to processes that change the structure of a car paint, e.g., weathering.5
The Scientific Working Group on Materials Analysis (SWGMAT) has published a guideline for paint analysis and comparison of paints that is used by forensic laboratories.6 In forensic applications, it is important to get as much information as possible, even from tiny amounts of sample, and thus a combination of several analysis methods should be used, preferably methods that are non-destructive and which do not require much sample preparation. The most important techniques that have been used so far are vibrational spectroscopy, pyrolysis-gas chromatography (combined with mass spectrometry), scanning electron microscopy–energy dispersive X-ray analysis and X-ray fluorescence spectrometry.
At first, a visual and macroscopic evaluation is performed and—if possible—samples are physically matched.6 In more complex cases, further examination is necessary and this starts with microscopic analysis to visualize the different layers and layer structure. Further techniques may be used to get as much information as possible. Some of these techniques require separation and isolation of the several layers (vide infra).
Vibrational spectroscopy, most often infrared (IR) spectroscopy, provides information on binders, pigments and additives in the coatings, although the information obtained on pigments is limited.7–10 Raman spectroscopy, on the other hand, which is known to be complementary to IR spectroscopy, is particularly useful for obtaining information on the pigments.11 The most important disadvantage of IR spectroscopy is, however, that it is not possible to obtain results for each individual layer without physically separating the layers from each other, so time consuming sample preparation is necessary.
To obtain information on binder type, pyrolysis-gas chromatography (PGC) is the recommended technique. If the pyrolysis and chromatographic conditions remain constant over time, comparison of the chromatograms with reference chromatograms permits the identification of binder types.12–15 Especially when combined with mass spectrometry, this technique is also powerful in providing information on additives, organic pigments and impurities.16 This qualitative information is very useful for discrimination purposes, but it is impossible to obtain quantitative information, which is necessary for databases. Other disadvantages of PGC are that it is a destructive technique and that the layers should again be physically separated to get information on one specific layer.
To characterize the metals present in the car paints, electron probe microanalysis (EPMA) with either energy dispersive (EDS) or wavelength dispersive (WDS) detection can be used. The preferred choice for such samples is scanning electron microscopy with energy dispersive X-ray analysis (SEM-EDS).17 Since the limit of detection of SEM-EDS is approximately 0.1%, the technique is unfortunately not sensitive enough for the detection of trace elements, which often play an important role in forensic science.
Also, X-ray fluorescence spectrometry (XRF) can be used for obtaining elemental information, although it is less spatially discriminating than SEM-EDS.18,19 A disadvantage of this technique is again that information for several or all of the layers will be obtained, unless they are physically separated. A promising solution for this problem is the use of μXRF, which provides a spatial resolution of 50 μm. An additional disadvantage of both SEM-EDS and XRF is the fact that with these techniques, the information depth depends on the energy of the X-ray photons and, hence, on the analyte element.
Laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) is a powerful technique for elemental analysis, combining high spatial resolution, enabling (quasi) non-destructive sampling, with limits of detection in the low to sub-ppb range.20 Elemental information is obtained and major, minor and trace elements present can be identified. The car paint can be ablated from top to bottom, which implies that subsequent information on the several layers is obtained without the necessity of physical separation of the layers.
Several other applications of LA-ICP-MS in the forensic sciences have also been reported on. Glass and steel are physical evidence very often found at crime scenes and LA-ICP-MS plays an important role in the characterization and evaluation of this evidence.21 The technique has, for example, also been used in the fingerprinting of both cannabis and gold. Fingerprinting of cannabis can potentially trace this product back to the specific geological environments of the crops,22 while the fingerprinting of gold facilitates the identification of the provenance of stolen gold or gold used in salting activities.23
The capabilities of LA-ICP-MS for discriminating between automotive paints by comparing the (trace) elemental compositions of the different layers in different samples have already been explored and reported on.24 The technique has been shown to complement the more commonly used techniques in the forensic investigation of car paints. Matrix-matched standards have been used successfully for the quantification of lead in the different paint layers and the elemental composition of 18 car paint samples has been used to evaluate the discriminating power of the technique. Unfortunately, spectral interferences have not been taken into account. However, as a result of the complex composition of these materials, the occurrence of spectral interferences is an important pitfall and neglecting their existence can lead to misinterpretation of the analysis data obtained (Table 1). The explorative research we performed with a quadrupole-based mass spectrometer revealed serious spectral overlap, hampering the use of a substantial number of important elements. The quadrupole-based ICP-MS instrument used is equipped with a dynamic reaction cell, and hence chemical resolution can be used to overcome the problem of spectral interferences.25–28 Nevertheless, this method was not explored because, inevitably, chemical resolution leads to some loss in the multi-element capabilities and because of the tiny fragments sometimes found at hit-and-run accidents, this has to be avoided at all costs. Therefore, an ICP-MS instrument equipped with a double-focusing sector field mass spectrometer was used to identify possible interferences and to study the discrimination power of this approach. The capabilities for forensic investigation of automotive paints offered by quadrupole-based and sector field instruments were also compared and possibilities and limitations of both approaches are discussed.
| Main compounds | Interfering ionsb | Nuclides affected |
|---|---|---|
| a For example, melamine, styrene, polyurethane, nitrocellulose, epoxy-resin. b Non-restrictive list (REE nuclides with an isotopic abundance ≤15% were not taken into account). | ||
| Organica | 12C2+ | 24Mg+ |
| 12C14N+ | 26Mg+ | |
| 12C16O+, 14N2+ | 28Si+ | |
| 13C16O+ | 29Si+ | |
| 14N16O+ | 30Si+ | |
| 40Ar12C+ | 52Cr+ | |
| 40Ar14N+ | 54Cr+, 54Fe+ | |
| 40Ar16O+ | 56Fe+ | |
| 40Ar14N14N+, 40Ar12C16O+ | 68Zn+ | |
| Inorganic | 24Mg2+ | 12C+ |
| Al2O3.2SiO2.2H2O | 26Mg2+ | 13C+ |
| 3MgO.4SiO2.H2O | 46Ti2+, 46Ca2+ | 23Na+ |
| BaSO4 | 48Ti2+, 48Ca2+ | 24Mg+ |
| TiO2 | 50Ti2+ | 25Mg+ |
| CaCO3 | 40Ar+, 24Mg16O+ | 40Ca+, 40K+ |
| Fe-containing | 25Mg16O+, 24Mg16O1H+ | 41K+ |
| pigments | 26Mg16O+, 25Mg16O1H+ | 42Ca+ |
| 27Al16O+, 26Mg16O1H+ | 43Ca+ | |
| 27Al16O1H+, 28Si16O+ | 44Ca+ | |
| 28Si16O1H+ | 45Sc+ | |
| 46Ti+ | 46Ca+ | |
| 32S16O+ | 48Ti+, 48Ca+ | |
| 32S16O1H+ | 49Ti+ | |
| 50Ti+ | 50Cr+, 50V+ | |
| 54Fe+ | 54Cr+ | |
| 40Ar16O+, 40Ca16O+ | 56Fe+ | |
| 40Ar16O1H+, 40Ca16O1H+ | 57Fe+ | |
| 42Ca16O+ | 58Fe+, 58Ni+ | |
| 43Ca16O+, 42Ca16O1H+ | 59Co+ | |
| 44Ca16O+, 43Ca16O1H+ | 60Ni+ | |
| 44Ca16O1H+ | 61Ni+ | |
| 46Ti16O+, 46Ca16O+ | 62Ni+ | |
| 47Ti16O+, 46Ti16O1H+, 46Ca16O1H+ | 63Cu+ | |
| 48Ti16O+, 48Ca16O+, 40Ar24Mg+, 47Ti16O1H+ | 64Ni +, 64Zn+ | |
| 130Ba2+, 49Ti16O+, 48Ti16O1H+, 48Ca16O1H+, 40Ar25Mg+ | 65Cu+ | |
| 132Ba2+, 50Ti16O+, 40Ar26Mg+, 49Ti16O1H+ | 66Zn+ | |
| 134Ba2+, 40Ar27Al+, 50Ti16O1H+ | 67Zn+ | |
| 136Ba2+, 40Ar28Si+, 32S16O2+ | 68Zn+ | |
| 138Ba2+ | 69Ga+ | |
| 54Fe16O+ | 70Zn+ | |
| 54Fe16O1H+ | 71Ga+ | |
| 56Fe16O+, 40Ar32S+ | 72Ge+ | |
| 57Fe16O+, 56Fe16O1H+ | 73Ge+ | |
| 58Fe16O+, 57Fe16O1H+ | 74Ge+, 74Se+ | |
| 58Fe16O1H+ | 75As+ | |
| 40Ar2+, 40Ar40Ca+ | 80Se+ | |
| 40Ar42Ca+ | 82Se+ | |
| 40Ar44Ca+ | 84Sr+ | |
| 40Ar46Ca+, 40Ar46Ti+ | 86Sr+ | |
| 40Ar47Ti+ | 87Sr+, 87Rb+ | |
| 40Ar48Ca+, 40Ar48Ti+ | 88Sr+ | |
| 40Ar49Ti+ | 89Y+ | |
| 40Ar50Ti+ | 90Zr+ | |
| 40Ar54Fe+ | 94Mo+, 94Zr+ | |
| 40Ar56Fe+ | 96Mo+, 96Ru+, 96Zr+ | |
| 40Ar57Fe+ | 97Mo+ | |
| 40Ar58Fe+ | 98Ru+, 98Mo+ | |
| 130Ba+ | 130Te+ | |
| 136Ba+ | 136Ce+ | |
| 138Ba+ | 138Ce+, 138La+ | |
| 130Ba16O+ | 146Nd | |
| 135Ba16O+, 134Ba16O1H+ | 151Eu+ | |
| 136Ba16O+, 135Ba16O1H+ | 152Sm+ | |
| 137Ba16O+, 136Ba16O1H+ | 153Eu+ | |
| 138Ba16O+, 137Ba16O1H+ | 154Sm+ | |
| 40Ar132Ba+ | 172Yb+ | |
| 40Ar134Ba+ | 174Yb+ | |
| 40Ar135Ba+ | 175Lu+ | |
| 40Ar137Ba+ | 177Hf+ | |
| 40Ar138Ba+ | 178Hf+ | |
Experimental
Samples
Two types of car paint are frequently used, ‘solid paints’ and ‘effect paints’.29,30 Both paints consist of a first primer (FP), a primer surfacer (PS) and a top coat. The first primer attaches the paint to the underlying steel substrate of the vehicle and protects this steel against weathering, while the primer surfacer, which is attached to the first primer, protects the first layer against ultraviolet radiation. The top coat is responsible for the colour of the vehicle and thus contains pigments. In ‘effect paints’ (Fig. 1), this top coat is divided into two separate layers, the base coat (BC), which contains the pigments, and a protective clear coat (CC). In addition to the pigments, effect base coats contain aluminium flakes, mica pigments and/or other effect pigments which give the vehicle its metallic sheen. To preserve these metal particles from fast degradation, a relatively thick (∼40 μm) transparent layer is applied upon the base coat, the clear coat. Effect car paints are typically approximately 100 μm thick, with the clear coat being the thickest layer. The base coat is approximately 15 μm thick, the primer surfacer 35 μm and the first primer about 20 μm (Fig. 1).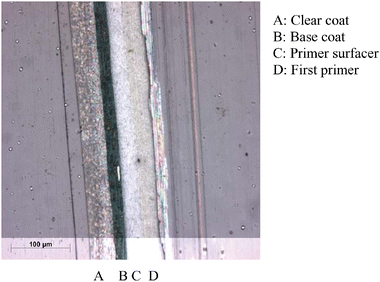 | ||
| Fig. 1 Visible microscopy image with reflected light darkfield observation of a transverse section (cutting angle of ±13°) of an effect paint. | ||
A set of six of these effect paints from different manufacturers was analyzed. These samples were obtained from the Belgian NICC (National Institute for Criminology and Criminalistics). The fragments obtained contained all four layers and were typically 100 mm2 large. No sample preparation was carried out prior to LA-ICP-MS analysis.
Instrumentation
A 193 nm GeoLas ArF excimer-based laser ablation system, commercially available from MicroLas (Germany), coupled to either a PerkinElmer Sciex (Thornhill, ON, Canada) DRCplus quadrupole-based ICP-mass spectrometer or a double-focusing ICP-sector field mass spectrometer (ICP-SF-MS, Element2, Thermo Electron Corporation, Germany) was used for the analyses. In this ArF excimer laser ablation system, the UV laser beam coming from the Compex102 laser unit (LambdaPhysik, Germany) undergoes homogenization,31 enabling flat-bottomed and straight-walled craters to be obtained, which makes the set-up well suited for depth profiling studies.32,33 The laser was used in single hole drilling mode with a 120 μm laser beam diameter, a repetition rate of 3 Hz and a constant fluence. The ablation cell was coupled to the ICP torch via a 3 mm internal diameter Tygon tube. At Ghent University, argon was used as the carrier gas, while at the ETH Zurich, helium was preferred since it leads to less redeposition of the ablated particles on the sample surface.34 Prior to introducing the helium gas flow, containing the laser-induced aerosol, into the ICP, an argon gas flow was admixed after the ablation cell using a bulb.35The instrument parameters (rf-power, gas flow settings, lens settings) of the quadrupole-based ICP-MS unit were optimized for maximum signal intensity (24Mg+, 115In+ and 238U+), low background (m/z = 8.5 and 220 < 2 cps), low formation of oxide (140Ce16O+/140Ce < 3%) and doubly charged species (138Ba2+/138Ba+ < 3%). Although the quadrupole-based instrument used is equipped with a dynamic reaction cell (DRC), which enables spectral interferences to be dealt with by means of chemical resolution,25–28 this approach was not further investigated due to the complexity of the matrix and the full multi-element capabilities required.
The ICP-SF-MS instrument was operated at medium mass resolution (R = 4000) throughout all experiments. Improved features, like fast scan-speed and optimized ion extraction from the ICP, were utilized to increase the detection power of the ICP-MS instrument in combination with high spatially resolved laser sampling.36 The optimization of the ICP-SF-MS conditions (rf-power, gas flow settings, and lens settings) was performed by ablating a standard reference glass material (NIST SRM 612) with the laser settings mentioned above. Parameters were optimized aiming at maximum signal-to-noise ratio for 7Li+, 115In+, and 238U+, low formation of oxide and doubly charged species, monitored via the 232Th16O+/232Th+ (<0.1%) ratio and the 42Ca2+/42Ca+ ratio (<0.5%), respectively, and a 238U+/232Th+ ratio close to unity (1.05).35
A summary of both the laser and ICP-MS parameters used with the different systems is given in Table 2 and Table 3, respectively.
| Laser ablation parameters | |
| Crater diameter/μm | 120 |
| Repetition rate/Hz | 3 |
| Energy density at sample/J cm−2 | 11.7–15.8 |
| Ablation cell volume/cm3 | 42 |
| Quadrupole-based ICP-MS parameters | |
| RF power/W | 1200 |
| Argon auxiliary gas flow rate/L min−1 | 1.2 |
| Plasma gas flow rate/L min−1 | 17 |
| Carrier gas flow rate/L min−1 | 1.4 |
| Sampling cone | Pt |
| Diameter of central opening/mm | 1.1 |
| Skimmer | Pt |
| Diameter of central opening/mm | 0.9 |
| Autolens | On |
| Scan mode | Peak hop |
| Dwell time/ms | 50 |
| Dead time correction/ns | 52 |
| Settling time/ms | 3 |
| Sweeps | 1 |
| Readings | 1 |
| Replicates | 500 |
| Number of nuclides monitored | 56 |
| Nuclides monitored: | |
| 13C, 23Na, 24Mg, 25Mg, 26Mg, 27Al, 28Si, 29Si, 30Si, 31P, 32S, 34S, 44Ca, 47Ti, 48Ti, 50V, 51V, 52Cr, 53Cr, 54Cr, 55Mn, 56Fe, 57Fe, 59Co, 58Ni, 60Ni, 62Ni, 63Cu, 65Cu, 64Zn, 66Zn, 67Zn, 68Zn, 69Ga, 71Ga, 85Rb, 86Sr, 88Sr, 111Cd, 118Sn, 120Sn, 134Ba, 135Ba, 136Ba, 137Ba, 138Ba, 139La, 140Ce, 142Ce, 200Hg, 202Hg, 204Pb, 206Pb, 208Pb, 209Bi | |
| Laser ablation parameters | |
| Crater diameter/μm | 120 |
| Repetition rate/Hz | 3 |
| Energy density at sample/J cm−2 | 25 |
| Ablation cell volume/cm3 | 28 |
| He carrier gas flow rate/L min−1 | 0.72 |
| ICP-SF-MS parameters | |
| RF power/W | 1200 |
| Argon make up gas flow rate/L min−1 | 1.01 |
| Argon auxiliary gas flow rate/L min−1 | 10.6 |
| Argon cooling gas flow rate/L min−1 | 15 |
| Sampling and skimmer cones | Al |
| Guard electrode | Pt (off) |
| Mass resolution | m/Δm = 4000 |
| Mass window (%) | 50 |
| Scan type | E-scan |
| Sample time/ms | 1.1 |
| Number of samples | 10 |
| Segment duration per isotope/ms | 11 |
| Runs and passes | 300 × 1 |
| Analysis time/min | 3.5 |
| Duty cycle per isotope (%) | 1.5 |
| Measurement efficiency (%) | 36.4 |
| Acquisition mode | E-scan, combined with 8 magnet jumps |
| Total settling time/ms | 461 |
| Number of nuclides monitored | 24 |
| Nuclides monitored: | |
| 13C, 23Na, 24Mg, 27Al, 28Si, 31P, 32S, 44Ca, 49Ti, 52Cr, 56Fe, 59Co, 60Ni, 63Cu, 64Zn, 71Ga, 85Rb, 88Sr, 109Ag, 118Sn, 137Ba, 139La, 208Pb, 209Bi | |
Measurements
In the first phase of the work, a multi-element method was developed for the depth profiling analysis of the multilayered car paints using the combination of LA and quadrupole-based ICP-MS.In Fig. 2, some intensity versus time profiles are shown for sample E. Fig. 2A shows the result when the automotive paint was ablated from top (clear coat) to bottom (first primer), with a beam diameter of 120 μm at one specific spot. The first problem encountered is that, although several layers can be distinguished from each other by monitoring the different signals, it is impossible to see at what point of the ablation the bottom (i.e., the end of the first primer) is reached. To overcome this problem, the automotive paints were mounted directly upon silver foil (99.95% purity, Goodfellow Metals, UK) and the 109Ag+ signal was monitored as well.37 A huge increase in the silver signal intensity is used as an indication for reaching the bottom of the paint, as shown in Fig. 2A.
 | ||
| Fig. 2 Intensity (log scale, cps) versus time (s) profiles for sample E (A: ablation from CC to FP; B: ablation from FP to CC) (CC: clear coat; BC: base coat; PS: primer surfacer; FP: first primer). | ||
Another problem finds its origin in the limitation of the depth resolution. From a certain depth onwards, the signal intensities of elements from subsequent layers are mixed together and, therefore, some layers—especially the deeper laying primer surfacer and first primer—are harder to distinguish from each other. To overcome this problem, all of the automotive paints were also turned upside down and ablated from first primer to clear coat. The corresponding result is shown in Fig. 2B, where the transition from the first primer to the primer surfacer can be easily deduced from the signal profile for 24Mg+, while this transition is much harder to discern from Fig. 2A. The transition between the base coat and the clear coat is more difficult to see, because the clear coat contains no measurable amounts of mineral elements, so no significant increase of the measured signals is expected and, as already mentioned, no clear decrease in signal intensities can be expected at this greater depth. This boundary can, however, be easily observed from Fig. 2A. The increases in signal intensities present in the clear coat in Fig. 2A are caused by both memory effects and surface contamination.
All further depth profiling measurements were performed by ablating at a single spot of the car paint from both clear coat to first primer as well as from first primer to clear coat, using the silver signal, originating from the foil, as an indication for penetration of the entire sample. The depth profiling analysis was performed at least twice for reproducibility purposes.
To ensure that no erroneous conclusions were drawn as a result of interferences, additional measurements were performed whereby—when possible—several isotopes were monitored for each element. These measurements revealed the occurrence of many spectral interferences, caused by the complex composition of the different layers of car paints. The layers are composed mainly of organic components, but can contain high concentrations of inorganic compounds, such as kaolin (Al2O3.2SiO2.2H2O), talc (3MgO.4SiO2.H2O), barite (BaSO4) and/or Fe-containing pigments. To investigate the occurrence of spectral interferences in more detail, measurements were also performed with a combination of LA and ICP-SF-MS.
Results and discussion
Spectral interferences
Measured isotope ratios were plotted as a function of time or ablation depth and compared with the corresponding ‘true’ value.38 If the bias between the two values exceeds ∼1–2% at mid-mass range, it is too pronounced to be attributed to mass discrimination and one may assume that it is caused by spectral interference(s).With a quadrupole-based instrument, a better insight into the origin of the spectral overlap can only be obtained by careful monitoring of the parent ions, possibly at the origin of the interference, and the signals at other mass-to-charge ratios that should also be affected by the suspect polyatomic or doubly charged ions. Given the complex composition of the layers (Table 1), this is a difficult task which hardly allows unequivocal conclusions to be drawn, especially for the transition metals. Therefore, the interferences were systematically studied using ICP-SF-MS. Several isotopes of Mg, Ti, Cu and Zn were measured by using a mass spectral window substantially larger than the analyte peak width at medium mass resolution (R = 4000). These measurements were also performed for the glass reference material SRM NIST 612 for comparison purposes. All of the samples were measured while ablating from clear coat to first primer as well as the other way around. The signal intensities were monitored as a function of time and average spectra were taken for every individual layer. In this way, the interferences can be visualized and were identified whenever possible. Fig. 3 shows the average spectra obtained in this way for all of the layers of sample C at mass-to-charge ratios of 24 (Mg), 25 (Mg), 48 (Ti), 49 (Ti), 63 (Cu), 64 (Zn), 65 (Cu), 66 (Zn), 67 (Zn), 68 (Zn), 137 (Ba) and 138 (Ba). Earlier measurements revealed that neither La nor Ce was detected in the car paints investigated. Therefore, the 138Ba+ signal can be measured without any significant interferences. For Zn it was impossible to find any differences in the clear coat compared with the base coat, so the boundary between the two layers could not be located and therefore an average spectrum over both layers was made.
 | ||
| Fig. 3 Mass spectra at mass-to-charge ratios of 24, 25, 48, 49, 63, 64, 65, 66, 67, 68, 137 and 138 for all of the layers of sample C. | ||
It should be stressed that the intensities are plotted on a log-scale. To get an idea of the importance of the different interferences, the fractions of the total signal intensity resulting from interfering ion(s) are listed in Table 4 for sample C. Because of the very high amount of Ba, Ba2+ has an important impact on the signal intensities at several mass-to-charge ratios when measuring at low mass resolution for the primer surfacer, which explains the problem encountered when measuring with the quadrupole-based instrument.
| Nominal m/z | Measured isotope or polyatomic ion | Clear coat | Base coat | Primer surfacer | First primer | |
|---|---|---|---|---|---|---|
| 24 | 48Ti2+ | 1.1 | 8.7 | 15.7 | 73.9 | |
| 24Mg+ | 97.5 | 91.3 | 84.3 | 25.6 | ||
| 12C12C+ | 1.4 | 0.0 | 0.0 | 0.5 | ||
| 25 | 50Ti2+ | 3.1 | 4.7 | 10.5 | 64.5 | |
| 25Mg+ | 96.9 | 95.3 | 89.5 | 35.5 | ||
| 26 | 26Mg+ | 41.9 | 78.7 | 89.0 | 53.3 | |
| 12C14N + | 58.1 | 21.3 | 11.0 | 46.7 | ||
| 47 | 47Ti+ | 100 | 100 | 100 | 100 | |
| 48 | 48Ti+, 48Ca+ | 93.0 | 100 | 99.9 | 100 | |
| 32S16O+ | 7.0 | 0.0 | 0.1 | 0.0 | ||
| 49 | 49Ti+ | 100 | 100 | 100 | 100 | |
| 63 | 63Cu+ | 42.9 | 99.6 | 5.2 | 10.3 | |
| 47Ti16O+, 40Ar23Na + | 57.1 | 0.4 | 94.8 | 89.7 | ||
| 64 | 64Zn+, 64Ni+ | 0 | 53.4 | 74.7 | ||
| 48Ti(Ca)16O+, 40Ar24Mg+ | 100 | 36.6 | 25.3 | |||
| 65 | 65Cu+ | 26.9 | 99.3 | 1.4 | 6.5 | |
| 49Ti16O+, 40Ar25Mg+ | 53.9 | 0.5 | 52.9 | 93.5 | ||
| 130Ba2+ | 19.2 | 0.3 | 45.8 | 0.0 | ||
| 66 | 66Zn+ | 0 | 89.0 | 96.2 | ||
| 40Ar26Mg+, 48(50)Ti18(16)O+ | 100 | 9.3 | 3.8 | |||
| 132Ba2+ | 0 | 4.7 | 0.0 | |||
| 67 | 67Zn+ | 0 | 8.0 | 100 | ||
| 40Ar27Al+ | 0 | 0.0 | 0.0 | |||
| 134Ba2+ | 0 | 92.0 | 0.0 | |||
| 68 | 68Zn+ | 0 | 9.1 | 69.5 | ||
| 136Ba2+ | 0 | 79.2 | 0.0 | |||
| 40Ar14N14N+, 40Ar12C16O+ | 100 | 11.8 | 30.5 |
Depth profiling analysis and discriminating power
By drilling continuously through the sample at one spot, while monitoring the signal intensities for the target elements with the ICP-MS instrument, an intensity versus time profile can be registered, which is in fact also an intensity versus depth profile. However, the ablation rate depends on both the experimental parameters and the matrix composition and, hence, may vary from one layer to the next. Additionally, obtaining this type of information requires the use of other techniques, such as profilometry, SEM (to some extent) or white-light interferometry. This type of investigation was considered to be beyond the scope of the present study.To explore the capabilities of LA-ICP-MS for discriminating between car paints originating from different vehicles, a multi-element method was used and measurements were performed as described before. Typical depth profiles obtained with the sector field instrument for three of the samples (samples D, E and F) are shown in Fig. 4. The different layers can be easily distinguished from each other and identified. For sample D, ablated from clear coat to first primer for instance, the transition from clear coat to base coat is indicated by an increase of the signal intensities for 27Al+, 48Ti+ and 63Cu+. An increase in the signal intensity of 137Ba+ indicates the point at which the laser beam penetrates into the primer surfacer, while the transition to the first primer is indicated by an increase in the signal from 208Pb+. All layers could be distinguished in a similar way for the other samples.
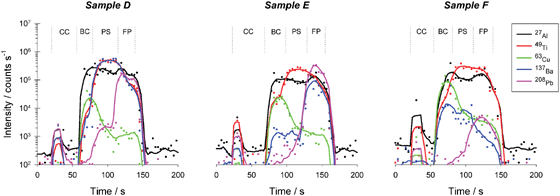 | ||
| Fig. 4 Depth profiles for three green car paint samples, obtained with ICP-SF-MS: intensity (log scale, cps) versus time. (CC: clear coat; BC: base coat; PS: primer surfacer; FP: first primer.) | ||
Although car paints and thus the corresponding depth profiles show great similarities to each other, there are enough differences for successful discrimination between different samples. Self-evidently, the actual discrimination can already start visually: samples with different colours obviously originate from different cars. It is, however, barely possible to visually discriminate between samples that have the same colour. In the set of car paints investigated, three green samples were present (samples D, E and F). In Fig. 4, the depth profiles of these three samples are shown. Only a small fraction of the isotopes measured are plotted for clarity. The selected signals suffice to discriminate between the three samples.
With the profile of Ba alone, for instance, all three samples can be distinguished from each other. Looking at the profiles in Fig. 4, it is obvious that Ba is present in high concentrations in the first primer of sample E, while in the other samples the amount of Ba is negligible in that specific layer. Sample D on the other hand, obviously contains the highest concentration of Ba in the primer surfacer. Of course, the 137Ba+ signal will not be sufficient in case of a larger series of green car paint samples. Therefore, also other signals and combinations thereof have to be taken into account. From the three graphs of Fig. 4, it is clear that for instance 208Pb+ also leads to a clear discrimination of these three car paints when considering the relative intensities in the primer surfacer and the first primer.
This illustrates that a comparison of two car paints originating from different vehicles could already be performed with only a small set of elements. This type of comparison is actually also possible with quadrupole-based ICP-MS since no quantitative information is necessary. To set up a database, however, quantitative information has to be obtained for as many elements as possible. As for this purpose the signals have to be interference-free, ICP-SF-MS is preferred in this context.
As a first step towards obtaining quantitative results, the ratio of the signal intensity for the target elements to that of an internal reference is suggested. Although still somewhat dependent on the instrument settings, these ratios are definitely more robust than the signal intensities themselves and, hence, comparison is facilitated. In Fig. 5(a) and (b), the results of this method using Ti—present at high concentrations in both the first primer as the primer surfacer—as internal reference is illustrated for the primer surfacer and the first primer of all six samples. Graphs like Fig. 5(a) and (b) facilitate discrimination between different samples. If two samples originate from the same vehicle, the contributions they make to each of the bars representing one of the ratios are equal for both. Samples originating from different vehicles, on the other hand, will have at least one element for which the ratio to Ti is significantly different.
 | ||
| Fig. 5 (a) Ratio of the signal intensities of the most relevant elements to the signal intensity of 49Ti+ for six samples (A–F) for the primer surfacer. (b) Ratio of the signal intensities of the most relevant elements to the signal intensity of 49Ti+ for six samples (A–F) for the first primer. | ||
Conclusions and outlook
LA-ICP-MS was demonstrated to be very promising for layer-specific elemental characterization of automotive paint fragments, found as material evidence at the scene of hit-and-run accidents. Further optimization of the method is, however, still needed. Often, only a tiny paint fragment is available, such that a method has to be developed in which one measurement suffices to obtain the required information for all four of the layers—instead of having to turn the sample around—without limitation in terms of multi-element capabilities.In real-life cases, it is very important to correctly identify the vehicle that caused the accident. Two approaches can be followed. One is to compare the evidence found at the scene of the hit-and-run accident with a paint fragment of the suspect vehicle(s), the other is to compare the layer-specific elemental composition of a car paint sample with a database. Although for the pairwise comparison of two car paint samples originating from different vehicles, qualitative information might be sufficient to draw conclusions, quantitative information is preferred for both approaches. A first step towards quantitative analysis was made by using the ratio to Ti as an internal reference signal, but further investigation still has to be performed.
For the pairwise comparison of a car paint fragment of a suspect vehicle with a fragment found at the hit-and-run accident, either quadrupole-based ICP-MS or ICP-SF-MS can be used. Of course, when using quadrupole-based ICP-MS, some of the signal intensities will be (partially) caused by interferences. However, if both samples originate from the same car and are investigated using the same instrumental set-up, the contribution from interferences will be equal, such that comparison will still be possible. It cannot be excluded, however, that a situation might arise in which equal signal intensities are found for a given nuclide, despite the fact that the corresponding element concentrations in the two car paints under investigation differ significantly. Different relative contributions of the analyte element and the interfering ion may indeed coincidentally result in the same observed signal intensity. However, even such a coincidental situation should not remain unnoticed, as it can be identified by comparing the data for several isotopes of the element under consideration and/or the presence of parent elements forming polyatomic or doubly charged ions interfering at the mass-to-charge ratio(s) of interest. Moreover, even if the analyst fails to recognise this situation, it is very hard to imagine that the difference between two car paint samples would not be revealed by any of the other data obtained in the multi-elemental approach used. From a theoretical point of view, a time-of-flight analyzer would be ideally suited for such purposes, but up to now instrumental limitations such as lower sensitivity hamper their widespread use in LA-ICP-MS.
To prove that the car paint samples match each other, chemometric techniques will be necessary. One possibility is to calculate the likelihood of a match between both samples based upon quantitative information. Introducing a limit of similarity, e.g., 99%, will enable drawing of the conclusion as to whether the samples originate from the same vehicle or not. Another possibility is to use the Hotelling T2 test, a versatile multivariate control chart statistic, which can be used for determining similarity between two samples.39 Whatever the method used, the conclusiveness and the similarity regarded as meaningful will always depend upon the accuracy and reproducibility of the quantitative method.
For the second approach, a database has to be set up and, therefore, accurate quantitative information on as many elements as possible is necessary for each layer. This goal can be reached most easily by measuring all elements interference-free. To find out from which vehicle(s) a paint fragment found at the scene of a hit-and-run accident originates, quantitative information on a large set of elements has to be compared with the database by means of chemometric techniques for each layer. Again, calculating the similarity between the unknown sample and all of the paints in the database should enable the obtaining of a ranking of, for example, the ‘top ten’ of samples that show the highest similarity with the unknown sample. Comparison of the list obtained by LA-ICP-MS with information from the other techniques mentioned above will then substantially help in the identification of the vehicle involved in the hit-and-run accident.
Acknowledgements
The authors wish to thank the Fund for Scientific Research—Flanders (FWO—Vlaanderen, Research Project G.0037.01), the Swiss National Science Foundation and ETH Zurich for their financial support.References
- Belgisch Staatsblad, 28/05/2002, 23106–23122, http://www.ejustice.just.fgov.be/cgi/welcome.pl.
- N. Giliot, Masters Thesis, Catholic University of Leuven, 2004.
- EUCAP: European Collection of Automotive Paint, European Network of Forensic Science Institutes, European Paint Group, Bundeskriminalamt, Germany.
- PDQ: Paint Data Query, International Automotive Paint Database, Royal Canadian Mounted Police.
- Handbook of Material Weathering, ed. G. Wypych, ChemTec Publishing, Ontario, 3rd edn., 2003 Search PubMed.
- US Department of Justice—Federal Bureau of Investigation—Scientific Working Group on Materials Analysis (SWGMAT), Forensic Sci. Commun., 1999, 1, http://www.fbi.gov/hq/lab/fsc/backissu/july1999/painta.htm Search PubMed.
- E. M. Suzuki, in Forensic Science Handbook: Forensic Applications of Infrared Spectroscopy, ed. R. Saferstein, Regents/Prentice Hall, Englewood Cliffs, NJ, 1993, vol. 3, pp. 71–195 Search PubMed.
- F. T. Tweed, R. Cameron, J. S. Deak and P. G. Rodgers, Forensic Sci., 1974, 4, 211–218 Search PubMed.
- T. J. Allen, Vib. Spectrosc., 1992, 3, 217–237 CrossRef CAS.
- E. M. Suzuki and W. P. Marshall, J. Forensic Sci., 1997, 42, 619–648 CAS.
- A. H. Kuptsov, J. Forensic Sci., 1994, 39, 305–318 CAS.
- A. R. Cassista and P. M. L. Sandercock, Can. Soc. Forensic Sci. J., 1994, 27, 209–233 Search PubMed.
- P. Burke, C. J. Curry, L. M. Davies and D. R. Cousins, Forensic Sci. Int., 1985, 28, 201–219 CrossRef CAS.
- R. D. Blackledge, Forensic Sci. Rev., 1992, 4, 2–15 Search PubMed.
- W. J. Irwin, J. Anal. Appl. Pyrolysis, 1979, 1, 3–25 CrossRef CAS.
- D. G. McMinn, T. L. Carlson and T. O. Munson, J. Forensic Sci., 1985, 30, 1064–1073 CAS.
- J. I. Goldstein, D. E. Newbury, P. Echlin, D. C. Joy, A. D. Romig, C. E. Lyman, C. Fiori and E. Lifshin, in Scanning Electron Microscopy and X-ray Microanalysis, Plenum Press, New York, 1992, pp. 79–90, 334–336 Search PubMed.
- C. R. Howden, R. J. Dudley and K. W. Smalldon, J. Forensic Sci. Soc., 1977, 17, 161–167 Search PubMed.
- P. J. Potts, A. T. Ellis, P. Kregsamer, J. Marshall, C. Streli, M. West and P. Wobrauschek, J. Anal. At. Spectrom., 2001, 16, 1217–1237 RSC.
- D. Günther, S. E. Jackson and H. P. Longerich, Spectrochim. Acta, Part B, 1999, 54, 381–409 CrossRef.
- R. J. Watling, B. F. Lynch and D. Herring, J. Anal. At. Spectrom., 1997, 12, 195–203 RSC.
- R. J. Watling, J. Anal. At. Spectrom., 1998, 13, 917–926 RSC.
- R. J. Watling, H. K. Herbert, D. Delev and I. D. Abell, Spectrochim. Acta, Part B, 1994, 49, 205–219 CrossRef.
- A. L. Hobbs and J. R. Almirall, Anal. Bioanal. Chem., 2003, 376, 1265–1271 CAS.
- S. D. Tanner, V. I. Baranov and D. R. Bandura, Spectrochim. Acta, Part B, 2002, 57, 1361–1452 CrossRef.
- S. D. Tanner and V. I. Baranov, At. Spectrosc., 1999, 20, 45–46 CAS.
- F. Vanhaecke, M. Resano, E. Garcia-Ruiz, L. Balcaen, K. R. Koch and K McIntosh, 2004, 19, 532–638.
- L. I. L. Balcaen, J. Lenaerts, L. Moens and F. Vanhaecke, J. Anal. At. Spectrom., 2005, 20, 417–423 RSC.
- Automotive Paints and Coatings, ed. G. Fettis, VCH, Weinheim, 1995 Search PubMed.
- http://www.automotive.dupont.com/en/productServices/paintCoatings/metalExterior.html .
- D. Günther, R. Frischknecht, C. A. Heinrich and H. J. Kahlert, J. Anal. At. Spectrom., 1997, 12, 939–944 RSC.
- P. R. D. Mason and A. J. G. Mank, in Laser-Ablation-ICPMS in the Earth Sciences, ed. P. Sylvester, St. John’s, Newfoundland, 2001, vol. 29, ch. 7, pp. 93–103 Search PubMed.
- D. Bleiner, A. Plotnikov, C. Vogt, K. Wetzig and D. Günther, Fresenius’ J. Anal. Chem., 2000, 368, 221–226 CrossRef CAS.
- S. M. Eggins, L. P. J. Kinsley and J. M. G. Shelley, Appl. Surf. Sci., 1998, 129, 278–286 CrossRef.
- B. Hattendorf, C. Latkoczy and D. Günther, Anal. Chem., 2003, 75, 341A–347A CrossRef CAS.
- C. Latkoczy and D. Günther, J. Anal. At. Spectrom., 2002, 17, 1264–1270 RSC.
- T. E. Jeffries, S. E. Jackson and H. P. Longerich, J. Anal. At. Spectrom., 1998, 13, 935–940 RSC.
- K. J. R. Rosman and P. D. P. Taylor, J. Anal. At. Spectrom., 1999, 14, 5N–24N Search PubMed.
- R. L. Mason, Y. M. Chou and J. C. Young, J. Qual. Technol., 2001, 33, 466–479 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
