Sample matrix-assisted photo-induced chemical vapor generation: a reagent free green analytical method for ultrasensitive detection of mercury in wine or liquor samples
Yuan
Li
a,
Chengbin
Zheng
a,
Qian
Ma
a,
Li
Wu
ab,
Changwei
Hu
a and
Xiandeng
Hou
*ab
aKey Lab of Green Chemistry & Technology of MOE at College of Chemistry, Chengdu, Sichuan, 610064, China
bAnalytical & Testing Center, Sichuan University, Chengdu, Sichuan, 610064, China. E-mail: houxd@scu.edu.cn; houxiandeng@yahoo.com.cn; Fax: +86 28 8541 2907
First published on 9th November 2005
Abstract
A new and unique photo-induced mercury cold/chemical vapor generation (PI-CVG), which directly uses sample matrix as a reductant, was proposed for atomic fluorescence spectrometric detection of trace mercury in wine or liquor samples. The new method was thus termed as sample matrix-assisted PI-CVG. With the ultraviolet radiation (UV), the sample matrix (ethanol) can reduce mercury compounds or ions to atomic mercury, Hg0, which is subsequently swept (by argon carrier gas) into an atomic fluorescence spectrometer for the measurements. Under the optimized experimental conditions, the LOD for mercury was found to be 70 pg mL−1 with ethanol. The standard addition method was used for the real sample analysis to achieve the reagent free goal. The proposed method features high sensitivity, simplicity (no sample pre-treatment), rapidness, freedom of reagent (the sample matrix as the reductant), cost-effectiveness, and environmental cleanness. Indeed, this is the simplest approach to generate mercury vapor from alcohol-containing samples, and it is expected to have a wide application in the analysis of wines, liquors, and the like. Also, the PI-CVG can be coupled to other analytical atomic spectrometers.
1. Introduction
Mercury is toxic and non-degradable, and its concentration can build up in food chains to the toxic level to human being. Wine or liquor may contribute to mercury intake due to its everyday life’s nature. Since mercury is usually present at trace levels in wine or liquor, the determination of mercury requires sensitive analytical techniques. There are a number of analytical techniques for quantitative determination of mercury in wine or liquor: inductively coupled plasma mass spectrometry (ICP-MS);1 anodic stripping voltammetry (ASV);2 atomic absorption spectrometry (AAS)3 and atomic fluorescence spectrometry (AFS).4 Cold vapor generation has been widely used in the determination of mercury at trace levels because of its high selectivity, high sensitivity and simplicity. However, most of these methods may require an expensive instrument or a large amount of chemical reagents and matrix interferences are sometimes serious. In addition, the sample pre-treatment for total mercury determination is traditionally mandatory. The most common method for the pre-treatment of the samples is to mineralize and vaporize it with nitric acid and hydrogen peroxide by heating on a hot plate, and this is both time consuming and labor intensive.5Chemical vapor generation (CVG) is considered as one of most popular derivation procedures for the determination of trace and ultratrace amounts of elements. It has been hyphened to various atomic spectrometric techniques. Production of volatile covalent hydrides (hydride generation, HG) of a number of elements (traditionally, As, Bi, Ge, Sn, Sb, Se, Te and Pb) by reacting with NaBH4 in acidic media is well established as a sample introduction scheme in analytical atomic spectrometric techniques, including atomic absorption or fluorescence or emission. The transport efficiency of analyte is very high, compared to solution nebulization; and the advantages also include separation and preconcentration of the analyte element and thus high sensitivity and selectivity. However, HG depends on the oxidation state of the analyte.6,7 For example, arsenic hydride generation with NaBH4 requires that arsenic be in the As3+ oxidation state. Hence, the pre-reduction of As(V) to As(III) is necessary. This leads to a time-consuming procedure, plus more potential contamination sources and higher cost. Further, the co-production of metals in the CVG process catalytically decomposes NaBH4 or adsorbs and/or decomposes the organometallic hydrides. Many approaches, such as matrix separation, increasing the acidity of the reaction medium and addition of chemical modifiers or chelating agents, have been used to overcome the interferences. But, again, all these methods are time-consuming and may add contamination risk. In addition, NaBH4 aqueous solution should be prepared daily prior to use. Therefore, it is of great interest to find new alternative CVG techniques. One of the new techniques is electrochemical hydride generation of volatile compounds.8–14 To some extent, it is capable of circumventing some disadvantages associated with the NaBH4 reduction system.12,13 Fewer reagents are required and experimental conditions may be less critical. However, transition metal ions can also be reduced and deposited on the surface of the electrode, and this can result in poor reproducibility.
Photo-generated reactive free radicals can be used as oxidizing or reducing agents.15–18 Recently, PI-CVG has been introduced to the analytical atomic spectrometric area for sample introduction, but most of the previous work used UV-induced photo-reduction for pre-reduction and NaBH4 was still needed. Guo et al.19–22 pioneered its application in analytical atomic spectrometry. Studies in environmental science and physical chemistry have shown that mercury vapor can be generated in the presence of low molecular weight organic acid and TiO2 catalyst under the irradiation of UV.23–25 Its analytical application has been demonstrated in our previous work, by using formic acid to reduce Hg2+ to Hg0 in the presence of UV light but without TiO2 as a catalyst.26 Here, we report the details of mercury PI-CVG with ethanol as a reductant under the UV irradiation and its unique application in the determination of trace mercury in wine or liquor samples. Since alcohol is a main ingredient of these samples, sample matrix is made use of reductant for generating mercury vapor from them without using any additional reagent. The new method is, therefore, termed as sample matrix-assisted PI-CVG. The beauty of this new approach is that it removes the sample pretretment step and does not need any chemical reagent, thus achieving cost effectiveness, simplicity, and environmental cleanness.
2. Experimental
2.1 Reagents and samples
All chemicals were of analytical-reagent grade. Working standard solutions of mercury were prepared prior to use by appropriate step dilutions of mercury stock standard solution of 1000 mg L−1. Working solutions of ethanol were diluted from their analytical-reagent grade with distilled deionized water (DDW). The wine or liquor samples used in this work were purchased from local markets. Spiked wine samples were prepared fresh daily by adding a certain amount of inorganic mercury (Hg2+) standard solution.2.2 Instrumentation
Detailed instrumentation was described in our previous work,26 but briefly summarized here. The photochemical reactor, as shown in Fig. 1, consists of a coiled quartz pipe (250 mm in length and 3.0 mm id) and a quartz container (250 mm in length and 15.0 mm id). The coiled quartz pipe wraps the quartz container inside which is a high-pressure Hg vapor UV lamp (125 W, Shanghai Yaming Bulb Co., Shanghai, China). The quartz container is used to protect the lamp from cooling water. Mercury cold vapor is detected with an atomic fluorescence spectrometer (AFS-2202, Beijing Haiguang Instrument Co., Beijing, China). The instrumental conditions for mercury vapor detection were optimized previously:26 Hg hollow cathode lamp current 30 mA, photomultiplier tube voltage −250 V, argon gas flow rate 500 mL min−1, and observation height 10 mm. For comparison, a Varian SpectrAA 220FS AAS instrument was also used for the determination of mercury in liquor samples, with the cold vapor AAS mode under the following main experimental conditions: 20% HCl, 25% SnCl2; peak area absorbance, analytical line 253.7 nm, 0.5 nm spectral bandwidth, lamp current 4 mA, and deuterium source for background correction.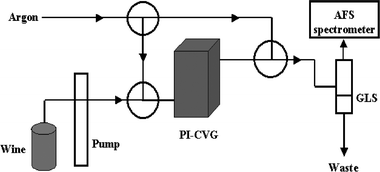 | ||
| Fig. 1 A schematic diagram of the matrix-assisted PI-CVG-AFS instrumentation. GLS: gas–liquid separator; PI-CVG: water-cooled UV photo-induced chemical vapor generator. | ||
2.3 Procedure
The analyte solution (i.e., mercury- and ethanol-containing solution) was sucked into the reactor for 20 s in step 1. In step 2, the reactor was irradiated with the UV for 20 s with argon flow bypassing the reactor. In step 3, the argon flow carried the reacted mixture to the gas–liquid separator of the AFS instrument, and mercury vapor was separated and introduced into the AFS spectrometer for detection. The signal intensity was integrated for 20 s in this step. Step 4 was a 6 s stop for making ready for the next measurement.3 Results and discussion
3.1 Selection of irradiation time
No signal can be detected without the UV irradiation. This is because that there are no radicals generated when the reaction is not irradiated by the UV. On the other hand, the fluorescence intensity was not obviously affected by the irradiation time when the UV was present, as shown in Fig. 2. This implied that the intensity of the UV light was strong enough to finish the reaction in very short time. Considering the intensity and stability of the fluorescence and sufficient time for the CVG reaction, the irradiation time of 20 s was selected for later use. | ||
| Fig. 2 The effect of irradiation time on the PI-CVG, with 10 μg L−1 Hg2+ solution. | ||
3.2 Selection of reductant concentration
It was found that the efficiency of mercury vapor generation depended on the reductant concentration. As shown in Fig. 3, the intensity of fluorescence increased with the concentration of ethanol and a plateau appeared when its concentration was around 10%. The reductant generated radicals that reduced Hg2+ to Hg0 under the irradiation of UV. The amounts of radicals increased with the concentration of reductant. Hence, the rate of reaction accelerated and resulted in the increase in the intensity of fluorescence. In consideration of the intensity and stability, 10% ethanol was chosen for use.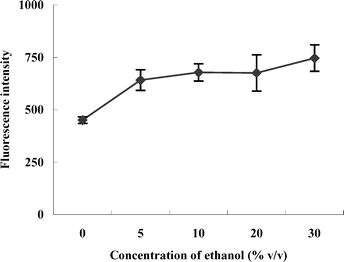 | ||
| Fig. 3 The effect of concentration of ethanol on the PI-CVG, with 10 μg L−1 Hg2+ solution. | ||
3.3 Influence of nano-TiO2 particles
It was found that the intensity of fluorescence increased when TiO2 was added in the reaction. Electrons and holes can be generated on the surface of TiO2. Mercury can be reduced by the electrons on the surface of nano-TiO2, which has very high surface area. It can be seen from Fig. 4 that when the concentration of TiO2 increased, the fluorescence intensity of mercury was accordingly enhanced. However, high concentrations of TiO2 can result in bubbling in the gas–liquid separator. The optimized nano-TiO2 concentration was therefore 3 g L−1. The signal enhancement means that the efficiency or yield of the mercury cold vapor from the ethanol–mercury reaction is apparently far less than 100%. Obviously, the efficiency directly affects the sensitivity of the method, and it can be estimated.19,22 With 10% alcohol or wine as samples (both mercury spiked), the mercury in the after-analysis waste solution was determined by the KBH4 scheme,27 and the PI-CVG efficiency was obtained at around 20% regardless of pure alcohol or wine matrix. Further, with the mercury standard solutions of the same concentration (5 μg L−1), the AFS signals were obtained by this method and the KBH4 scheme;27 the efficiency was 23%, which was calculated as the ratio of the PI-CVG-AFS signal to the KBH4 scheme AFS signal, assuming the efficiency for KBH4 scheme is 100%. Therefore, the efficiency of the PI-CVG for mercury is fairly low. | ||
| Fig. 4 The effect of TiO2 nano-material on the PI-CVG, with a solution of 10 μg L−1 Hg2+ and TiO2 in 10% ethanol. | ||
3.4 Calibration curve and limits of detection
Under the optimized experimental conditions, a calibration curve was constructed with a series of Hg(II) standards (2–10 μg L−1). Sensitivity was the slope of the calibration curve. The details about sensitivities and LODs are shown in Table 1. The sensitivity of the system with nano-TiO2 is higher than without, but the LOD is similar for both cases. The LOD of this method is worse than, but close to that of HG-AFS for mercury, as reported in our previous work with the same AFS instrument.27 To achieve a reagent-free green analytical method, nano-TiO2 was not used in the real sample analysis.3.5 Interference of transition metals
In traditional HG-AFS method, Cu, Co, Ni can seriously affect the determination of mercury. The experimental results here indicated that there are no interferences from Fe, Co, Cu, and Ni, even at concentrations as high as 50 mg L−1 for Fe, 10 mg L−1 for Co and Cu, and 1 mg L−1 for Ni, in the mixed solution that contained the interfering ions and 5 μg L−1 Hg2+.3.6 Determination of mercury in wine or liquor samples
The standard addition method was used for the determination of mercury in the sample analysis. No mercury was found in the wine samples, so the wine samples were spiked with Hg2+ standard solution to the level of 2 μg L−1. Then, 5 mL spiked wine was pipetted into each of five 25 mL volumetric flasks, before mercury standard solution was added. The amounts of mercury standard added were 0, 2, 4, 8 and 16 μg L−1. Table 2 shows satisfactory analytical results for the trace level mercury-spiked wine samples. Since there are no appropriate certified reference materials available for the evaluation of the accuracy of this method, three liquor samples were analyzed with the analytical results listed in Table 3. The results are in good agreement with those obtained by traditional cold vapor AAS. These samples all contain 10% or higher ethanol. Here, it should be pointed out that it is difficult to apply this method for the determination of mercury in samples of low alcohol concentration, such as beer.| Sample | This method/μg L−1a | Cold vapor AAS/μg L−1a |
|---|---|---|
| a Average ±3 × standard deviation of three trials. | ||
| Angelica liquor | 0.5 ± 0.1 | 0.6 ± 0.02 |
| Liquor | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Liquor extract of mixed medicinal herbs | 1.2 ± 0.1 | 1.3 ± 0.4 |
To conclude, the matrix-assisted PI-CVG method has the following advantages: (1) wine or liquor samples are directly detected without any additional reagent; (2) it is a simple, fast and cost-effective method; (3) because of no pre-treatment, it can easily achieve on-line analysis when used with flow injection analysis; (4) it is a green analytical method since the by-products are environment-friendly and the produced mercury vapor is extremely low in concentration; (5) this is the simplest approach to generate mercury vapor from alcohol-containing samples, and it is expected to have a wide application in the analysis of wines, liquors, and the like; and (6) the new PI-CVG system can be hyphened with other analytical spectrometric techniques, such as AAS, ICP-MS and ICP-AES. Finally, the new concept can be developed for the determination of mercury or other elements in varied types of samples via different PI-CVG.
Acknowledgements
XH acknowledges the financial support of this project from the National Natural Science Foundation of China through Grant No.20375026 and the Ministry of Education of China through Grant NCET-04-0869. The cold vapor AAS data for liquor samples were provided by our colleague, Mr Guanglei Cheng of the Analytical & Testing Center of Sichuan University.References
- M. Y. Pérez-Jordán, J. Soldevila, A. Salvador, A. Pastor and M. de la Guardia, J. Anal. At. Spectrom., 1999, 14, 33–39 RSC.
- E. A. Zakharova, V. M. Pichugina and T. P. Tolmacheva, J. Anal. Chem., 1996, 51, 918–923 CAS.
- R. Enkelmann, H. Eschnauer, K. May and M. Stoeppler, Fresenius’ Z. Anal. Chem., 1984, 317, 478–480 CrossRef CAS.
- L. Liang, N. S. Bloom and M. Hovart, Clin. Chem., 1994, 40, 602–607 CAS.
- J. L. Capelo, H. A. Pedro and A. M. Mota, Talanta, 2003, 61, 485–491 CrossRef CAS.
- W. W. Ding and R. E. Sturgeon, J. Anal. At. Spectrom., 1996, 11, 225–230 RSC.
- W. W. Ding and R. E. Sturgeon, Spectrochim. Acta, Part B, 1996, 51, 1325–1334 CrossRef.
- V. I. Rigin, Zh. Anal. Khim., 1978, 33, 1966–1971 CAS.
- V. I. Rigin, Zh. Anal. Khim., 1979, 34, 1569–1573 CAS.
- V. I. Rigin and G. N. Verkhoturov, Zh. Anal. Khim., 1977, 32, 1965–1968 CAS.
- F. Laborda, E. Bolea and J. R. Castillo, J. Anal. At. Spectrom., 2000, 15, 103–107 RSC.
- Y. H. Lin, X. R. Wang, D. X. Yuan, P. Y. Yang, B. L. Huang and Z. X. Zhuang, J. Anal. At. Spectrom., 1992, 7, 287–291 RSC.
- A. Brockmann, C. Nonn and A. Colloch, J. Anal. At. Spectrom., 1993, 8, 397–401 RSC.
- E. Denkhaus, A. Golloch, X. M. Guo and B. L. Huang, J. Anal. At. Spectrom., 2001, 16, 870–878 RSC.
- R. Glazewski and G. M. Morrison, Sci. Total Environ., 1996, 189/190, 327–333 CrossRef.
- K. Kawano, M. Komatsu, Y. Yajima, H. Haneda, H. Maki and T. Yamamoto, Appl. Surf. Sci., 2002, 189, 265–270 CrossRef CAS.
- C. R. Chenthamarekshan and K. Rajeshwar, Electrochem. Commun., 2000, 2, 527–530 CrossRef CAS.
- K. Hayashi, S. Kawai, T. Ohno and Y. Maki, J. Chem. Soc., Chem. Commun., 1997, 158–189 Search PubMed.
- X. M. Guo, R. E. Sturgeon, Z. Mester and G. J. Gardner, Anal. Chem., 2003, 75, 2092–2099 CrossRef CAS.
- X. M. Guo, R. E. Sturgeon, Z. Mester and G. J. Gardner, Anal. Chem., 2004, 76, 2401–2405 CrossRef CAS.
- X. M. Guo, R. E. Sturgeon, Z. Mester and G. J. Gardner, Appl. Organomet. Chem., 2003, 17, 575–579 CrossRef CAS.
- X. M. Guo, R. E. Sturgeon, Z. Mester and G. Gardner, Appl. Organomet. Chem., 2004, 18, 205–211 CrossRef CAS.
- L. R. Skubal and N. K. Meshkov, J. Photochem. Photobiol. A: Chem., 2002, 148, 211–214 CrossRef CAS.
- L. B. Khalil, M. W. Rophael and W. E. Mourad, Appl. Catal. B: Ennviron., 2002, 36, 125–130 Search PubMed.
- X. L. Wang, S. O. Penkonen and A. K. Ray, Electrochim. Acta, 2004, 49, 1435–1444 CrossRef.
- C. B. Zheng, Y. Li, Y. H. He, Q. Ma and X. D. Hou, J. Anal. At. Spectrom., 2005, 20, 746–750 RSC.
- Z. Long, J. J. Xin and X. D. Hou, Spectrosc. Lett., 2004, 37, 263–274 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
