Down-scaling narrowbore LC-ICP-MS to capillary LC-ICP-MS: a comparative study of different introduction systems
Zs. Stefánkaa, G. Koellenspergerb, G. Stingederb and S. Hann*b
aHungarian Academy of Sciences, Institute of Isotopes, Department of Radiation Safety, Konkoly-Thege Miklós út. 29-33, 1121, Budapest, Hungary
bDepartment of Chemistry, Division of Analytical Chemistry, BOKU – University of Natural Resources and Applied Life Sciences, Vienna, Muthgasse 18, A-1190, Vienna, Austria. E-mail: stephan.hann@boku.ac.at
First published on 11th November 2005
Abstract
Narrowbore high performance liquid chromatography (HPLC) was scaled down stepwise to capillary HPLC employing 2.1, 1 and 0.32 mm id separation columns. Provided that each column was operated at optimum chromatographic conditions (eluent velocity, injection volume) and provided that system connection (tubing, unions) was optimal, the influence of different HPLC-ICP-MS interfaces on extra column dispersion could be investigated. Several pneumatic nebulizers i.e. PFA-ST, MCN6000, AriMist, direct injection high efficiency nebulizer (DIHEN), CEI-100 and a costume made PFA005 nebulizer were tested. It could be shown that the contribution to extra column dispersion is minimal for the DIHEN interface. As a major advantage most of the microbore and capillary hyphenations were not prone to signal suppression due to organic solvents. The 1 mm id microbore set-up employing flow rates of 60 μL min−1 offered the best compromise in terms of sensitivity, peak dispersion, resolution, peak asymmetry and compatibility with organic solvent.
Introduction
Miniaturisation in separation techniques, particularly in high performance liquid chromatography (HPLC), has become one of the most dominant trends in the last ten years. Generally the use of microbore (<2.1 mm id) and capillary columns (<0.5 mm id) instead of narrowbore (2.1–3 mm id) and analytical columns (≥4 mm id) has several advantages e.g. reduced sample volume and low eluent consumption.1 Although ICP-MS is an amount dependent detector, miniaturized HPLC-ICP-MS systems profit from the fact that both increasing transport efficiency of the analyte to the plasma and increasing ionization efficiency of the analyte in the plasma is realized with decreasing flow rates through microconcentric nebulizers.2,3 Several papers have been published discussing the capabilities of miniaturized HPLC-ICP-MS,4–7 whereas the reduction of signal suppression by organic solvents has been found to be a key advantage of such systems.8,9In the present methodological study we critically discuss the benefits and limitations of downscaling HPLC in hyphenated ICP-MS analysis. We compare the analytical figures of merit and extra column dispersion of several introduction systems using the same stationary phase packed in columns with different internal diameters (2.1 mm, 1 mm and 0.32 mm). The scope of our study was to identify the interface with highest sensitivity and minimum contribution to extra-column dispersion. Moreover, as reversed phase chromatography often implies the use of high concentration of organic eluents, compatibility of the diverse hyphenations with respect to signal suppression and signal stability at increasing methanol concentrations of the mobile phase was investigated.
Experimental
Reagents
For preparation of HPLC eluents suprapure formic acid (VWR, Darmstadt, Germany), subboiled ammonium hydroxide (Sigma-Aldrich, Milwaukee, WI, USA) and methanol (HPLC gradient grade, VWR) were used. All solutions and eluents were prepared using ultrapure subboiled water. All bottles used were made of polyethylene except of the eluent containers, which were made of glass.Standards
Carboplatin (10 mg mL−1) and oxaliplatin (5 mg mL−1) stock solutions were kindly provided by Prof. Dr Robert Mader/Vienna University Hospital. Standards for flow injection experiments and HPLC separation were obtained by diluting an appropriate amount of the stock solutions in subboiled water immediately before measurement. For flow injection a carboplatin solution containing 26 μg L−1 Pt was used. All HPLC measurements were conducted employing a mixture of carboplatin and oxaliplatin (12 and 13 μg L−1 Pt).HPLC-ICP-MS
An inert HPLC gradient system (Rheos 2000, Flux Instruments AG, Basel, Switzerland) in combination with a metal-free autosampler (CTC Analytics AG, Zwingen, Switzerland) was used throughout the study. Reversed phase chromatography (X-Terra stationary phase, Waters, MA, USA) was employed to separate carboplatin and oxaliplatin. Baseline separation of the two compounds was achieved under isocratic conditions using 10 mmol L−1 ammonium formate (pH 3.75) containing 2 v/v% methanol. The column temperature was kept constant at 20 °C (Mistral column oven, Micro-Tech Scientific Inc., Vista, CA, USA) during the measurements. Three columns with different internal diameter (2.1, 1 and 0.32 mm id) were used. In order to preserve column dispersion the column length and particle diameter of the stationary phase were kept constant at 150 mm and 3 μm, respectively. Moreover, injection volumes and flow rates were down scaled according to eqn (1)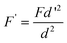 | (1) |
Extra column peak dispersion caused by connecting tubing and injection loops was kept constant by appropriate down scaling of the internal diameter of polyether ether ketone (PEEK) tubing (Upchurch Scientific, Oak Harbor, Washington, USA) in proportion to the column diameters. 127 μm (0.005 inch) id tubing and 63 μm (0.0025 inch) id tubing was used for the 2.1 and 1 mm id hyphenations, respectively. For capillary chromatography (0.32 mm id column) the eluent flow of 63 μL min−1 delivered by the HPLC pump was split after the injector immediately in front of the separation column using a micro flow splitter (Upchurch) to achieve an eluent flow of 4 μL min−1.
The HPLC system was coupled to a quadrupole based ICP-MS (Elan DRC II, PE-SCIEX, Ontario, Canada). Different types of nebulizers were hyphenated with the different columns as detailed in Table 1. The HPLC effluent was connected via PEEK tubing to the nebulizers. The distance between the column outlet and the nebulizer inlet was 65 cm. During the study two types of cyclonic spray chambers with a volume of approximately 40 mL (PE-SCIEX) and 17 mL (Glass Expansion, Hawthorn, Melbourne, Australia) were used to study their influence on peak dispersion.
| Column id/mm | Eluent flow rate/μL min−1 | Injection volume/μL | Injected amount of carboplatin/pg | Introduction system | Peak width σa/min | Resolution Rb | Peak asymmetry Aa | LODb/μg L−1 | LODb/pg |
|---|---|---|---|---|---|---|---|---|---|
| a Determined for oxaliplatin.b Determined for carboplatin shape. | |||||||||
| 2.1 | 250 | 12.5 | 150 | PFA-ST – Cyclonic spray chamber | 0.067 ± 0.001 | 3.93 ± 0.02 | 1.26 ± 0.01 | 0.09 | 1.13 |
| 1 | 60 | 3 | 36 | PFA-ST – Cyclonic spray chamber | 0.071 ± 0.002 | 3.18 ± 0.06 | 1.06 ± 0.01 | 0.27 | 0.810 |
| PFA-ST – MCN6000 | 0.106 ± 0.003 | 2.08 ± 0.03 | 1.68 ± 0.04 | 0.29 | 0.870 | ||||
| AriMist – Cyclonic spray chamber | 0.077 ± 0.003 | 2.60 ± 0.10 | 1.35 ± 0.03 | 0.22 | 0.660 | ||||
| DIHEN | 0.067 ± 0.001 | 3.67 ± 0.04 | 1.01 ± 0.01 | 0.13 | 0.390 | ||||
| 0.32 | 4 | 0.2 | 2.4 | PFA005 – Cyclonic spray chamber | 0.083 ± 0.006 | 3.22 ± 0.07 | 1.18 ± 0.05 | 0.83 | 0.166 |
| PFA005 – Microcyclonic spray chamber | 0.085 ± 0.005 | 3.32 ± 0.05 | 1.19 ± 0.05 | 0.45 | 0.090 | ||||
| PFA005 – MCN6000 | 0.113 ± 0.004 | 1.97 ± 0.04 | 1.64 ± 0.07 | 1.83 | 0.366 | ||||
| CEI-100 | 0.085 ± 0.007 | 2.89 ± 0.07 | 1.33 ± 0.06 | 1.40 | 0.280 | ||||
| AriMist – Cyclonic spray chamber | 0.073 ± 0.011 | 3.25 ± 0.10 | 1.29 ± 0.09 | 3.86 | 0.772 | ||||
To minimize the void volume of the DIHEN a 75 μm id/360 μm od fused silica capillary was inserted into the sample capillary of the nebulizer.
ICP-MS parameters were optimized for each investigated hyphenation to achieve the best signal to noise ratio using a platinum standard solution (10 ng mL−1) with the same composition as the eluent. Typical working parameters were: forward power 1250 W, plasma gas 15 L min−1, auxiliary gas 1–1.5 L min−1, sample gas 0.9–1.1 L min−1. For the DIHEN a sample gas flow rate of 0.25 L min−1 revealed optimum signal intensity and stability.
Generation and export of chromatograms was carried out using Chromlink (Version 2.1, PE-SCIEX) in combination with Turbochrom (Version 6.2, PE-SCIEX). Chromeleon software (Version 6.4, Dionex, Sunnyvale, CA, USA) was used for integration and evaluation of all chromatographic data. Prior to calculation of peak with σ, resolution R and peak asymmetry A all chromatograms were smoothed using a moving average filter. Subsequently, σ was determined at 88.2% peak height, whereas R and A were determined according to eqns (2) and (3)
 | (2) |
 | (3) |
Results and discussion
Extra column dispersion
First, the contribution to extra column dispersion caused by different sample introduction systems used to connect narrowbore, microbore and capillary HPLC to ICP-MS was compared. Moreover, its effect on detection limits was studied for each interface. According to the “rate theory” of chromatography the total dispersion σtotal of a chromatographic system arises from diffusion and flow phenomena. The different sources of dispersion are regarded as independent and therefore their variances are additive. Hence, the total dispersion of a chromatographic system is be described by eqn (4)| σ2total = σ2col + σ2conn + σ2inj + σ2det + σ2el | (4) |
In order to evaluate the peak dispersion caused by different sample introduction systems (σ2det), all other possible sources of dispersion were kept constant via appropriate down scaling of the corresponding system components. First of all column contribution to peak dispersion was preserved by using columns with a constant length containing the same stationary phase and by appropriate downscaling of the injection volumes and flow rates (eqn (1)) resulting in a constant peak concentration of approximately 1 μg L−1 carboplatin expressed as platinum. A constant linear velocity of the eluent and therefore a constant σ2conn was obtained via accurate down scaling of connecting tubing diameter. σ2inj was kept constant by down scaling of the internal diameter of the injection loops and the contribution caused by electronics (σ2el) was maintained using identical instrument settings for processing the signals obtained by the different hyphenations.
Table 1 lists the values observed for peak width, chromatographic resolution and peak asymmetry employing narrowbore, microbore and capillary HPLC. As can be readily seen chromatographic resolution does not increase upon reducing column id. In contrast, interfacing HPLC with ICP-MS at flow rates of 60 and 4 μL min−1 deteriorates the peak resolution compared to the 250 μL min−1 flow rate for all investigated set-ups. The MCN6000 with the highest dead volume after aerosol generation showed the worst values for resolution due to extra column peak dispersion. Whereas peak widths of 0.067 to 0.085 min could be obtained for all microconcentric nebulizers in connection with cyclonic or miniaturized spray chambers, the MCN6000 ranged at 0.1 min. An additional criterion qualifying an HPLC-ICP-MS interface is peak asymmetry reflecting memory effects. Here, the best values were observed for the DIHEN interface demonstrating again the essential role of interface dead volume after aerosol generation.
Limits of detection
Limits of detection (LOD) obtained for the different introduction systems were calculated as three times the standard deviation of the baseline signal quantified by peak height calibration using carboplatin standards. In Table 1 relative LODs are expressed as μg L−1 platinum. Multiplication of relative LODs by the injection volume results in absolute LODs expressed as pg platinum. It can be seen that the lowest relative LODs were obtained for narrowbore HPLC using a 2.1 mm id column. Moreover, the relative LODs are increasing with decreasing column diameter, although the peak concentration of carboplatin is kept constant at 1 μg L−1 platinum. As a matter of fact this observation is characteristic for amount dependent detectors such as ICP-MS and can not be compensated by the increasing transport efficiency at decreasing nebulizer flow rates. Additionally, the standard deviation of the baseline signal (noise) is nearly independent of the eluent flow rate, revealing a lower signal to noise ratio and higher relative LODs at lower column diameters.However, the higher sensitivity at lower flow rates due to higher transport and ionization efficiency of low flow nebulizers is reflected by the lower absolute LODs. In the case of the microbore hyphenations transport efficiency could be increased to 100% by using the DIHEN interface. Accordingly the best absolute and relative LODs were obtained by this set-up. Moreover the effect of reduced dead volume can be observed for the three capillary systems employing the PFA005 nebulizer with different spray chambers. The combination of the PFA005 nebulizer and the microcyclonic spraychamber revealed the absolute LOD of 90 fg platinum.
Compatibility with organic solvents
Reversed phase chromatography often requires the addition of organic solvent such as methanol or acetonitrile. Unfortunately, ICP shows a low tolerance against organic solvents leading to enhanced signal suppression and unsatisfactory long-term stability. Often the transfer of HPLC methods developed for electrospray mass spectrometry to ICP-MS is impeded by this fact. To overcome these problems plasma stability can be improved by reducing the amount of methanol using capillary HPLC, as has been reported by Yanes and Miller-Ihli.12To investigate the effect of methanol on signal intensity, a flow injection experiment was designed at flow rates of 250, 60 and 4 μL min−1 using the different introduction systems listed in Table 1. An—appropriately down scaled—volume of a carboplatin standard (see Table 1) was injected (n = 5) into a continuous flow of 10 mmol L−1 ammonium formiate containing 0, 5, 10, 25, and 50% v/v methanol, respectively. As can be seen in Fig. 1 the platinum signal decreased dramatically with increasing methanol content using the flow rate typical for 2 mm id columns. This loss in sensitivity has been described elsewhere13 and may be attributed to the local cooling of the central channel of the plasma by the high methanol amount. This effect was less pronounced for micro and capillary columns. Even at the highest methanol content the maximum reduction was 40%. Moreover, for some introduction systems even signal enhancement was observed (see Fig. 1). This phenomenon could be explained as an effect of improved mass transport efficiency for methanol due to smaller average droplet size and higher evaporation rates than for aqueous solutions.14
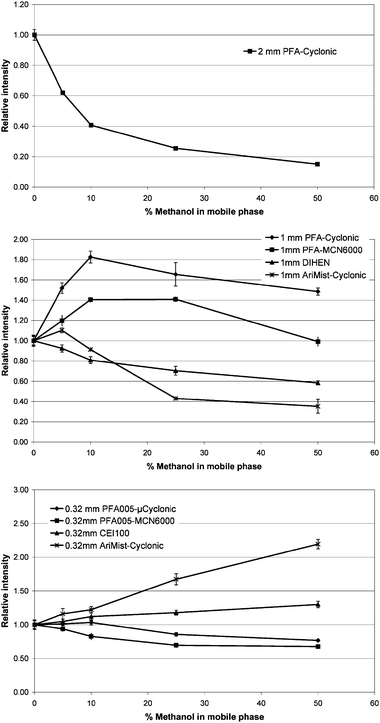 | ||
| Fig. 1 Effect of methanol concentration of the HPLC eluent on HPLC-ICP-MS signal intensity observed for narrowbore (2.1 mm id), microbore (1.0 mm id) and capillary (0.32 mm id) columns. The flow injection peaks obtained at different methanol concentrations were integrated and normalised to those obtained for the aqueous eluent. | ||
The results obtained in the present work indicate that there are three major reasons to choose for reduced column diameters for hyphenation of HPLC and ICP-MS, i.e. (i) limitation of sample mass, (ii) the higher compatibility of the interface with organic solvents at lower flow rates or (iii) the performance of an on-line pre-concentration step using a trapping column. Decreasing the diameter of a chromatographic column does not increase the number of theoretical plates. Therefore no improvement of chromatographic resolution, peak width or sensitivity can be achieved. Contrariwise, the step from normal- and narrowbore columns to microbore, capillary or even nanobore hyphenations often reduces sensitivity and resolution, unless all sources of extra column dispersion are carefully evaluated and minimized.
Acknowledgements
Financial support was granted through the Austrian Science Fund (FWF-ProjectP16089-N03: “Speciation of cancerostatic Pt compounds in the environment”). Zs. Stefánka is grateful to the Hungarian Scholarship Board for granting the Hungarian State Eötvös Scholarship.References
- Y. Shen and R. D. Smith, Electrophoresis, 2002, 23, 3106 CrossRef CAS.
- J. W. Olesik, J. A. Kinzer and B. Harkleroad, Anal. Chem., 1994, 66, 2022 CrossRef CAS.
- J. L. Todoli, V. Hernandis, A. Canals and J. M. Mermet, J. Anal. At. Spectrom., 1999, 14, 1289 RSC.
- E. G. Yanes and N. J. Miller-Ihli, Spectrochim. Acta, Part B, 2004, 59, 891 CrossRef.
- B. P. Jensen, B. Gammelgaard, S. H. Hansen and J. V. Andersen, J. Anal. At. Spectrom., 2003, 18, 891 RSC.
- D. Schaumlöffel, J. R. Encinar and R. Lobinski, Anal. Chem., 2003, 75, 6837 CrossRef.
- M. Wind, M. Edler, N. Jakubowski, M. Linscheid, H. Wesch and W. D. Lehmann, Anal. Chem., 2001, 73, 29 CrossRef CAS.
- K. L. Ackley, K. L. Sutton and J. A. Caruso, J. Anal. At. Spectrom., 2000, 15, 1069 RSC.
- L. Bendahl and B. Gammelgaard, J. Anal. At. Spectrom., 2005, 5, 410–416 RSC.
- J. W. Dolan, LC-GC Eur., 2001, 14, 14 CAS.
- V. L. Mcguffin, in Chromatography 6th edition: Fundamentals and applications of chromatography and related differential migration methods, ed. E. Heftmann, Elsevier, Amsterdam, 2004, p. 33 Search PubMed.
- E. G. Yanes and N. J. Miller-Ihli, Spectrochim. Acta, Part B, 2004, 59, 883 CrossRef.
- C. N. Ferrarello, J. Ruiz Encinar, G. Centineo, J. I. Garcıa Alonso, M. R. Fernandez de la Campa and A. Sanz-Medel, J. Anal. At. Spectrom., 2002, 17, 1024 RSC.
- J. W. Olesik and J. A. W. Moore, Anal. Chem., 1990, 62, 840 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
