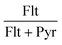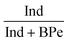Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking
Siao
Wei See
a,
Sathrugnan
Karthikeyan
a and
Rajasekhar
Balasubramanian
*ab
aDepartment of Chemical and Biomolecular Engineering, National University of Singapore, Block E5, 4 Engineering Drive 4, 117576, Singapore. E-mail: eserbala@nus.edu.sg; Fax: (65) 6779 1936; Tel: (65) 6874 5135
bDivision of Environmental Science and Engineering, National University of Singapore, Block E5, 4 Engineering Drive 4, 117576, Singapore
First published on 19th January 2006
Abstract
Food cooking using liquefied petroleum gas (LPG) has received considerable attention in recent years since it is an important source of particulate air pollution in indoor environments for non-smokers. Exposure to organic compounds such as polycyclic aromatic hydrocarbons (PAHs) contained in particles is of particular health concern since some of these compounds are suspected carcinogens. It is therefore necessary to chemically characterize the airborne particles emitted from gas cooking to assess their possible health impacts. In this work, the levels of fine particulate matter (PM2.5) and 16 priority PAHs were determined in three different ethnic commercial kitchens, specifically Chinese, Malay and Indian food stalls, where distinctive cooking methods were employed. The mass concentrations of PM2.5 and PAHs, and the fraction of PAHs in PM2.5 were the highest at the Malay stall (245.3 μg m−3, 609.0 ng m−3, and 0.25%, respectively), followed by the Chinese stall (201.6 μg m−3, 141.0 ng m−3, and 0.07%), and the Indian stall (186.9 μg m−3, 37.9 ng m−3, and 0.02%). This difference in the levels of particulate pollution among the three stalls may be attributed to the different cooking methods employed at the food stalls, the amount of food cooked, and the cooking time, although the most sensitive parameter appears to be the predominant cooking method used. Frying processes, especially deep-frying, produce more air pollutants, possibly due to the high oil temperatures used in such operations. Furthermore, it is found that frying, be it deep-frying at the Malay stall or stir-frying at the Chinese stall, gave rise to an abundance of higher molecular weight PAHs such as benzo[b]fluoranthene, indeno[1,2,3-cd]pyrene and benzo[g,h,i]perylene whereas low-temperature cooking, such as simmering at the Indian stall, has a higher concentration of lower molecular weight PAHs. In addition, the correlation matrices and diagnostic ratios of PAHs were calculated to determine the markers of gas cooking. To evaluate the potential health threat due to inhalation exposure from the indoor particulate pollution, excess lifetime cancer risk (ELCR) was also calculated for an exposed individual. The findings suggest that cooking fumes in the three commercial kitchens pose adverse health effects.
1. Introduction
Exposure to airborne particulate matter (PM) has been recognized as an important factor affecting human health. For example, inhalation exposure to particulate air pollution has been linked to a range of different health problems, especially cardiorespiratory diseases, leading to increased morbidity as well as premature mortality.1–2 Since most people spend about 80 to 90% of their time indoors, indoor PM has received increased scrutiny. These particles can come from migration of pollutants from outdoors, or from indoor sources.3 Therefore, it is necessary to study the origin and transport of PM indoors, particularly PM2.5 (PM with an aerodynamic diameter of 2.5 μm or smaller), as fine particles are more strongly associated with adverse health effects.4 The particles of combustion origin are of particular concern because of their small sizes.5In many developed countries, gas cooking represents a significant indoor source of combustion-derived PM2.5,6,7 and has been specifically linked to respiratory ailments and lung cancer.8,9 The health effects can be attributed to the small physical size of particles10,11 and/or their inorganic and organic toxic chemical compound content, including mutagens and carcinogens,12–16 which are released from the incomplete combustion of fuel, cooking oil, and food. Hence, high concentrations of PM2.5 and chemical components emitted from gas cooking can be extremely hazardous to chefs and other workers in and around the kitchens, notably in kitchens with no effective ventilation. In addition, cooking emissions can migrate to outdoor environments and contribute significantly to organic particles in urban air.17
The recognition of the importance of gas cooking has resulted in the physical and chemical characterization of the combustion particles produced by doing controlled experiments, as well as in real-world kitchens.10–16,18–21 A few of these real-world studies have placed high emphasis on polycyclic aromatic hydrocarbons (PAHs),14,15,21 a major class of very stable organic molecules, some of which are probable or possible carcinogens according to United States Environmental Protection Agency (USEPA) and International Agency for Research on Cancer (IARC). However, most of these studies have measured PAHs in total suspended particles (TSP) collected using a high volume sampler, which are of less significance compared to PM2.5 from a health risk assessment point of view.
In spite of the serious health implications associated with PAHs from gas cooking, there have been no field investigations conducted in the Republic of Singapore, a densely populated country with a land area of 646 km2 and a population of over 4 million, which is popularly known to be a food paradise. Many of the commercial food courts have Chinese, Malay and Indian kitchens due to the presence of these three major ethnic groups in Singapore. Consequently, a systematic study was undertaken to determine PAHs in PM2.5 present in the indoor air of typical Chinese, Malay and Indian commercial food stalls. The quantitative analysis of PAHs is of health significance. In order to provide insights into possible health effects resulting from food cooking, the potential human health risk posed by the inhalation of carcinogenic PAHs was estimated.
2. Experimental
2.1. Description of sampling sites
One Chinese, Malay and Indian food stall in one of the canteens within the National University of Singapore (NUS) Kent Ridge campus were chosen as the sampling sites. These commercial food stalls are located in the same food court, and are not air-conditioned, i.e. no mechanical ventilation is employed. The stalls are small, and have an identical layout with a floor area of approximately 6 to 8 m2. As a result, they experience almost the same level of natural ventilation and also have similar air dispersion conditions. Although the three kitchens are situated in the same food court, the indoor air in each stall is unlikely to be influenced by pollution from other stalls as they are physically separated.All three food stalls are naturally ventilated through the front counter and the back door during their operating hours (also known as the cooking hours) from 0730 to 1930 and are completely closed otherwise. There are four LPG stoves below an exhaust fume extractor on the right hand side and the sampler was placed on the opposite side of the stoves, 1.5 m above the ground to simulate the breathing zone. Air particulate sampling was carried out during both cooking (0730 to 1930) and non-cooking hours (1930 to 0730 the following day) under identical sampling conditions to assess the contribution of cooking activities to the PM2.5 and PAHs concentrations measured indoors, and the corresponding health risk posed by the inhalation of cooking emissions.
2.2. Sample collection
Air particulate sampling was carried out under ambient conditions at these three locations over a period of two weeks, from 13 March 2005 to 26 March 2005. A MiniVol portable air sampler (Airmetrics, OR, USA) was calibrated with a Gilibrator-2 standard flow Primary Air Flow Calibrator (Gilian Instrument Co, NJ, USA) before and after sampling. This sampler was used in each of the kitchens to collect PM2.5 by drawing the ambient air at a flow rate of ∼0.005 m3 min−1 through size-selective inlets (PM10 and PM2.5 impactors) and subsequently through a 47 mm QMA pre-combusted quartz filter (Whatman International Ltd, Kent, UK).Before sampling, clean quartz filters were maintained in a dry box at a constant temperature of 25 °C and constant humidity of 35% for at least 24 h before weighing with a microbalance (readability to 1 μg; Sartorius AG, Goettingen, Germany) just before use. After sampling, the exposed filters were stored in sterile petri dishes (Gelman Sciences Inc., MI, USA) and again placed in the dry box for at least 24 h before final weight measurements, after which they were stored in the refrigerator at 4 °C until extraction and chemical analyses. The mass concentration of PM2.5 was calculated as
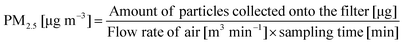 | (1) |
2.3. Extraction and chemical analyses
The quartz filters were extracted and analyzed for PAHs following the procedures described by Karthikeyan et al.22 Briefly, the filters were treated with 20 ml of 1 ∶ 1 v/v reagent grade acetone∶hexane in a MLS1200 MEGA closed vessel microwave digestion system (Milestone srl, Sorisole (BG), Italy) for 20 min at 150 W microwave irradiation. The extracts were concentrated to 3 ml using a rotary evaporator and then to near dryness with a gentle stream of nitrogen under low temperature at ∼20 °C and re-dissolved in 1 ml of the extraction solvent for PAHs analysis. The evaporative loss of low molecular weight PAHs was minimized by carrying out the drying process carefully at low temperature using a small steady flow of nitrogen. Acenaphthene-d10 was used as a surrogate, and its recovery efficiency evaluated, which was in the range of 90 ± 10%.16 PAHs which are regarded as priority pollutants by the USEPA were analyzed, namely naphthalene (Nap), acenaphthene (Ace), acenaphthylene (Acy), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (Ind), dibenz[a,h]anthracene (DBA) and benzo[g,h,i]perylene (BPe).
A Hewlett Packard 6890 series GC System and Mass Selective Detector (GC-MS) (Agilent Technologies, CA, USA) fitted with a DB-5MS 5%-phenyl-methylpolysiloxane 30 m long × 0.2 mm internal diameter × 0.25 μm film thickness capillary column (J & W Scientific, CA, USA) was used. The GC-MS was operated under the following conditions: splitless injection of 2 μl, split opening after 30 s and injector temperature at 280 °C; the oven temperature program was 50 °C (hold 2 min); 50 °C to 200 °C at 10 °C min−1 (hold 1 min); 200 °C to 300 °C at 5 °C min−1 (hold 8 min). The detector was run in electron impact mode with an electron energy of 70 eV and ion source temperature of 230 °C. Helium at a constant flow rate of 0.8 ml min−1 was used as carrier gas. PAHs were monitored using selected ion monitoring mode (SIM). In order to get maximum sensitivity, the 16 ions were divided into groups (seven intervals of retention time), and the detector monitors only the ions programmed for each group. The identification of individual PAHs was based on the comparison of retention times (chromatographic column) and mass spectra (mass detector) of PAHs in aerosol samples with those of PAH standards (full scan mode).
Prior to sample analyses, the GC-MS was calibrated with three different concentrations (200, 500 and 1000 times dilution) of an EPA 610 Polynuclear Aromatic Hydrocarbons Mix containing the 16 PAHs (Supleco, 100 ppm for Phe, Ant, Pyr, BaA, Chr, BkF, BaP, Ind; 200 ppm for Flu, Flt, BbF, DBA, BPe, 1000 ppm for Nap and Acy, 2000 ppm for Ace). In addition, the recoveries of PAHs were determined by processing four sets of SRM 1649a urban dust (National Institute of Standards and Technology, MD, USA) in the same manner as the samples and compared against the certified values. The retention times, major ions (m/z), regression coefficient for the calibration and the recoveries are listed in Table 1. Information on the limit of detection (LOD, 0.3 × 10−3 ppm (BaP) to 8.81 × 10−3 ppm (Flt)) and limit of quantification (LOQ, 0.59 × 10−3 ppm (BaP) to 17.63 × 10−3 ppm (Flt)) is given in one of our recent publications by Karthikeyan et al.22
| PAHs | Retention time/min | Major ion (m/z) | r 2 | Measured concentration (ppm) | Certified concentration (ppm) | Recovery (%) |
|---|---|---|---|---|---|---|
| Nap | 10.77 | 128 | 0.997 | 10.60 ± 3.80 | — | — |
| Ace | 14.50 | 152 | 0.994 | 0.42 ± 0.03 | — | — |
| Acy | 14.93 | 154 | 0.998 | 0.36 ± 0.03 | — | — |
| Flu | 16.16 | 166 | 0.999 | 0.56 ± 0.04 | — | — |
| Phe | 18.64 | 178 | 0.999 | 5.16 ± 0.40 | 4.14 ± 0.37 | 124.6 ± 9.7 |
| Ant | 18.81 | 178 | 1.000 | 0.54 ± 0.01 | 0.43 ± 0.09 | 125.3 ± 3.0 |
| Flt | 22.73 | 202 | 1.000 | 6.44 ± 0.46 | 6.45 ± 0.18 | 99.8 ± 7.2 |
| Pyr | 23.57 | 202 | 1.000 | 5.56 ± 0.43 | 5.29 ± 0.25 | 105.1 ± 8.1 |
| BaA | 28.66 | 228 | 0.999 | 2.09 ± 0.16 | 2.21 ± 0.07 | 94.5 ± 7.1 |
| Chr | 28.81 | 228 | 0.997 | 4.02 ± 0.28 | 3.05 ± 0.06 | 131.7 ± 9.3 |
| BbF | 33.16 | 252 | 0.995 | 6.81 ± 0.47 | 6.45 ± 0.64 | 105.6 ± 7.2 |
| BkF | 33.27 | 252 | 0.990 | 1.63 ± 0.13 | 1.91 ± 0.03 | 85.3 ± 6.8 |
| BaP | 34.40 | 252 | 0.995 | 2.37 ± 0.18 | 2.51 ± 0.09 | 94.6 ± 7.3 |
| Ind | 38.44 | 276 | 0.999 | 3.37 ± 0.28 | 3.18 ± 0.72 | 106.0 ± 8.7 |
| DBA | 38.61 | 278 | 0.999 | 0.31 ± 0.07 | 0.29 ± 0.02 | 107.0 ± 24.0 |
| BPe | 39.35 | 276 | 0.990 | 3.59 ± 0.27 | 4.01 ± 0.91 | 89.5 ± 6.7 |
2.4. Health risk estimates
A complete human health risk assessment encompasses 4 distinct stages: hazard identification, exposure assessment, dose-response assessment and risk characterization.23| PAHs | USEPA classificationa | IARC classificationa | TEF | IUR/mg−1 m3 | ISF/mg−1 kg d |
|---|---|---|---|---|---|
| a USEPA Class B2 and IARC Class 2A: probable human carcinogens; USPEA Class C and IARC Class 2B: possible human carcinogens; USEPA Class D and IARC Class 3: not classifiable as to human carcinogenicity. | |||||
| Nap | C | 2B | 0.001 | 3.4 × 10−2 | 1.2 × 10−1 |
| Ace | — | — | 0.001 | ||
| Acy | D | — | 0.001 | ||
| Flu | D | 3 | 0.001 | ||
| Phe | D | 3 | 0.001 | ||
| Ant | D | 3 | 0.01 | ||
| Flt | D | 3 | 0.001 | ||
| Pyr | D | 3 | 0.001 | ||
| BaA | B2 | 2A | 0.1 | 8.8 × 10−2 | 3.1 × 10−1 |
| Chr | B2 | 3 | 0.01 | 8.8 × 10−4 | 3.1 × 10−3 |
| BbF | B2 | 2B | 0.1 | 8.8 × 10−2 | 3.1 × 10−1 |
| BkF | B2 | 2B | 0.1 | 8.8 × 10−3 | 3.1 × 10−2 |
| BaP | B2 | 2A | 1 | 8.8 × 10−1 | 3.1 × 100 |
| Ind | B2 | 2B | 0.1 | 8.8 × 10−2 | 3.1 × 10−1 |
| DBA | B2 | 2A | 1 | 8.8 × 10−1 | 3.1 × 100 |
| BPe | D | 3 | 0.01 | ||
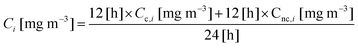 | (2) |
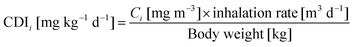 | (3) |
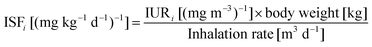 | (4) |
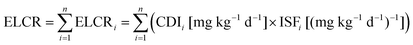 | (5) |
3. Results and discussion
3.1. Mass concentrations of PM2.5 and PAHs
The average mass concentrations of PM2.5 (reported in μg m−3) and PAHs (reported in ng m−3) during cooking and non-cooking hours are given in Table 3 in terms of their mean values with the corresponding standard deviation. The background concentrations measured for all the three stalls during non-cooking hours are quite similar, and the average concentrations are presented in the table. As can be seen from the table, both the mass concentrations of PM2.5 and PAHs, and the percentage of PAHs in PM2.5 were highest at the Malay stall (PM2.5: 245.3 μg m−3, PAHs: 609.0 ng m−3, PAHs in PM2.5: 0.25%), followed by the Chinese stall (201.8 μg m−3, 141.0 ng m−3, 0.07%) and then the Indian stall (186.9 μg m−3, 37.9 ng m−3, 0.02%). While the level of PM2.5 increased by factors of 8.3, 6.9 and 6.4, that of PAHs was enhanced by factors of 14.3, 61.6 and 3.8 compared to the background levels at the Chinese, Malay and Indian stalls, respectively. This difference among the three food stalls in terms of the particulate pollution levels may be explained by the different cooking activities that took place in the individual stalls.| Chinese stall | Malay stall | Indian stall | Background | ||
|---|---|---|---|---|---|
| PM2.5/μg m−3 | 201.8 ± 140.5 | 245.3 ± 77.1 | 186.9 ± 43.6 | 29.4 ± 7.6 | |
| Nap/ng m−3 | 1.9 ± 3.8 | 2.8 ± 5.1 | 3.9 ± 3.2 | 0.7 ± 1.0 | |
| Ace/ng m−3 | 1.0 ± 0.8 | 3.1 ± 2.8 | 1.1 ± 0.3 | 0.4 ± 0.1 | |
| Acy/ng m−3 | 2.4 ± 2.0 | 5.6 ± 5.0 | 2.7 ± 0.8 | 1.8 ± 1.1 | |
| Flu/ng m−3 | 3.8 ± 2.4 | 9.2 ± 9.3 | 3.9 ± 1.1 | 1.1 ± 0.3 | |
| Phe/ng m−3 | 11.5 ± 8.7 | 15.7 ± 10.5 | 9.5 ± 4.3 | 2.2 ± 0.4 | |
| Ant/ng m−3 | 3.0 ± 1.3 | 6.1 ± 4.9 | 2.6 ± 1.2 | 0.5 ± 0.1 | |
| Flt/ng m−3 | 6.9 ± 9.9 | 30.7 ± 47.3 | 1.6 ± 0.7 | 0.3 ± 0.0 | |
| Pyr/ng m−3 | 10.9 ± 14.5 | 18.1 ± 27.3 | 2.9 ± 1.1 | 0.6 ± 0.0 | |
| BaA/ng m−3 | 3.8 ± 5.1 | 23.1 ± 24.2 | 1.0 ± 0.5 | 0.2 ± 0.0 | |
| Chr/ng m−3 | 5.8 ± 8.1 | 48.7 ± 50.7 | 1.0 ± 0.5 | 0.1 ± 0.1 | |
| BbF/ng m−3 | 21.8 ± 34.8 | 122.4 ± 125.9 | 1.9 ± 1.4 | 0.4 ± 0.2 | |
| BkF/ng m−3 | 3.7 ± 5.9 | 23.1 ± 27.4 | 0.5 ± 0.5 | 0.1 ± 0.1 | |
| BaP/ng m−3 | 5.6 ± 7.6 | 16.0 ± 20.5 | 0.9 ± 0.6 | 0.3 ± 0.2 | |
| Ind/ng m−3 | 24.4 ± 41.9 | 105.9 ± 143.4 | 1.3 ± 1.0 | 0.4 ± 0.3 | |
| DBA/ng m−3 | 2.7 ± 4.3 | 8.3 ± 11.1 | 1.1 ± 1.4 | 0.1 ± 0.0 | |
| BPe/ng m−3 | 31.9 ± 52.9 | 170.1 ± 239.1 | 2.1 ± 1.5 | 0.6 ± 0.4 | |
It has been documented that the major factors contributing to the amount and type of pollutants released from food cooking include the type of fuel,24 oil,13 food25 and cooking methods18 employed during the operation. Dispersion of cooking emissions within each stall is also likely to affect the particulate concentrations. However, all the three food stalls have identical layouts and are naturally ventilated with almost equal air change rates. Therefore, the differences in the concentrations of PM2.5 and PAHs between the stalls are taken to be independent of dispersion conditions. In addition, as pointed out earlier, all the three stalls use only LPG as fuel and vegetable oil, for cooking. The three ethnic food stalls cooked a variety of vegetables, meat and fish, but in different amounts. Hence, the variables considered here are the relative quantity of food cooked, the relative amount of time spent on cooking, and the cooking methods used.
The quantity of food cooked and the total time spent on cooking on each day were estimated by the respective chefs. Food was cooked using one or more of the following five cooking methods: deep-frying (to fry by immersing in hot oil), stir-frying (to fry quickly in a small amount of oil at high heat while stirring continuously), pan-frying (to fry in a small amount of oil), simmering (to cook in a hot liquid kept just below its boiling point) and steaming (to cook over boiling water).
The Malay stall was found to be the most polluted. However, relatively less food was cooked in this stall on a daily basis compared to the other two stalls (∼30 kg compared to ∼45 kg at the Chinese stall and ∼40 kg at the Indian stall). The time spent on cooking was about 10 h while it was about 8 h at the Chinese stall and about 20 h at the Indian stall; this estimation was done based on the number of gas stoves used for cooking. It therefore appears that the higher mass concentrations of PM2.5 and PAHs at the Malay stall as compared to the other two stalls are associated with the cooking method used. Specifically, deep frying is the preferred cooking method at the Malay stall as it offers a number of deep fried snacks such as fried bread, banana cakes, bananas and curry puffs besides rice and side dishes. On the other hand, the most common cooking method at the Chinese stall was stir-frying where the ingredients are raw, or partially cooked by pan-frying, and the process itself takes only a few minutes. At the Indian stall, the cooking method is simmering as Indian curry is a very popular dish at the stall, and this recipe requires simmering until the ingredients are tender.
A comparison of the different cooking methods used at the three ethnic food stalls implies that deep frying generates more PM2.5 and PAHs than any other cooking method which could be due to the higher temperature maintained during cooking and the larger amount of oil used in deep frying. Acrylamide, a cancer causing chemical produced when starchy foods like potatoes are fried at high temperatures, has been reported to increase in concentration with temperature.26 Likewise both PM2.5 and airborne PAHs could follow the same trend when high temperature cooking is used. This postulation is supported by the higher mass concentrations of PM2.5 and PAHs, and percentage of PAHs in PM2.5 measured at the Chinese stall than at the Indian stall since the stir-frying cooked method involves a higher temperature and uses more oil than simmering. In addition, the larger quantity of food cooked at the Chinese stall could also contribute to the higher level of particulate pollution.
3.2. Chemical markers of cooking
The most abundant PAHs at the Chinese and Malay stalls were BbF, Ind and BPe, which are combustion-related; other combustion-related PAHs include Flt, Pyr, BaA, Chr, BkF and BaP.27 Hence, the three abundant PAHs could serve as markers of frying operations; stir-frying and deep-frying were the respective commonly used cooking methods at the two stalls. On the other hand, at the Indian stall, Nap, Flu and Phe were most abundant and this trend is similar to that seen in the background air where Acy, Flu and Phe showed the highest concentration. This trend could possibly be due to the low temperature cooking employed at the food stall and thus the more volatile PAHs were emitted in larger amounts than the less volatile ones with higher-molecular weight such as BkF, Ind, and BaP.In order to assess the inter-relationship among the PAHs measured in the food stall, a correlation matrix was constructed. Correlation matrix analysis is often used to find out whether airborne PAHs are derived from a distinct source or process.28,29Table 4 shows the Pearson product moment correlation coefficients (r) (at 95% confidence level) for all PAHs measured at the three different stalls during cooking hours. Correlation coefficients with values ≥0.7 are highlighted in bold. In general, high correlation values were observed for the ten combustion-related PAHs, especially at the Chinese food stall, which is consistent with their abundances. In order to provide further insights into the origin of PAHs, their diagnostic ratios were calculated.
| (a) Chinese stall | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | Nap | Ace | Acy | Flu | Phe | Ant | Flt | Pyr | BaA | Chr | BbF | BkF | BaP | Ind | DBA | BPe |
| Nap | 1 | |||||||||||||||
| Ace | 0.73 | 1 | ||||||||||||||
| Acy | 0.53 | 0.93 | 1 | |||||||||||||
| Flu | 0.36 | 0.85 | 0.84 | 1 | ||||||||||||
| Phe | 0.04 | −0.53 | −0.68 | −0.57 | 1 | |||||||||||
| Ant | 0.04 | 0.40 | 0.54 | 0.40 | −0.20 | 1 | ||||||||||
| Flt | −0.28 | −0.70 | −0.70 | −0.76 | 0.83 | 0.10 | 1 | |||||||||
| Pyr | −0.28 | −0.68 | −0.67 | −0.75 | 0.81 | 0.14 | 1.00 | 1 | ||||||||
| BaA | −0.28 | −0.70 | −0.74 | −0.75 | 0.84 | 0.07 | 0.99 | 0.99 | 1 | |||||||
| Chr | −0.29 | −0.71 | −0.79 | −0.74 | 0.86 | 0.00 | 0.97 | 0.96 | 0.99 | 1 | ||||||
| BbF | −0.30 | −0.73 | −0.80 | −0.75 | 0.87 | −0.02 | 0.97 | 0.96 | 0.99 | 1.00 | 1 | |||||
| BkF | −0.30 | −0.73 | −0.79 | −0.76 | 0.87 | −0.02 | 0.98 | 0.97 | 0.99 | 1.00 | 1.00 | 1 | ||||
| BaP | −0.32 | −0.61 | −0.79 | −0.55 | 0.75 | −0.11 | 0.76 | 0.74 | 0.84 | 0.90 | 0.88 | 0.87 | 1 | |||
| Ind | −0.28 | −0.73 | −0.76 | −0.78 | 0.86 | 0.01 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 0.99 | 0.81 | 1 | ||
| DBA | −0.30 | −0.73 | −0.80 | −0.76 | 0.87 | −0.03 | 0.98 | 0.96 | 0.99 | 1.00 | 1.00 | 1.00 | 0.88 | 0.99 | 1 | |
| BPe | −0.29 | −0.73 | −0.78 | −0.77 | 0.87 | 0.00 | 0.99 | 0.98 | 1.00 | 0.99 | 1.00 | 1.00 | 0.84 | 1.00 | 1.00 | 1 |
| (b) Malay stall | ||||||||||||||||
| Nap | 1 | |||||||||||||||
| Ace | 0.45 | 1 | ||||||||||||||
| Acy | 0.46 | 0.96 | 1 | |||||||||||||
| Flu | 0.19 | 0.79 | 0.84 | 1 | ||||||||||||
| Phe | −0.27 | 0.68 | 0.71 | 0.82 | 1 | |||||||||||
| Ant | −0.04 | 0.81 | 0.86 | 0.89 | 0.97 | 1 | ||||||||||
| Flt | 0.01 | 0.56 | 0.49 | 0.82 | 0.59 | 0.58 | 1 | |||||||||
| Pyr | −0.15 | 0.68 | 0.68 | 0.93 | 0.91 | 0.89 | 0.87 | 1 | ||||||||
| BaA | 0.09 | 0.64 | 0.62 | 0.91 | 0.66 | 0.68 | 0.98 | 0.91 | 1 | |||||||
| Chr | 0.28 | 0.82 | 0.83 | 0.98 | 0.74 | 0.82 | 0.87 | 0.90 | 0.94 | 1 | ||||||
| BbF | 0.57 | 0.42 | 0.40 | 0.60 | 0.06 | 0.18 | 0.75 | 0.43 | 0.76 | 0.70 | 1 | |||||
| BkF | 0.74 | 0.39 | 0.38 | 0.48 | −0.10 | 0.06 | 0.57 | 0.25 | 0.60 | 0.59 | 0.97 | 1 | ||||
| BaP | 0.40 | 0.89 | 0.88 | 0.93 | 0.66 | 0.77 | 0.83 | 0.82 | 0.89 | 0.96 | 0.72 | 0.65 | 1 | |||
| Ind | 0.41 | 0.35 | 0.29 | 0.56 | 0.07 | 0.14 | 0.82 | 0.46 | 0.79 | 0.67 | 0.97 | 0.90 | 0.69 | 1 | ||
| DBA | 0.78 | 0.34 | 0.34 | 0.40 | −0.19 | −0.02 | 0.49 | 0.15 | 0.51 | 0.52 | 0.94 | 0.99 | 0.58 | 0.87 | 1 | |
| BPe | 0.53 | 0.40 | 0.35 | 0.56 | 0.03 | 0.13 | 0.76 | 0.41 | 0.75 | 0.67 | 0.99 | 0.95 | 0.71 | 0.99 | 0.92 | 1 |
| (c) Indian stall | ||||||||||||||||
| Nap | 1 | |||||||||||||||
| Ace | 0.92 | 1 | ||||||||||||||
| Acy | 0.85 | 0.90 | 1 | |||||||||||||
| Flu | 0.51 | 0.46 | 0.78 | 1 | ||||||||||||
| Phe | 0.70 | −0.09 | 0.14 | 0.55 | 1 | |||||||||||
| Ant | 0.69 | 0.34 | 0.35 | 0.41 | 0.82 | 1 | ||||||||||
| Flt | 0.34 | 0.00 | −0.04 | 0.10 | 0.80 | 0.91 | 1 | |||||||||
| Pyr | 0.38 | −0.11 | −0.04 | 0.23 | 0.91 | 0.89 | 0.97 | 1 | ||||||||
| BaA | 0.78 | 0.83 | 0.94 | 0.81 | 0.32 | 0.53 | 0.17 | 0.17 | 1 | |||||||
| Chr | 0.72 | 0.76 | 0.66 | 0.46 | 0.44 | 0.82 | 0.58 | 0.50 | 0.79 | 1 | ||||||
| BbF | 0.65 | 0.65 | 0.66 | 0.63 | 0.60 | 0.84 | 0.59 | 0.57 | 0.82 | 0.96 | 1 | |||||
| BkF | 0.47 | 0.73 | 0.80 | 0.73 | 0.17 | 0.32 | −0.03 | −0.02 | 0.85 | 0.71 | 0.78 | 1 | ||||
| BaP | 0.47 | 0.76 | 0.81 | 0.70 | 0.06 | 0.23 | −0.14 | −0.14 | 0.83 | 0.65 | 0.71 | 0.99 | 1 | |||
| Ind | 0.60 | 0.18 | 0.33 | 0.63 | 0.94 | 0.90 | 0.80 | 0.87 | 0.54 | 0.70 | 0.83 | 0.45 | 0.34 | 1 | ||
| DBA | 0.37 | 0.66 | 0.76 | 0.72 | 0.05 | 0.13 | −0.23 | −0.20 | 0.77 | 0.54 | 0.63 | 0.98 | 0.99 | 0.31 | 1 | |
| BPe | 0.45 | 0.22 | 0.43 | 0.75 | 0.88 | 0.79 | 0.64 | 0.73 | 0.61 | 0.67 | 0.84 | 0.60 | 0.50 | 0.97 | 0.49 | 1 |
The diagnostic ratios of PAHs isomers are also frequently used to investigate the origin or the aging of aerosol particles as part of source apportionment studies.30–33 In addition, the diagnostic ratios can also serve as markers or tracers of pollution sources. Table 5 lists the ratios of Phe/(Ant + Phe) (structural isomers of molecular weight (MW) = 178), Flt/(Flt + Pyr) (MW = 202), BaA/(BaA + Chr) (MW = 228), and Ind/(Ind + BPe) (MW = 276) found at the three different stalls during cooking hours, and the ratios obtained were compared to those measured in other commercial kitchens.14–15,21 To be consistent with the other studies reported in the literature, all ratios were evaluated based on the mean concentrations. With the exception of Phe/(Phe + Ant), all the other ratios fall within the range of those found in other studies, confirming that the PAHs measured in the three kitchens originated from food cooking. The diagnostic ratios of PAHs released other common sources such as engine exhaust and biomass combustion are also included in the table for the sake of comparison. It can be seen that the ratios from the same source even varied over a range due to the type of particulate and/or gaseous emissions collected, the proximity to the sources, etc.
| PM | Type of kitchen |
|
|
|
|
Reference |
|---|---|---|---|---|---|---|
| Gas cooking | ||||||
| PM2.5 | Chinese | 0.21 | 0.32 | 0.40 | 0.43 | This study |
| PM2.5 | Malay | 0.28 | 0.38 | 0.32 | 0.38 | This study |
| PM2.5 | Indian | 0.21 | 0.43 | 0.50 | 0.39 | This study |
| TSP and gas | Chinese | 0.86 | 0.50 | 0.62 | 0.63 | 14 |
| TSP and gas | Western | 0.86 | 0.46 | 0.38 | 0.63 | 14 |
| TSP and gas | Fast food | 0.96 | 0.60 | 0.32 | 0.53 | 14 |
| TSP and gas | Japanese | 0.97 | 0.66 | 0.13 | 0.83 | 14 |
| PM2.5 | Chinese, Hunan | 0.96 | 0.44 | 0.51 | — | 15 |
| PM2.5 | Chinese, Cantonese | 1.00 | 0.36 | 0.47 | 0.19 | 15 |
| TSP and gas | Chinese | 0.51 | 0.18 | 0.74 | — | 21 |
| TSP and gas | Chinese | 0.41 | 0.19 | 0.18 | — | 21 |
| TSP and gas | Chinese | 0.37 | 0.23 | 0.22 | — | 21 |
| TSP and gas | Chinese | 0.51 | 0.23 | 0.38 | — | 21 |
| Diesel engines | ||||||
| TSP | — | 0.90 | 0.61 | 0.14 | — | 36 |
| PM2 | — | 0.88 | 0.37 | 0.27 | — | 37 |
| TSP and gas | — | 0.65 | 0.62 | 0.64 | 0.70 | 38 |
| PM10 | 0.97 | 0.38 | 0.73 | 0.96 | 39 | |
| Gasoline engines | ||||||
| TSP and gas | — | 0.51 | 0.38 | 0.17 | — | 38 |
| PM2.5 | — | — | 0.43 | 0.51 | 0.27 | 40 |
| PM10 | — | 0.68 | 0.31 | 0.42 | 0.79 | 41 |
| Biomass | ||||||
| TSP and gas | Oak wood | 0.38 | 0.49 | 0.36 | — | 38 |
| TSP and gas | Wood (eucalyptus chip) | 0.71 | 0.67 | 0.48 | 0.69 | 42 |
| TSP and gas | Coal briquettes | 0.45 | 0.32 | 0.15 | — | 42 |
| TSP and gas | Charcoal (mangrove) | 0.79 | 0.33 | 0.37 | — | 42 |
| TSP and gas | House coal | 0.60 | 0.48 | 0.59 | 0.37 | 43 |
| TSP and gas | Hardwood | 0.80 | 0.52 | 0.53 | 0.57 | 43 |
3.3. Occupational health risk assessment
To obtain a better estimate of the health risks associated with gas cooking at the different stalls, the mass concentrations of PM2.5 and PAHs were compared to the present regulatory standards which were established to protect public or workers’ health with adequate safety margin. In 1997, the USEPA established PM2.5 National Ambient Air Quality Standard (NAAQS) at 15 μg m−3 for the annual standard (3 year average of the annual arithmetic mean concentrations) and 65 μg m−3 (3 year average of the 98th percentile of 24 h concentrations) for the 24 h standard; other PM2.5 standards also exist for different countries. However, similar indoor air quality standards or guidelines do not currently exist for PM2.5. The PM2.5 levels measured at the food stalls over a 24 h period were 115.6 μg m−3, 137.4 μg m−3, 108.1 μg m−3 at the Chinese, Malay and Indian stall, respectively which are in far exceedance of the 24 h NAAQS, and are thus of health concern.Exposure to total PAHs is regulated by the National Institute for Occupational Safety and Health (NIOSH) with the recommended exposure limit (REL) at 0.1 mg m−3 for an 8 h time weighted average (TWA) exposure, and the Occupational Safety and Health Administration (OSHA) with the permissible exposure limit (PEL) at 0.2 mg m−3 for a 10 h TWA exposure. The total mass concentrations of PAHs measured at the stalls are much lower than the REL and PEL. However, it should be noted that these limits are much higher than the target annual mean values of BaP (most carcinogenic PAH) of 0.7 to 1.3 ng m−3 established by a few European countries.34
The equivalent mass concentrations of BaP (CBaPeq) in the food stall were calculated over a 24 hour period according to the following equation:
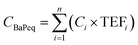 | (6) |
The inter-comparison of PM2.5 and PAHs mass concentrations with the regulatory standards or exposure limits is just a qualitative estimate. A better understanding of the associated health risks at the food stalls can be achieved by calculating the ELCR according to eqn (2) to (4). The ELCRs were estimated to be 4.08 × 10−3, 1.21 × 10−2 and 1.07 × 10−3 at the Chinese, Malay and Indian stalls, respectively. These values are much higher than the recommended acceptable limit of 10−6 for ELCR. These calculations suggest that the chefs and other workers in the commercial kitchens, and possibly the clients visiting the food stalls are exposed to an exceedingly large amount of fine particles containing carcinogenic PAHs. Thus, the human exposure to cooking emissions in the food stalls is of serious health concern.
Conclusions
In this study, emissions of fine airborne particulate matter and PAHs from three different types of ethnic food cooking were quantitatively evaluated in commercial kitchens in the Republic of Singapore for the first time. In addition, the molecular composition of PAHs was examined when different cooking methods were used. Both the statistical analysis of the analytical data and the diagnostic ratios of PAHs indicate that these organic compounds originated from gas cooking. PAHs could evaporate directly from the vegetable oil used during cooking as well as arising from the high temperature cracking of organic compounds through the recombination of free radicals formed as intermediates. The average mass concentrations of PM2.5 and PAHs were higher at the Malay food stall compared to those at both the Chinese and Indian food stalls. The increased emissions of fine particles and PAHs are due mainly to the deep frying method used at the former stall, which involves the heating of oil to high temperatures. The quantity of food cooked could also contribute to the increase in the level of particulate pollution in kitchens. The equivalent mass concentrations of BaP were calculated for all three food stalls, and they are higher than the European limit (0.7 to 1.3 ng m−3). The excess lifetime cancer risk analysis indicated that the occupational exposure to cooking emissions in the three commercial kitchens is of serious health concern. Effective protective measures should therefore be undertaken to reduce cooking emissions and/or minimize human exposure to such emissions especially when a large quantity of food is cooked using deep-frying or stir-frying.Acknowledgements
This study was funded by the NUS ARF through Grant No. RP-279-000-142-112. We are grateful to NUS for the financial support provided for the pursuit of this project.References
- V. H. Borja-Aburto, M. Castillejos, D. R. Gold, S. Bierzwinski and D. Loomis, Environ. Health Perspect., 1998, 106, 849–855 CrossRef CAS
.
- R. T. Burnett, M. Smith-Doiron, D. Stieb, S. Cakmak and J. R. Brook, Arch. Environ. Health, 1999, 54, 130–139 Search PubMed
.
- L. Wallace, J. Air Waste Manage. Assoc., 1996, 46, 98–126 CAS
.
- J. Schwartz, D. W. Dockery and L. M. Neas, J. Air Waste Manage. Assoc., 1996, 46, 927–939 CAS
.
- J. S. Lighty, J. M. Veranth and A. F. Sarofim, J. Air Waste Manage. Assoc., 2000, 50, 1565–1618 CAS
.
- E. Abt, H. H. Suh, G. Allen and P. Koutrakis, Environ. Health Perspect., 2000, 108, 35–44 CrossRef CAS
.
- C. M. Long, H. H. Suh and P. Koutrakis, J. Air Waste Manage. Assoc., 2000, 50, 1236–1250 CAS
.
- D. Jarvis, S. Chinn, C. Luczynska and P. Burney, Lancet, 1996, 347, 426–431 CrossRef CAS
.
- Y. C. Ko, L. S. Cheng, C. H. Lee, J. J. Huang, M. S. Huang, E. L. Kao, H. Z. Wang and H. J. Lin, Am. J. Epidemiol., 2000, 151, 140–147 CAS
.
- M. Dennekamp, S. Howarth, C. A. Dick, J. W. Cherrie, K. Donaldson and A. Seaton, Occup. Environ. Med., 2001, 58, 511–516 CrossRef CAS
.
- L. A. Wallace, S. J. Emmerich and C. Howard-Reed, Environ. Sci. Technol., 2004, 38, 2304–2311 CrossRef CAS
.
- W. F. Rogge, L. M. Hildemann, M. A. Mazurek, G. R. Cass and B. R. T. Simoneit, Environ. Sci. Technol., 1991, 25, 1112–1125 CAS
.
- P. F. Wu, T. A. Chiang, L. F. Wang, C. S. Chang and Y. C. Ko, Mutat. Res., 1998, 403, 29–34 CrossRef CAS
.
- C. T. Li, Y. C. Lin, W. J. Lee and P. J. Tsai, Environ. Health Perspect., 2003, 111, 483–487 CAS
.
- L. Y. He, M. Hu, X. F. Huang, B. D. Yu, Y. H. Zhang and D. Q. Liu, Atmos. Environ., 2004, 38, 6557–6564 CrossRef CAS
.
- T. Sugimura, K. Wakabayashi, H. Nakagama and M. Nagao, Cancer Sci., 2004, 95, 290–299 Search PubMed
.
- G. R. Cass, Trends Anal. Chem., 1998, 17, 356–366 CrossRef CAS
.
- S. C. Lee, W. M. Li and L. Y. Chan, Sci. Total Environ., 2001, 279, 181–193 CrossRef CAS
.
- S. C. Lee, W. M. Li and C. H. Ao, Atmos. Environ., 2002, 36, 225–237 CrossRef CAS
.
- L. Morawska, C. R. He, J. Hitchins, K. Mengersen and D. Gilbert, Atmos. Environ., 2003, 37, 4195–4203 CrossRef CAS
.
- L. Z. Zhu and J. Wang, Chemosphere, 2003, 50, 611–618 CrossRef CAS
.
-
S. Karthikeyan, R. Balasubramanian and S. W. See, Talanta, 2005, DOI:10.1016/j.talanta.2005.08.060
.
- United States Department of Energy, Guidance for Conducting Risk Assessments and Related Risk Activities for the DOE-ORO Environmental Management Program, BJC/OR-271, USDOE, 1999, http://risk.lsd.ornl.gov/homepage/rap_docs.shtml, 18th January 2006 Search PubMed.
- N. T. K. Oanh, L. B. Reutergardh and N. T. Dung, Environ. Sci. Technol., 1999, 33, 2703–2709 CrossRef CAS
.
- J. D. McDonald, B. Zielinska, E. M. Fujita, J. C. Sagebiel, J. C. Chow and J. G. Watson, J. Air Waste Manage. Assoc., 2003, 53, 185–194 CAS
.
- C. Gertz and S. Klostermann, Eur. J. Lipid Sci. Technol., 2003, 104, 762–771
.
- I. G. Kavouras, J. Lawrence, P. Koutrakis, E. G. Stephanou and P. Oyola, Atmos. Environ., 1999, 33, 4977–4986 CrossRef CAS
.
- S. S. Park, Y. J. Kim and C. H. Kang, Atmos. Environ., 2002, 36, 2917–2924 CrossRef CAS
.
- M. Tavares Jr, J. P. Pinto, A. L. Souza, I. S. Scarminio and M. C. Solci, Atmos. Environ., 2004, 38, 5039–5044 CrossRef
.
- X. H. Bi, G. Y. Sheng, P. A. Peng, Z. Q. Zhang and J. M. Fu, Sci. Total Environ., 2002, 300, 213–228 CrossRef CAS
.
- M. B. Yunker, R. W. Macdonald, R. Vingarzan, R. H. Mitchell, D. Goyette and S. Sylvestre, Org. Geochem., 2002, 33, 489–515 CrossRef CAS
.
- H. Guo, S. C. Lee, K. F. Ho, X. M. Wang and S. C. Zou, Atmos. Environ., 2003, 37, 5307–5317 CrossRef CAS
.
- G. C. Fang, C. N. Chang, Y. S. Wu, P. C. P. Fu, I. L. Yang and M. H. Chen, Sci. Total Environ., 2004, 327, 135–146 CrossRef CAS
.
- P. Perez Ballesta, E. De Salgar and D. Kotzias D, Fresenius’ Environ. Bull., 1999, 8, 499–505
.
- C. Nisbet and P. LaGoy, Regul. Toxicol. Pharmacol., 1992, 16, 290–300 CrossRef CAS
.
- R. N. Westerholm, J. Almen, H. Li, J. Ulf Rannug, K. E. Egeback and K. Gragg, Environ. Sci. Technol., 1991, 25, 332–338 CrossRef CAS
.
- W. F. Rogge, L. M. Hildemann, M. A. Mazurek and G. R. Caw, Environ. Sci. Technol., 1993, 27, 636–651 CAS
.
- N. R. Khalili, P. A. Scheff and T. M. Holsen, Atmos. Environ., 1995, 29, 533–542 CrossRef CAS
.
- E. Manoli, A. Kouras and C. Samara, Chemosphere, 2004, 56, 867–878 CrossRef CAS
.
- L. C. Marr, T. W. Kirchstetter, R. A. Harley, A. H. Miguel, S. V. Hering and S. K. Hammond, Environ. Sci. Technol., 1995, 33, 3091–3099
.
- H. H. Yang, S. M. Chien, M. R. Chao and C. C. Lin, J. Hazard. Mater., 2005, 125, 154–159 CrossRef CAS
.
- N. T. K. Oanh, L. B. Reutergardh and N. T. Dung, Environ. Sci. Technol., 1999, 33, 2703–2709 CrossRef CAS
.
- R. G. M. Lee, P. Coleman, J. L. Jones, K. C. Jones and R. Lohmann, Environ. Sci. Technol., 2005, 39, 1436–1447 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2006 |